Abstract
The Mtr respiratory pathway of Shewanella oneidensis strain MR-1 is required to effectively respire both soluble and insoluble forms of oxidized iron. Flavins (riboflavin and flavin mononucleotide) recently have been shown to be excreted by MR-1 and facilitate the reduction of insoluble substrates. Other Shewanella species tested accumulated flavins in supernatants to an extent similar to that of MR-1, suggesting that flavin secretion is a general trait of the species. External flavins have been proposed to act as both a soluble electron shuttle and a metal chelator; however, at biologically relevant concentrations, our results suggest that external flavins primarily act as electron shuttles for MR-1. Using deletion mutants lacking various Mtr-associated proteins, we demonstrate that the Mtr extracellular respiratory pathway is essential for the reduction of flavins and that decaheme cytochromes found on the outer surface of the cell (MtrC and OmcA) are required for the majority of this activity. Given the involvement of external flavins in the reduction of electrodes, we monitored current production by Mtr respiratory pathway mutants in three-electrode bioreactors under controlled flavin concentrations. While mutants lacking MtrC were able to reduce flavins at 50% of the rate of the wild type in cell suspension assays, these strains were unable to grow into productive electrode-reducing biofilms. The analysis of mutants lacking OmcA suggests a role for this protein in both electron transfer to electrodes and attachment to surfaces. The parallel phenotypes of Mtr mutants in flavin and electrode reduction blur the distinction between direct contact and the redox shuttling strategies of insoluble substrate reduction by MR-1.
Shewanella oneidensis strain MR-1 (MR-1) is a facultative anaerobe capable of respiring a variety of substrates, including various metals and metal oxides, a phenotype that is important for bioremediation and metal cycling in natural environments (22, 53). At near-neutral pH, Fe(III) and Mn(IV) often are present as insoluble oxide minerals. Dissimilatory metal-reducing bacteria such as MR-1 have developed pathways to transfer electrons from the interior of the cell to these external terminal electron acceptors. In some bacteria, these pathways also can transfer electrons to electrodes, which can be harnessed for renewable energy and remote biosensor applications (23, 26, 27). Beyond increasing our understanding of this unusual process, applying anaerobic microbial extracellular respiration to new technologies requires a thorough understanding of the molecular dynamics and cellular physiology of electron source utilization (substrate oxidation) and the reduction of insoluble terminal electron acceptor(s). There are four proposed mechanisms to explain how insoluble substrates are reduced by Shewanella: (i) direct contact, (ii) electron shuttling, (iii) chelation, and (iv) electrically conductive appendages (reviewed in reference 18). We will focus on the first three strategies here.
Flavins recently have been discovered to accelerate the reduction of both iron oxide minerals (51) and electrodes (30) by MR-1. Riboflavin (vitamin B2) is a precursor for the biosynthesis of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) (13). Riboflavin and FMN both can be observed to build up in the supernatant of anaerobic and aerobically grown cultures of MR-1 (30, 51). However, the mechanism by which flavins enhance the rate of iron oxide mineral or electrode reduction is unknown, although recent work is consistent with a critical role for these compounds in mediating solid Fe(III) reduction by MR-1 (42). Since soluble (chelated) Fe(III) is reduced faster than insoluble Fe(III) by MR-1 (6), one possible explanation for the enhancement of insoluble iron reduction by flavins is increased available soluble iron via chelation (1, 2, 30). Flavins also may be utilized as redox-active compounds to traffic electrons between extracellular reductases on the surface of the cell and insoluble substrates (30, 51), a process termed electron shuttling (18, 39, 41). The chelation of the terminal electron acceptor during electrode reduction is not relevant when the anode is composed of graphite. Therefore, electron shuttling likely is responsible for the flavin enhancement of current production on poised-potential electrodes (30). However, it is unclear if the chelation of metals by flavins influences insoluble metal reduction by S. oneidensis (30).
The Mtr pathway is required for the reduction of metals and electrodes (5, 6, 9, 17). Five primary protein components have been identified in this pathway: OmcA, MtrC, MtrA, MtrB, and CymA (47). Current models of electron transfer in MR-1 assume that electrons from carbon source oxidation are passed via the menaquinone pool to the inner membrane-anchored c-type cytochrome CymA (19, 31). These electrons then are transferred to a periplasmic c-type cytochrome, MtrA, and eventually to outer membrane (OM)-anchored c-type cytochromes MtrC and OmcA, which interact with an integral OM scaffolding protein, MtrB (32, 33, 43). These OM cytochromes then can reduce various substrates, including iron oxides and electrodes (8, 9, 12, 36, 47). Since the Mtr system is required by MR-1 to reduce many different substrates (18), it also could be capable of reducing extracellular flavins. Indeed, electron transfer to carbon electrodes is impaired in strains lacking Mtr pathway components (9, 17), which may be explained simply by a decreased ability to reduce extracellular flavins. The observation that Mtr mutants produce less current on electrodes than the wild type could be due to (i) less current generated per cell (either direct reduction or flavin mediated), (ii) decreased attachment to the electrode surface, (iii) differences in external flavin concentrations, or (iv) a combination of these three possibilities. Determining the specific activity (current produced per unit of attached biomass) of Mtr mutants on electrodes under conditions where flavin levels were controlled would allow for differentiation between these possibilities. To date, this kind of analysis has not been reported.
The results presented here extend our knowledge of how S. oneidensis catalyzes the reduction of insoluble substrates. Experiments using a model iron chelator and electron shuttle are consistent with electron shuttling being the primary mechanism by which flavins enhance insoluble iron oxide reduction rates. Moreover, we demonstrate that MR-1 reduces extracellular flavins at physiologically relevant rates and that the Mtr pathway accounts for at least 95% of this activity. The specific activities of various mutant strains lacking Mtr pathway components on poised-potential electrodes also are reported. Our data suggest that MtrC is responsible for most of the electron transfer to carbon electrodes, while OmcA is involved in attachment and has a lesser role in electron transfer. These observations could have broader implications regarding the role of OmcA in the reduction of soluble and insoluble substrates (8, 9, 36).
MATERIALS AND METHODS
Bacterial strains and media used.
