Results of this study suggest that treating patients according to evidence-based guidelines is a cost-effective strategy for delivering care to those with non–small-cell lung cancer treated in the outpatient community setting.
Abstract
Purpose:
The goal of this study was to evaluate the cost-effectiveness of Level I Pathways, a program designed to ensure the delivery of evidence-based care, among patients with non–small-cell lung cancer (NSCLC) treated in the outpatient community setting.
Patients and Methods:
We included patients with NSCLC initiating a chemotherapy regimen between July 1, 2006, and December 31, 2007, at eight practices in the US Oncology network. Patients were characterized with respect to age, sex, stage, performance status, and line of therapy and were classified by whether they were treated according to Level I Pathways guidelines. Twelve-month cost of care and overall survival were compared between patients treated on Pathway and off Pathway. A net monetary benefit approach and corresponding cost-effectiveness acceptability curves were used to evaluate the cost-effectiveness of Level I Pathways.
Results:
Overall, outpatient costs were 35% lower for on-Pathway versus off-Pathway patients (average 12-month cost, $18,042 v $27,737, respectively). Costs remained significantly less for patients treated on Pathway versus off Pathway in the adjuvant and first-line settings, whereas no difference in overall cost was observed in patients in the second-line setting. No difference in overall survival was observed overall or by line of therapy. In the net monetary benefit analysis, after adjusting for potential confounders, we found that treating patients on Pathway was cost effective across a plausible range of willingness-to-pay thresholds.
Conclusions:
Results of this study suggest that treating patients according to evidence-based guidelines is a cost-effective strategy for delivering care to those with NSCLC.
Introduction
Lung cancer is the second most common cancer diagnosed in the United States and is the leading cause of cancer-related deaths, with an estimated 219,440 new cases and 159,390 deaths in 2009.1 The economic cost of lung cancer is high, with an estimated cost of $9 billion per year.2 Non–small-cell lung cancer (NSCLC) makes up approximately 80% of lung cancer cases in the United States. More than 70% of patients are diagnosed with stage III to IV disease. Patients with stage III disease have an estimated 5-year survival of 9% to 24% versus only 2% for patients with stage IV disease.3 Because of the incidence, severity, and rising costs, it is becoming increasingly important to deliver consistent, high-quality, cost-effective care for NSCLC.
From 2003 to 2008, the number of oncology-related Investigational New Drug (IND) applications increased from 935 to more than 1,400, with the US Food and Drug Administration Office of Oncology Drug Products approving 53 new indications in the last 3 years.4 These advances are having a growing financial impact on patients and society. Cancer care costs are escalating at a rate of 15% per year, nearly three times the increase in overall health care spending.5
Various chemotherapeutic options are available for NSCLC. However, no single regimen has emerged as the superior choice for treatment of patients with advanced disease,6–8 and there is limited evidence regarding the cost-effectiveness of newer treatment strategies.9 The Level I Pathways program is a physician-led initiative that encourages the consistent delivery of value-driven, evidence-based treatment. The goal of this program is to delineate treatment options that meet the following criteria: maximize survival benefit, minimize toxicities, and provide cost-saving advantages. Level I Pathways are developed and regularly updated by a multidisciplinary task force in collaboration with a network of more than 1,200 practicing community oncologists. To promote standardized and predictable care that meet the abovementioned criteria, Level I Pathways recommendations have been incorporated into the iKnowMed (iKM) electronic medical record (EMR) system, which is currently used by 83% of practices in the US Oncology network. The purpose of this study was to evaluate the cost-effectiveness of treating patients with NSCLC according to Level I Pathways recommendations.
Patients and Methods
Patient Identification and Characterization
Using a retrospective cohort design, we identified all patients with NSCLC initiating a chemotherapy regimen between July 1, 2006, and December 31, 2007, at eight practices in the US Oncology network. Using clinical data from the US Oncology iKM EMR system and online Pathways reporting system, we characterized patients by age, sex, stage at diagnosis, performance status, line of therapy, and vital status. Age, performance status, and line of therapy were measured at initiation of first chemotherapy regimen in the study period. Patients were assigned a Pathway status depending on whether they were treated according to Level I Pathways for NSCLC (Appendix Table A1, online only). Patients were classified as treated: on Pathway, if they received care according to Level I Pathways recommendations for the entire study period; off Pathway, if they were treated with regimens not 0included in Level I Pathways; or both on and off Pathway, if they were switched from being treated on to off Pathway or vice versa during the study period. Because the group of patients treated both on and off Pathway was small (n = 109; 6%), and because of the difficulty in attributing costs and outcome to Pathway status among these patients, they were excluded. To avoid likely confounding of costs and survival estimates, we also excluded patients enrolled onto clinical trials (n = 128; 7%) or patients who had a diagnosis of a cancer other than NSCLC (n = 130; 7%).