S. oneidensis strain MR-1 originally was isolated from Oneida Lake in New York (34, 50). Overnight cultures were made from single colonies freshly streaked from a frozen stock culture inoculated into 2 ml of Luria-Bertani (LB) medium and aerobically grown for 16 h. The minimal medium used for experiments was Shewanella basal medium (SBM) as previously described (21). Anaerobic cultures were flushed with nitrogen gas for 15 min in Balch anaerobic tubes sealed with a butyl rubber stopper (3). All anaerobic cultures were supplemented with 0.05% Casamino Acids and contained 40 mM fumarate or 5 mM iron oxide (ferrihydrite) prepared as previously described (45) as the electron acceptor, where indicated. S. baltica strain OS155, S. putrefaciens strain CN-32, S. loihica strain PV-4, S. frigidimarina strain NCIMB 400, S. denitrificans strain OS217T, and S. amazonensis strain SB2B were obtained from Jim Fredrickson (Pacific Northwest National Laboratories).
Total flavin quantification.
Overnight cultures of different strains of Shewanella were grown aerobically for 16 h in LB medium, and the optical density (OD) was read at 600 nm. Cultures were centrifuged, and oxidized supernatants were analyzed in a 96-well plate in a Molecular Devices SpectraMax Pro reader with an excitation wavelength of 440 nm and an emission wavelength of 525 nm. Standard curves were made from stock solutions of oxidized riboflavin or FMN in LB medium for comparison. High-performance liquid chromatography (HPLC) was performed as previously described (30) on the same supernatants to confirm that fluorescence was due specifically to riboflavin and FMN.
Flavin reduction assay.
OD readings of overnight cultures were taken at 600 nm, and cells were washed once with SBM and resuspended to an OD of 4.0 (verified by monitoring diluted samples). For cuvette experiments, 100 μl of this suspension was injected into a sealed cuvette containing 1 ml of SBM with vitamins and minerals to obtain a final concentration of 12 μM riboflavin and either 18 mM lactate or no electron donor. The sealed cuvette was purged with nitrogen gas for 5 min prior to cell addition. For reoxidation experiments the same protocol was followed, except that a 1:10 dilution of cells was used (for a final OD600 of 0.4). After 1 h, the cuvette was removed from the spectrophotometer and vortexed thoroughly without the stopper for ∼10 s and resealed, and then readings were resumed.
For 96-well plate assays, 20 μl of mutant or wild-type cultures prepared as described above were aliquoted into a black 96-well plate in a Coy anaerobic chamber containing an atmosphere of 5% hydrogen, 20% carbon dioxide, and 75% nitrogen. Reactions were initiated with 280 μl SBM to obtain a final concentration of 120 μM riboflavin and 18 mM lactate, and fluorescence (excitation, 440 nm; emission, 525 nm) was monitored over time. High concentrations of flavins were used in these experiments to obtain accurate and reproducible reduction rate measurements for the various strains tested.
Iron reduction.
MR-1 was grown overnight aerobically in LB medium, washed once, and resuspended in SBM to an OD of 1.30. Thirty microliters of this suspension was added to 270 μl of SBM containing vitamins; minerals; final concentrations of 18 mM lactate, 4.5 mM ferrihydrite, and 12 μM of 9,10-anthraquinone-2,6-disulfonic acid (AQDS); 12 mM or 12 μM sodium citrate; or 12 μM riboflavin in 96-well plates. Plates were kept in a GasPak System anaerobic petri dish holder that was flushed for 15 min with nitrogen gas between time points. At each time point, the oxidation of Fe(II) was prevented by adding 50 μl of 5 M hydrochloric acid (HCl). Thirty microliters was taken from this well and diluted in 270 μl of 0.5 M HCl to yield concentrations within the range of standard curves. Fifty microliters of the diluted sample was mixed with 300 μl ferrozine reagent (48) and read at 562 nm. Standard curves were made from ferrous sulfate dissolved in 0.5 N HCl.
Mutant construction/verification.
Mutant strains lacking mtrC, mtrB, mtrA, and omcA have been described previously (21) and were generated by the ligation of regions flanking target genes into the suicide vector pSMV3. For the strain lacking both omcA and mtrC, the upstream and downstream primers were as follows: DCdelUP1, NNNGAGCTCTTATGCTAACTGGGGAACC; DCdelUP2, NNNGAATTCACAGCATCATCTGAGTTCCT; DCdelDOWN1, NNNGGTCTCGTAATTTGCCCAAGCAGG; and DCdelDOWN2, NNNACGAGTCATTCACACTACCGTGAG. PCR using primers flanking target genes confirmed that deletions had occurred, and all single deletion strains were complemented using the expression vector pBBR1MCS-2 (24). Quantitative reverse transcription-PCR (RT-PCR) was performed on all mutant strains using a DyNAmo Sybr green kit according to the manufacturer's protocol and read using an Applied Biosystems 7900 HT sequence detection system.
Operation of the three-electrode bioreactor.
Bioreactors were constructed as previously described (30). Cells inoculated from a fresh LB medium overnight culture were grown aerobically for 24 h in SBM with 0.05% Casamino Acids, 30 mM lactate, and 40 mM fumarate. A 10% transfer into anaerobic SBM with vitamins, minerals, 0.05% Casamino Acids, 20 mM lactate, and 40 mM fumarate was performed, and the cells were allowed to grow until an OD between 0.3 and 0.4 was achieved. Ten milliliters of this anaerobic culture was used to inoculate sterile and nitrogen-degassed electrochemical cells. The chamber was flushed continuously with oxygen-free nitrogen gas, and the electrodes were maintained at oxidizing potential (+0.242 V versus SHE [standard hydrogen electrode]) using a 16-channel VMP potentiostat (Bio-Logic SA). To ensure that all strains were exposed to similar initial flavin concentrations, the bioreactor medium was supplemented with 250 nM riboflavin. Additional lactate at 10 mM also was added at the start of the experiment to prevent carbon source limitation during growth.
CA measurements.
The electron transfer rate at the poised (+0.242 V versus SHE) working electrode was measured during a period of 96 h by recording amperometry data points every 900 s. Chronoamperometry (CA) growth curves were collected four times for each strain to demonstrate the repeatability of the procedure and increase statistical significance. To obtain data from fully colonized electrodes under identical flavin and lactate concentrations, the medium was replaced when strains reached a phase of maximum current production. Within 6 h of this medium replacement (containing 20 mM lactate and 0.5 μM riboflavin), current production stabilized, and electrodes were harvested for protein determination.
BCA protein measurement.
Attached biomass was quantified using a bicinchoninic acid (BCA) assay (Pierce). Electrodes were detached from bioreactors and placed in a 1.5-ml tube containing 1 ml of 0.2 N NaOH. The tubes were heated at 96°C in a heat block for 20 min to lyse and detach cells attached to the electrode surface. The tubes were cooled to room temperature and assayed according to the manufacturer's protocol at 562 nm using a SpectraMax Pro (Molecular Devices). Standard curves were generated using bovine serum albumin.
Confocal microscopy.
Biofilm coverage and thickness were analyzed by confocal imaging. Carbon working electrodes prepared as described above displaying steady-state current production were harvested and rapidly placed in amber 1.5-ml centrifuge tubes containing 1 ml SBM with 60 μM propidium iodide and 10 μM Syto 9 from a Live/Dead BacLight bacterial viability kit (Invitrogen, Carlsbad, CA). The sample was stained for 20 min, rinsed, and placed in the center of a microscope slide surrounded by a gasket. The basin was filled with 50 μl SBM, and a large coverslip was sealed in place. The stained biofilm was viewed with a Nikon Eclipse C1 confocal microscope using 488- and 561-nm filters. The morphology and coverage of the biofilm were evaluated at several locations, and representative sites were chosen for the collection of three-dimensional images using 0.5-μm intervals, beginning approximately 20 μm above the top of the film and continuing until 20 μm beyond the electrode/biofilm interface. The resulting slices were rendered using EZ-C1 3.20 FreeViewer software. Confocal images were picked as the best representatives of at least n = 3 experiments for each strain, with z projections performed at multiple locations on each electrode to ensure accurate results. All averaged protein data are the results of at least n = 5 measurements for each strain.
RESULTS
Flavin production by Shewanella strains.
MR-1, Shewanella sp. strain MR-4, and Shewanella strains isolated from textile waste facilities have been shown previously to produce and excrete flavins (30, 51). We tested other Shewanella strains closely related to MR-1, and several much more distantly related strains (as determined by 16S rRNA gene phylogeny [22]), to determine if flavin accumulation in supernatants is a common feature of these bacteria. The strains tested were S. baltica strain OS155, S. putrefaciens strain CN-32, S. loihica strain PV-4, S. frigidimarina strain NCIMB 400, S. denitrificans strain OS217T, and S. amazonensis strain SB2B. Of the strains tested, all have been reported to reduce iron oxide, except S. denitrificans (10, 22), and all accumulated flavins at levels near the value observed for MR-1 (Table 1). Flavins accumulated to ∼1.0 μM in cultures grown in rich medium, and this accumulation appears to be a general trait among the shewanellae (as previously suggested by von Canstein et al. [51]), and it may have additional functionality beyond accelerating the rates of insoluble substrate reduction in certain species such as S. denitrificans.
TABLE 1.
Flavin production in rich medium by various Shewanella speciesa
| Strain | Flavin concn. measured as: |
|
|---|---|---|
| μM (in culture) | μmol/mg protein | |
| S. oneidensis MR-1 | 1.31 ± 0.20 | 2.8 ± 0.4 |
| S. frigidimarina NCIMB 400 | 1.01 ± 0.10 | 2.4 ± 0.2 |
| S. loihica PV-4 | 1.05 ± 0.16 | 5.3 ± 0.8 |
| S. baltica OS155 | 0.56 ± 0.04 | 2.8 ± 0.2 |
| S. amazonensis SB2B | 0.87 ± 0.28 | 1.9 ± 0.6 |
| S. denitrificans OS217T | 0.54 ± 0.03 | 3.0 ± 0.2 |
Strains were grown for 16 h aerobically, and supernatant fluorescence was quantified to determine total flavin excreted. PV-4, OS155, NCIMB 400, and OS217T were grown for 30 h to reach optical densities similar to those of other strains.
Chelation and electron shuttling.
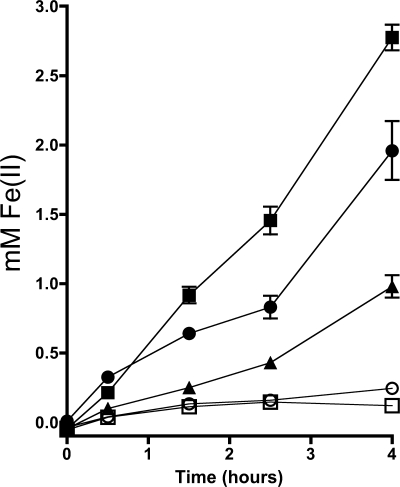
We previously hypothesized that flavins have two distinct mechanisms to accelerate rates of insoluble iron reduction by MR-1: chelation and electron shuttling (30). To observe the effects of extracellular chelators and electron shuttles on insoluble iron reduction in MR-1, we conducted experiments using AQDS (a synthetic analogue of the redox active moieties in humic acids) as a model electron shuttle and citrate as a model iron chelator. We quantified the degree to which these compounds increased iron oxide reduction rates when added exogenously to MR-1 cultures. With no addition, MR-1 reduced iron oxide at an initial rate of 75.7 μM/h under the conditions tested. AQDS at 12 μM increased this rate to 183 μM/h (Fig. 1), while the same amount of riboflavin resulted in a rate of 317 μM/h. Using FMN instead of riboflavin yielded identical results (data not shown). Citrate addition did not have a significant effect at the same concentration (12 μM), but at concentrations 1,000 times higher (12 mM) the initial rate of iron reduction was increased to 613 μM/h. The binding constant of citrate for iron is four orders of magnitude higher than that of riboflavin (2, 15), suggesting that at μM concentrations, the chelation of iron by flavins does not significantly contribute to the increased rate of iron reduction. These data are consistent with the electron shuttling activity of flavins as the primary mechanism for the enhancement of insoluble iron reduction by MR-1.
FIG. 1.
Acceleration of S. oneidensis iron oxide reduction by electron shuttles versus that by a strong chelator. MR-1 cells were inoculated into SBM containing lactate, iron oxide, and 12 μM AQDS (▴), 12 μM riboflavin (•), 12 μM sodium citrate (○), 12 mM sodium citrate (▪), or no addition (□) and incubated anaerobically. Fe(II) formation was monitored over time using a standard ferrozine assay. Error bars indicate standard errors from three replicates.
Flavin reduction by wild-type cells.