Cost Analysis
We compared 12-month cost of care for patients on Pathway versus off Pathway using the Kaplan-Meier Sample Average (KMSA) approach, a robust method for estimating costs over time when complete follow-up is not available and when costs are likely to vary over time.10 Variances and 95% CIs were calculated by applying the percentile method to 10,000 bootstrap samples.11 The cost analysis was conducted from a payer's perspective and considered only direct costs. Discounting was not applied because of the short duration of the cost period. Outpatient charges were extracted from the US Oncology Claims Data Warehouse (CDW), a repository of all claims for services provided in the network. Data included Healthcare Common Procedure Coding System (HCPCS) and Common Procedural Terminology (CPT) codes, date of services, quantities, amounts billed, and primary payers. All charges (except diagnostic services and surgery costs) from the initiation of chemotherapy regimen through 12 months of follow-up were included. We excluded costs related to radiologic services (including radiation therapy) because these services were not provided uniformly across all practices. To normalize cost estimates, unadjusted 2007 Medicare reimbursement rates (Geographic Practice Cost Index 93) were applied. Costs for oral chemotherapy agents were estimated using 2007 average wholesale prices. In addition to directly measuring costs incurred in the US Oncology network, we conducted a sensitivity analysis to estimate the impact of hospitalizations on total cost for patients treated on versus off Pathway. Because claims data captured in the CDW do not capture total hospitalization costs, we calculated an average total cost for an inpatient stay for patients with lung cancer using national claims and reimbursement data from Aetna. This average total hospitalization cost was then applied to the total cost (as calculated using CDW data) for patients who were identified as having been hospitalized to determine if and to what extent inpatient costs might have affected observed cost differences. Hospitalization events were identified by searching for evaluation and management charges in the CDW data corresponding to inpatient admissions and consultations.
To identify underlying cost drivers, cost was broken into several categories (eg, outpatient visits, chemotherapy/nonchemotherapy medications, laboratory services, and ancillary services/therapies) using HCPCS and CPT codes. To address likely confounding that may be associated with Pathway status and cost, stratified analyses were conducted by line of therapy.
Survival Analysis
Twelve-month overall survival was compared between patients treated on Pathway and patients treated off Pathway using the Kaplan-Meier method and corresponding log-rank test. Vital status was supplemented for patients who were lost to follow-up by querying the Social Security Death Master File. Cox proportional hazards regression analysis was used to explore the impact of Pathway status on risk of mortality after adjusting for other relevant clinical and demographic characteristics. The proportionality assumption was confirmed visually.
Net Monetary Benefit Analysis
To estimate the overall cost effectiveness of treating patients with NSCLC according to Level I Pathways, we calculated the net monetary benefit (NMB) of Pathways. This metric overcomes the limitations of estimating cost effectiveness using cost-effectiveness ratios in the presence of small differences in effectiveness and also provides a less biased estimate of cost effectiveness in the presence of differences in baseline measures between treatment groups often seen in observational studies.12–14 The basic model for deriving the NMB is presented:
In this equation, Bij = survival time for patient i; Cij = cost of treatment for patient i; X = demographic and clinical characteristics; and λ = specified ceiling ratio (willingness to pay). The uncertainty of the estimated NMB was illustrated using cost-effectiveness acceptability curves.15–16
Results
A total of 1,409 patients with NSCLC were identified who met the eligibility criteria for this study. Of these, 1,095 (78%) were treated on Pathway, and 314 (22%) were treated off Pathway. During the 12-month follow-up period, 735 patients (52%) died. Of those who lived, 494 (73%) had a full 12 months of follow-up time. Median follow-up time was similar for patients on pathway (median, 8.8 months) and off Pathway (median, 9.0 months).