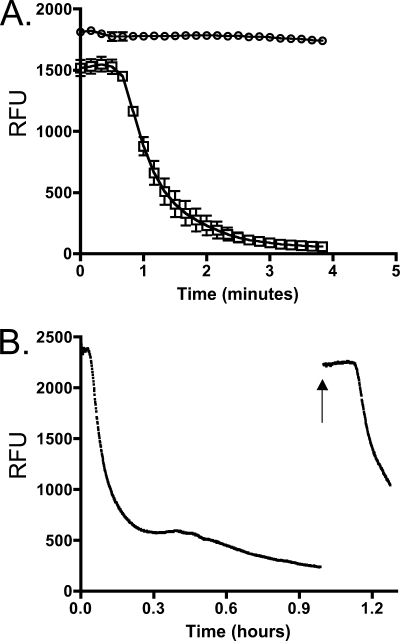
If flavins shuttle electrons between cells and solid surfaces, extracellular flavins must be reduced directly by MR-1. Flavins fluoresce when oxidized but not when reduced (16), making it possible to monitor the oxidation state of riboflavin over time. When wild-type cells were anaerobically incubated with riboflavin and lactate, fluorescence decreased over time, which is consistent with the reduction of flavins (Fig. 2A). When lactate was omitted from the reaction, fluorescence did not change over time, demonstrating that this loss of fluorescence was dependent on electron donor availability (Fig. 2A). The fluorescence change of cultures incubated with FMN occurred at the same rate as that of riboflavin (data not shown), indicating that FMN is reduced at the same rate as that of riboflavin. After fluorescence readings had reached a minimum value, samples were exposed to oxygen. Fluorescence readings returned to the same value as that at the beginning of the experiment (Fig. 2B) and could be reduced again when returned to anaerobic conditions. These experiments confirmed that flavins were not consumed or destroyed and could be recycled. Therefore, MR-1 is able to couple the reduction of flavins to the oxidation of lactate in a manner consistent with its use as a continuously regenerated electron shuttle.
FIG. 2.
Development of flavin reduction assay by S. oneidensis. (A) MR-1 cells were washed in minimal medium and injected to an anaerobic cuvette containing minimal medium and riboflavin as the electron acceptor and given either 18 mM lactate (□) or no carbon source (○). Relative fluorescence units (RFU) were measured over time as described in the text. Error bars represent standard deviations from triplicate experiments. (B) MR-1 cells were examined as described for panel A, only a lower cell density was used. The arrow indicates when the culture was exposed to the atmosphere and vortexed for ∼10 s. Culture then was resealed and readings were resumed. This figure is representative of three independent experiments.
Flavin reduction by Mtr mutants.
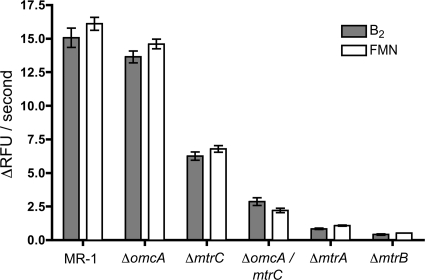
To determine if the Mtr pathway is used in the reduction of flavins, gene deletion mutants were constructed. Strains lacking the genes omcA (ΔomcA), mtrC (ΔmtrC), mtrA (ΔmtrA), and mtrB (ΔmtrB), as well as a strain lacking both omcA and mtrC (ΔomcA ΔmtrC), were tested for their ability to reduce both riboflavin and FMN. Both flavins were tested here, because they have been detected previously in wild-type cultures of electrode (28) and iron-reducing cultures (49) of S. oneidensis strain MR-1. Quantitative RT-PCR confirmed that in all constructed mutants, the expression of the remaining mtr genes was not altered more than twofold compared to that of the wild type (see Table S1 in the supplemental material). Mutants in the Mtr pathway did not reduce flavins at the same rate as the wild type, indicating that this pathway is involved in the reduction of riboflavin and FMN by MR-1 (Fig. 3). All single gene deletion strains were complemented, and flavin reduction activity was restored to near-wild-type rates (data not shown). Both MtrA and MtrB were essential for this process, as ΔmtrA and ΔmtrB reduced flavins at less than 5% of the rate of MR-1. Mutants lacking OmcA reduced flavins at nearly the same rate as the wild type, while ΔmtrC reduced flavins at about half the rate of the wild type. ΔomcA ΔmtrC reduced flavins at only ∼10% of the rate of MR-1 (Fig. 3). This suggests that OmcA can reduce riboflavin and FMN in the absence of MtrC, but it does not significantly contribute to the observed rate in wild-type cells. We conclude that MR-1 transfers electrons from the interior of the cell to flavins using the Mtr pathway, and approximately 90% of this activity occurs at the outer membrane using the OM proteins OmcA and MtrC.
FIG. 3.
Reduction rates of riboflavin and FMN by mutants in the Mtr pathway. Rates were calculated over the linear portion of the initial reduction curves, such that all r2 values were greater than 0.95. Error bars indicate standard errors of reduction rates from three independent cultures. Under these experimental conditions, 1 μM flavin is equal to approximately 166 relative fluorescence units (RFU).
Specific activity of Mtr mutants in three-electrode bioreactors.
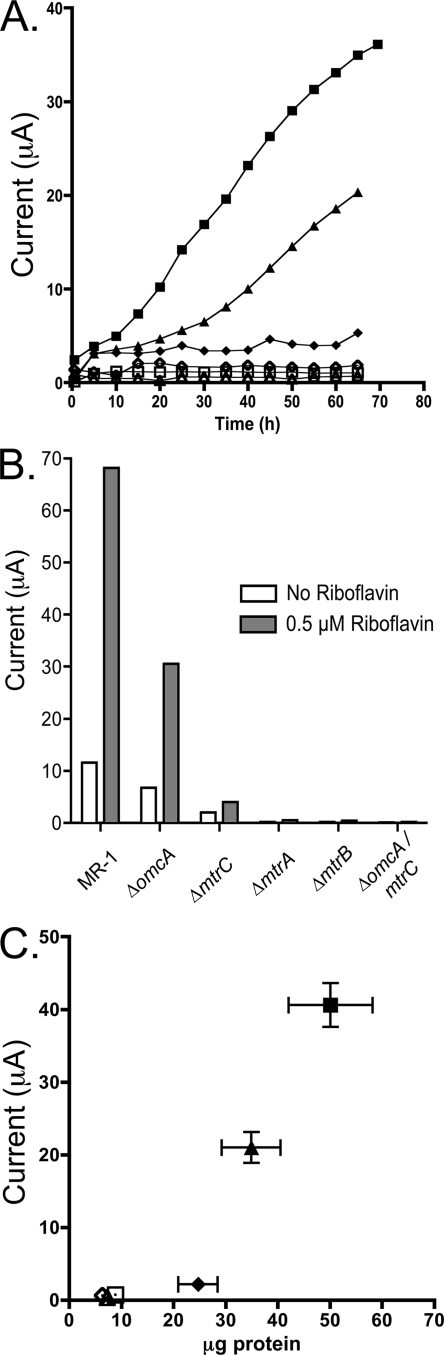
The presence of flavins has been shown to significantly accelerate current production when MR-1 utilizes carbon electrodes as a terminal electron acceptor (30). If the Mtr pathway is responsible for flavin-mediated electrode reduction as well, the ability of strains to reduce flavins should mirror rates of current production, especially in a poised-electrode system where the electron acceptor is not limiting. To address this, current production was monitored over time for all mutant strains when grown in a three-electrode bioreactor until a maximum current was obtained (Fig. 4A). Consistently with defects in flavin reduction, ΔmtrC, ΔmtrA, ΔmtrB, and ΔomcA ΔmtrC all grew slower and produced less maximum current than the wild type. Strikingly, ΔomcA produced only 50% of the maximum current of the wild type, although it had only a slight defect in flavin reduction (Fig. 3).
FIG. 4.
Analysis of electrode reduction capacity of Mtr pathway mutants. (A) Example current production over time by Mtr mutants, using controlled-potential 2-cm2 graphite electrodes. The single curves shown are representative of four replicates. Experiments were performed with MR-1 (▪), ΔomcA (▴), ΔmtrC (⧫), ΔmtrA (□), ΔmtrB (▵), and ΔomcA ΔmtrC (⋄). (B) Flavin acceleration of electrode reduction by S. oneidensis requires the Mtr pathway. Cultures were grown on electrodes to a maximum current production rate (72 to 96 h) and were washed free of external flavins. Current produced at electrodes was recorded after a 6-h equilibration period (white bars). Riboflavin at 0.5 μM was added and current was again recorded after a 6-h equilibration period (gray bars). Representative data from at least two experiments are shown. (C) Maximum current production versus protein attached to electrode surface for different strains. Current production data were recorded for each strain at a flavin concentration of 0.5 μM. Experiments were performed using MR-1 (▪), ΔomcA (▴), ΔmtrC (⧫), ΔmtrA (□), ΔmtrB (▵), and ΔomcA ΔmtrC (⋄). Error bars indicate standard errors from triplicate experiments.
As all of these observations were collected at identical levels of extracellular flavins, differences in flavin concentrations could not explain the observed differences between strains. In addition, measurements also were collected for electrode-attached cells that were washed free of extracellular flavins. The depletion of flavins decreased current production by all wild-type and mutant cultures by more than 70% (Fig. 4B), demonstrating that flavin shuttling was the dominant route of electron transfer in all of these incubations.
It was unknown if Mtr mutant strains produced lower current at electrodes due to lower per-cell rates of electron transfer or simply less attachment by cells to electrode surfaces, both of which are required for current production (30). To differentiate between these two possibilities, total protein attached to electrode surfaces was quantified after measuring the maximum current for each of the mutant strains and the wild type under identical flavin concentrations. The most electrode-attached protein was observed with wild-type biofilms (50.1 ± 6.1 μg total protein per electrode), while strains lacking omcA (34.9 ± 4.3 μg total protein per electrode) and mtrC (24.7 ± 1.4 μg total protein per electrode) attached to electrodes 70 and 50% as effectively as the wild type, respectively (Fig. 4C). Protein quantification revealed that ΔmtrA, ΔmtrB, and ΔomcA ΔmtrC did not significantly colonize electrode surfaces (Fig. 4C). Calculating electron transfer rates as specific activities revealed the current production per unit of attached protein to be 0.81 μA/μg protein for MR-1, 0.60 μA/μg protein for ΔomcA, and 0.090 μA/μg protein for ΔmtrC (Fig. 4C). The complementation of ΔomcA returned iron reduction and current production to wild-type levels (see Fig. S2 in the supplemental material). These results showed that while ΔomcA produced less current per electrode, this difference was due more to a colonization defect than an electron transfer defect per se.
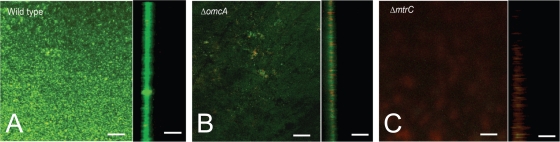
Confocal microscopy of wild-type, ΔomcA, and ΔmtrC strains on electrodes harvested after measuring the maximum current and visualized using live/dead fluorescent staining agreed with total protein differences observed between these strains (Fig. 5). Total volume calculations of biofilms estimated using the Image Structure Analyzer software from the Center for Biofilm Engineering at Montana State University (57) were consistent with attached biomass measurements. Surface plots to aid the visualization of the very thin (1 to 2 μm) biofilms formed by the mutant strains are provided in the supplemental material (see Fig. S2). Interestingly, the small amount of ΔmtrC biomass on the electrode surface stained red, suggesting that these cells possessed compromised membranes, and this explained the very low current output per unit protein observed (Fig. 4C). These results were consistent with the hypothesis that OmcA is required for attachment and/or biofilm formation on electrodes, while MtrC is the primary terminal reductase for electron transfer under electrode-reducing conditions.
FIG. 5.
Confocal microscopy of Shewanella biofilms attached to electrode surfaces. For each panel, the left side is a top-down maximum projection of the electrode surface and the right side is a single representative cross-section (z) view. Bars indicate a length of 40 μm. (A) S. oneidensis strain MR-1 wild-type cells; (B) omcA mutant cells; (C) mtrC mutant cells.
DISCUSSION
Although it was known that secreted flavins increased the rates of electrode (30) and insoluble iron (51) reduction by S. oneidensis, the exact mechanism of this enhancement remained elusive prior to this study. In every species of Shewanella we have tested to date, flavins are found to accumulate in culture supernatants. Intriguingly, one of the few characterized Shewanella species unable to reduce insoluble iron oxide minerals, S. denitrificans, accumulated flavins to similar levels. The genome analysis of S. denitrificans strain OS217 indicates that the genes encoding the Mtr respiratory pathway (omcA, mtrC, mtrB, and mtrA) are absent (14). One possibility is that S. denitrificans recently lost the Mtr pathway, and the secretion of flavins is a metabolic relic. On the other hand, extracellular flavins may be critical for a non-respiratory role, such as iron acquisition (7, 52, 55), the sensing of redox gradients, or promoting associations with other organisms. Such multiple roles for extracellularly secreted molecules may help explain the metabolic investment of producing these compounds.
Several mechanisms have been proposed for the transfer of electrons from the cytoplasm to insoluble extracellular electron acceptors in Shewanella. One explanation involves direct contact by OM proteins, where OmcA and/or MtrC directly reduces substrates unable to diffuse into the periplasm. Indeed, recent research has shown that purified OM cytochromes can reduce electrodes in vitro (12, 54). Electrically conductive appendages also have been observed in MR-1 cultures under certain conditions and have been proposed to be involved in electron transfer (17). When proteins cannot directly contact metals, siderophore production is a common strategy to increase available soluble iron (38), and MR-1 could use a similar mechanism to make soluble iron available to the cell for respiration (18, 49). Small redox-active molecules also can accept electrons from cells and pass them to insoluble substrates, a process termed electron shuttling (18). The observation that MR-1 can reduce insoluble iron at a distance (25) suggests that chelation and/or electron shuttling are utilized, as these mechanisms do not require contact between MR-1 and the iron surface. The work presented here supports the model of electron shuttling using excreted flavins by MR-1. Chelators could increase rates of insoluble iron reduction at high concentrations, but the effect of chelation on iron oxide reduction near natural flavin concentrations appears to be unimportant (Fig. 1). Consistently with this observation, the addition of 12 μM citrate did not stimulate reduction rates of resting cells containing 12 μM AQDS (data not shown).
To verify that flavins can act as electron shuttles, we demonstrated that MR-1 reduces extracellular flavins, and this activity is dependent on the electrons gained from carbon source oxidation. We also demonstrated that the reduction of flavins by MR-1 requires components of the Mtr respiratory pathway. The finding that OmcA does not contribute to the flavin reduction rate in incubations with wild-type cells but can partially compensate for the loss of MtrC suggests a level of functional redundancy or modularity between these OM cytochromes. Importantly, flavins stimulated insoluble iron reduction more effectively than AQDS, suggesting that MR-1 has evolved to use flavins for electron shuttling and that acceleration seen from other exogenous shuttles is a consequence of pathway promiscuity (18).
Though strains lacking OmcA had no defect in flavin reduction in washed-cell incubations, by standardizing for levels of attached protein, controlling for external flavin concentrations, and maintaining a constant electrode potential, we identified a new phenotype for this mutant. Lower current production (per unit of surface area) by strains could be ascribed to both a lower current production rate per cell and a lower degree of attachment by ΔomcA strains. These experiments highlighted the importance of accounting for all variables when using electrodes as electron acceptors. OmcA previously was thought to be only a terminal reductase of metals, which was supported by in vitro biochemical evidence that it can reduce various forms of iron (46, 47, 56). OmcA previously was shown to bind insoluble forms of iron in vitro with a high affinity (56), a greater affinity than that of MtrC (28). We suggest that while OmcA plays a small role in electron transfer in vivo, it also is involved in cellular attachment to solid surfaces or other cells. Our observation is consistent with atomic force microscope experiments demonstrating the unfolding of proteins in the size range of OmcA and MtrC on the outer surface of S. oneidensis bound to iron oxide-coated cantilevers (29). Indeed, mutants lacking OmcA have a marked deficiency in the reduction of insoluble substrates such as electrodes (Fig. 4A) and manganese oxide (36) but not for the reduction of soluble substrates like flavins (Fig. 3), cobalt(III)-EDTA (21), and ferric citrate (8, 36).
Another observation made in this study is that strains lacking MtrC attach to electrodes but produce only 10% of the wild-type current when expressed per unit of attached protein (Fig. 4C). This conflicts with the observation that cell suspensions of ΔmtrC are able to reduce flavins at nearly half the rate of MR-1 (Fig. 3). Several factors may account for this discrepancy; most important is the fact that flavin reduction assays were performed for a short period of time using resting cells, while all electrode experiments require continuous growth and colonization by the strains being tested. Another difference between these assays was the concentration of flavins used: 250 nM in the bioreactor and as much as 120 μM in the resting-cell experiments (which were used to obtain a robust signal for rate calculations). OmcA may replace MtrC more effectively under conditions of high flavin concentrations. It also is clear that a majority of ΔmtrC cells simply were dead (or at least unable to maintain a sufficient membrane potential), although they remained attached to the electrode surface (as shown via confocal microscopy) (Fig. 5), diluting the per-cell respiration rates of ΔmtrC. Regardless, the respiration rates observed for wild-type resting cells (15 relative fluorescence units/s = ∼4 nmol electrons/mg protein/s) were similar to those observed for colonized electrodes (0.8 μA/μg = ∼8 nmol electrons/mg protein/s). Thus, our resting-cell assay accurately reflected the maximal rates of electron transfer in both cases.
The availability of OmcA to promote electrode interaction also may be impaired in cells lacking MtrA (D. Richardson, personal communication) and MtrB (32), as strains lacking MtrB mislocalize the outer membrane proteins MtrC and OmcA. MtrC mutants are able to reduce soluble substrates such as ferric citrate and cobalt(III)-EDTA much more efficiently than mutants lacking MtrA, MtrB, or both OmcA and MtrC (5, 6, 8, 21). If the same trend follows for electrode reduction, cells lacking MtrC may be able to respire enough to begin the colonization of the electrode, whereas ΔmtrA, ΔmtrB, and ΔomcA ΔmtrC cannot. The defect in the electrode reduction of strains lacking MtrA and MtrB could be linked to the fact that these proteins form a complex with MtrC in MR-1 (43). Thus, if any of the components are missing, the entire complex could be inactivated or mislocalized. Overall, this model could explain the observation that while mutant strains lacking OmcA are deficient only in solid substrate reduction, MtrC mutants are defective in the reduction of both soluble and insoluble substrates (8, 9, 35, 36).
We recently demonstrated that OM cytochromes can reduce electrode surfaces in the absence of exogenous flavins, although these rates are dramatically lower than flavin-assisted rates (4), a finding that corroborates experiments that showed that flavins were needed to accelerate the turnover of purified membrane cytochromes to explain observed reduction rates of whole cells (42). While purified cytochromes (MtrC and OmcA) are able to reduce electrodes in the absence of electron shuttles (12, 20), under cell growth conditions, the majority of the current produced in three electrode bioreactors requires the presence of flavins and is critically dependent on the Mtr pathway (Fig. 4). Both direct contact and electron-shuttling strategies absolutely depend on close cell surface contact, because electron shuttles are predicted to be effective only across very short distances (∼1 μm) (40).
We have shown in this study that the Mtr pathway is essential for growth on electrodes and accounts for at least 90% of flavin reduction activity. The specific contribution of flavin shuttling in the reduction of iron oxide, manganese oxide, and many other insoluble substrates by Shewanella still has not been quantified and will require mutants or isolates unable to actively secrete flavins. While previous work suggests that the Mtr pathway accounts for most of the insoluble substrate reduction in S. oneidensis, it remains unknown if these studies were monitoring deficiencies in direct iron/manganese/electrode reduction (5, 6, 9, 11, 37, 44) or simply reflecting deficiencies in flavin reduction by these mutant strains.
Supplementary Material
Acknowledgments
This work was supported by the Office of Naval Research by grants awarded to J.A.G. (N000140810166) and D.R.B. (N000140810162). D.C. was supported by a Biotechnology Training Grant awarded to the University of Minnesota by the National Institutes of Health (2T32-GM008347-16).
Footnotes
Published ahead of print on 6 November 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Albert, A. 1953. Quantitative studies of the avidity of naturally occurring substances for trace metals. III. Pteridines, riboflavin and purines. Biochem. J. 54:646-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert, A. 1950. The metal-binding properties of riboflavin. Biochem. J. 47:xxvii. [PubMed] [Google Scholar]
- 3.Balch, W. E., and R. S. Wolfe. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol. 32:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron, D., E. LaBelle, D. Coursolle, J. A. Gralnick, and D. R. Bond. 2009. Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1. J. Biol. Chem. 284:28865-28873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beliaev, A. S., and D. A. Saffarini. 1998. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J. Bacteriol. 180:6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beliaev, A. S., D. A. Saffarini, J. L. McLaughlin, and D. Hunnicutt. 2001. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722-730. [DOI] [PubMed] [Google Scholar]
- 7.Boretsky, Y. R., O. V. Protchenko, T. M. Prokopiv, I. O. Mukalov, D. V. Fedorovych, and A. A. Sibirny. 2007. Mutations and environmental factors affecting regulation of riboflavin synthesis and iron assimilation also cause oxidative stress in the yeast Pichia guilliermondii. J. Basic Microbiol. 47:371-377. [DOI] [PubMed] [Google Scholar]
- 8.Borloo, J., B. Vergauwen, L. De Smet, A. Brige, B. Motte, B. Devreese, and J. Van Beeumen. 2007. A kinetic approach to the dependence of dissimilatory metal reduction by Shewanella oneidensis MR-1 on the outer membrane cytochromes c OmcA and OmcB. FEBS J. 274:3728-3738. [DOI] [PubMed] [Google Scholar]
- 9.Bretschger, O., A. Obraztsova, C. A. Sturm, I. S. Chang, Y. A. Gorby, S. B. Reed, D. E. Culley, C. L. Reardon, S. Barua, M. F. Romine, J. Zhou, A. S. Beliaev, R. Bouhenni, D. Saffarini, F. Mansfeld, B. H. Kim, J. K. Fredrickson, and K. H. Nealson. 2007. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 73:7003-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brettar, I., R. Christen, and M. G. Hofle. 2002. Shewanella denitrificans sp. nov., a vigorously denitrifying bacterium isolated from the oxic-anoxic interface of the Gotland Deep in the central Baltic Sea. Int. J. Syst. Evol. Microbiol. 52:2211-2217. [DOI] [PubMed] [Google Scholar]
- 11.DiChristina, T. J., C. M. Moore, and C. A. Haller. 2002. Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type II protein secretion gene. J. Bacteriol. 184:142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firer-Sherwood, M., G. S. Pulcu, and S. J. Elliott. 2008. Electrochemical interrogations of the Mtr cytochromes from Shewanella: opening a potential window. J. Biol. Inorg. Chem. 13:849-854. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, M., and A. Bacher. 2008. Biosynthesis of vitamin B2: structure and mechanism of riboflavin synthase. Arch. Biochem. Biophys. 474:252-265. [DOI] [PubMed] [Google Scholar]
- 14.Fredrickson, J. K., M. F. Romine, A. S. Beliaev, J. M. Auchtung, M. E. Driscoll, T. S. Gardner, K. H. Nealson, A. L. Osterman, G. Pinchuk, J. L. Reed, D. A. Rodionov, J. L. Rodrigues, D. A. Saffarini, M. H. Serres, A. M. Spormann, I. B. Zhulin, and J. M. Tiedje. 2008. Towards environmental systems biology of Shewanella. Nat. Rev. Microbiol. 6:592-603. [DOI] [PubMed] [Google Scholar]
- 15.Furia, T. E. 1972. CRC handbook of food additives, 2nd ed. CRC Press, Boca Raton, FL.
- 16.Ghisla, S., V. Massey, J. M. Lhoste, and S. G. Mayhew. 1974. Fluorescence and optical characteristics of reduced flavines and flavoproteins. Biochemistry 13:589-597. [DOI] [PubMed] [Google Scholar]
- 17.Gorby, Y. A., S. Yanina, J. S. McLean, K. M. Rosso, D. Moyles, A. Dohnalkova, T. J. Beveridge, I. S. Chang, B. H. Kim, K. S. Kim, D. E. Culley, S. B. Reed, M. F. Romine, D. A. Saffarini, E. A. Hill, L. Shi, D. A. Elias, D. W. Kennedy, G. Pinchuk, K. Watanabe, S. Ishii, B. Logan, K. H. Nealson, and J. K. Fredrickson. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 103:11358-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gralnick, J. A., and D. K. Newman. 2007. Extracellular respiration. Mol. Microbiol. 65:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gralnick, J. A., H. Vali, D. P. Lies, and D. K. Newman. 2006. Extracellular respiration of dimethyl sulfoxide by Shewanella oneidensis strain MR-1. Proc. Natl. Acad. Sci. USA 103:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartshorne, R. S., B. N. Jepson, T. A. Clarke, S. J. Field, J. Fredrickson, J. Zachara, L. Shi, J. N. Butt, and D. J. Richardson. 2007. Characterization of Shewanella oneidensis MtrC: a cell-surface decaheme cytochrome involved in respiratory electron transport to extracellular electron acceptors. J. Biol. Inorg. Chem. 12:1083-1094. [DOI] [PubMed] [Google Scholar]
- 21.Hau, H. H., A. Gilbert, D. Coursolle, and J. A. Gralnick. 2008. Mechanism and consequences of anaerobic respiration of cobalt by Shewanella oneidensis strain MR-1. Appl. Environ. Microbiol. 74:6880-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hau, H. H., and J. A. Gralnick. 2007. Ecology and biotechnology of the genus Shewanella. Annu. Rev. Microbiol. 61:237-258. [DOI] [PubMed] [Google Scholar]
- 23.Kim, B., H. J. Kim, M. S. Hyun, and D. H. Park. 1999. Direct electrode reaction of Fe(III)-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9:127-131. [Google Scholar]
- 24.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, Jr., and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 25.Lies, D. P., M. E. Hernandez, A. Kappler, R. E. Mielke, J. A. Gralnick, and D. K. Newman. 2005. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl. Environ. Microbiol. 71:4414-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd, J. R., D. R. Lovley, and L. E. Macaskie. 2003. Biotechnological application of metal-reducing microorganisms. Adv. Appl. Microbiol. 53:85-128. [DOI] [PubMed] [Google Scholar]
- 27.Lovley, D. R., D. E. Holmes, and K. P. Nevin. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:219-286. [DOI] [PubMed] [Google Scholar]
- 28.Lower, B. H., L. Shi, R. Yongsunthon, T. C. Droubay, D. E. McCready, and S. K. Lower. 2007. Specific bonds between an iron oxide surface and outer membrane cytochromes MtrC and OmcA from Shewanella oneidensis MR-1. J. Bacteriol. 189:4944-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lower, S. K., M. F. Hochella, Jr., and T. J. Beveridge. 2001. Bacterial recognition of mineral surfaces: nanoscale interactions between Shewanella and alpha-FeOOH. Science 292:1360-1363. [DOI] [PubMed] [Google Scholar]
- 30.Marsili, E., D. B. Baron, I. D. Shikhare, D. Coursolle, J. A. Gralnick, and D. R. Bond. 2008. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 105:3968-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers, C. R., and J. M. Myers. 1997. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J. Bacteriol. 179:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers, C. R., and J. M. Myers. 2002. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1. Appl. Environ. Microbiol. 68:5585-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers, C. R., and J. M. Myers. 2004. The outer membrane cytochromes of Shewanella oneidensis MR-1 are lipoproteins. Lett. Appl. Microbiol. 39:466-470. [DOI] [PubMed] [Google Scholar]
- 34.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 35.Myers, J. M., and C. R. Myers. 2002. Genetic complementation of an outer membrane cytochrome omcB mutant of Shewanella putrefaciens MR-1 requires omcB plus downstream DNA. Appl. Environ. Microbiol. 68:2781-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers, J. M., and C. R. Myers. 2001. Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl. Environ. Microbiol. 67:260-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers, J. M., and C. R. Myers. 2000. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J. Bacteriol. 182:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neilands, J. B. 1984. Siderophores of bacteria and fungi. Microbiol. Sci. 1:9-14. [PubMed] [Google Scholar]
- 39.Newman, D. K., and R. Kolter. 2000. A role for excreted quinones in extracellular electron transfer. Nature 405:94-97. [DOI] [PubMed] [Google Scholar]
- 40.Picioreanu, C., I. M. Head, K. P. Katuri, M. C. van Loosdrecht, and K. Scott. 2007. A computational model for biofilm-based microbial fuel cells. Water Res. 41:2921-2940. [DOI] [PubMed] [Google Scholar]
- 41.Rabaey, K., and W. Verstraete. 2005. Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol. 23:291-298. [DOI] [PubMed] [Google Scholar]
- 42.Ross, D. E., S. L. Brantley, and M. Tien. 2009. Kinetic characterization of OmcA and MtrC, terminal reductases involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 75:5218-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross, D. E., S. S. Ruebush, S. L. Brantley, R. S. Hartshorne, T. A. Clarke, D. J. Richardson, and M. Tien. 2007. Characterization of protein-protein interactions involved in iron reduction by Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 73:5797-5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saffarini, D. A., S. L. Blumerman, and K. J. Mansoorabadi. 2002. Role of menaquinones in Fe(III) reduction by membrane fractions of Shewanella putrefaciens. J. Bacteriol. 184:846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwertmann, U., and R. M. Cornell. 2000. Iron oxides in the laboratory. Wiley VHC, New York, NY.
- 46.Shi, L., B. Chen, Z. Wang, D. A. Elias, M. U. Mayer, Y. A. Gorby, S. Ni, B. H. Lower, D. W. Kennedy, D. S. Wunschel, H. M. Mottaz, M. J. Marshall, E. A. Hill, A. S. Beliaev, J. M. Zachara, J. K. Fredrickson, and T. C. Squier. 2006. Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1. J. Bacteriol. 188:4705-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi, L., T. C. Squier, J. M. Zachara, and J. K. Fredrickson. 2007. Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol. Microbiol. 65:12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stookey, L. L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 49.Taillefert, M., J. S. Beckler, E. Carey, J. L. Burns, C. M. Fennessey, and T. J. DiChristina. 2007. Shewanella putrefaciens produces an Fe(III)-solubilizing organic ligand during anaerobic respiration on insoluble Fe(III) oxides. J. Inorg. Biochem. 101:1760-1767. [DOI] [PubMed] [Google Scholar]
- 50.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49:705-724. [DOI] [PubMed] [Google Scholar]
- 51.von Canstein, H., J. Ogawa, S. Shimizu, and J. R. Lloyd. 2008. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microbiol. 74:615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vorwieger, A., C. Gryczka, A. Czihal, D. Douchkov, J. Tiedemann, H. P. Mock, M. Jakoby, B. Weisshaar, I. Saalbach, and H. Baumlein. 2007. Iron assimilation and transcription factor controlled synthesis of riboflavin in plants. Planta 226:147-158. [DOI] [PubMed] [Google Scholar]
- 53.Wall, J. D., and L. R. Krumholz. 2006. Uranium reduction. Annu. Rev. Microbiol. 60:149-166. [DOI] [PubMed] [Google Scholar]
- 54.Wigginton, N. S., and K. M. Rosso, Jr. 2007. Mechanisms of electron transfer in two decaheme cytochromes from a metal-reducing bacterium. J. Phys. Chem. B 111:12857-12864. [DOI] [PubMed] [Google Scholar]
- 55.Worst, D. J., M. M. Gerrits, C. M. Vandenbroucke-Grauls, and J. G. Kusters. 1998. Helicobacter pylori ribBA-mediated riboflavin production is involved in iron acquisition. J. Bacteriol. 180:1473-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong, Y., L. Shi, B. Chen, M. U. Mayer, B. H. Lower, Y. Londer, S. Bose, M. F. Hochella, J. K. Fredrickson, and T. C. Squier. 2006. High-affinity binding and direct electron transfer to solid metals by the Shewanella oneidensis MR-1 outer membrane c-type cytochrome OmcA. J. Am. Chem. Soc. 128:13978-13979. [DOI] [PubMed] [Google Scholar]
- 57.Yang, X., H. Beyenal, G. Harkin, and Z. Lewandowski. 2000. Quantifying biofilm structure using image analysis. J. Microbiol. Methods 39:109-119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.