Table 1 shows the patient characteristics overall and by Pathway status. The overall median age of patients was 67 years (range, 30 to 94 years), and 54% (n = 766) were male. A majority of patients (n = 931; 72%) were diagnosed with advanced disease (stage IIIB to IV). Approximately two thirds of patients were initially treated with first-line regimens, 14% (n = 200) were treated in the adjuvant/neoadjuvant setting, 13% (n = 184) were treated with second-line care, and 7% (n = 105) were treated with third-line care or greater. Patients treated on versus off Pathway were similar with respect to age, sex, stage at diagnosis, and Eastern Cooperative Oncology Group performance status; however, they differed by line of therapy. Of patients treated on Pathway, 87% (n = 951) received adjuvant/neoadjuvant or first-line care, compared with 54% (n = 169) of off Pathway patients. By definition, patients treated with chemotherapy beyond third line were considered off Pathway (n = 47).
Table 1.
Patient Demographic and Clinical Characteristics
| Factor | Total (N = 1,409) |
Pathway Status |
||||
|---|---|---|---|---|---|---|
| No. | % | On (n = 1,095) |
Off (n = 314) |
|||
| No. | % | No. | % | |||
| Age, years | ||||||
| Median | 67 | 67 | 66 | |||
| Range | 30-94 | 30-94 | 35-90 | |||
| <60 | 360 | 26 | 274 | 25 | 86 | 27 |
| 60-69 | 468 | 33 | 362 | 33 | 106 | 34 |
| 70-79 | 454 | 32 | 366 | 33 | 88 | 28 |
| ≥80 | 127 | 9 | 93 | 9 | 34 | 11 |
| Sex | ||||||
| Female | 643 | 46 | 492 | 45 | 151 | 48 |
| Male | 766 | 54 | 603 | 55 | 163 | 52 |
| Stage IIIB to IV at diagnosis | ||||||
| No | 368 | 28 | 278 | 28 | 90 | 31 |
| Yes | 931 | 72 | 731 | 72 | 200 | 69 |
| Unknown | 110 | 86 | 24 | |||
| ECOG performance status | ||||||
| 0 | 574 | 49 | 465 | 50 | 109 | 45 |
| 1 | 523 | 44 | 409 | 44 | 114 | 47 |
| 2-3 | 83 | 7 | 64 | 7 | 19 | 8 |
| Unknown | 229 | 157 | 72 | |||
| Initial line of therapy | ||||||
| Adjuvant/neoadjuvant | 200 | 14 | 166 | 15 | 34 | 11 |
| First | 920 | 65 | 785 | 72 | 135 | 43 |
| Second | 184 | 13 | 138 | 13 | 46 | 15 |
| Third | 58 | 4 | 6 | 1 | 52 | 17 |
| Fourth to sixth | 47 | 3 | — | — | 47 | 15 |
| No. of involved clinics | 126 | 122 | 105 | |||
| Distribution of patients by state | ||||||
| Colorado | 69 | 5 | 44 | 4 | 25 | 8 |
| Missouri | 417 | 30 | 388 | 35 | 29 | 9 |
| New York | 152 | 11 | 122 | 11 | 30 | 10 |
| Ohio | 59 | 4 | 45 | 4 | 14 | 4 |
| Texas | 385 | 27 | 273 | 25 | 112 | 36 |
| Virginia | 282 | 20 | 185 | 17 | 97 | 31 |
| Washington | 45 | 3 | 38 | 3 | 7 | 2 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Cost Analysis
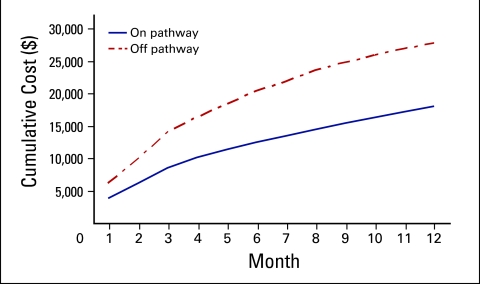
Figure 1 presents the 12-month cumulative costs of patients treated on Pathway versus patients treated off Pathway. Outpatient costs were 35% lower for on-Pathway (average 12-month cost, $18,041.62) versus off-Pathway patients (average 12-month cost, $27,736.51; on/off cost ratio, 0.65; 95% CI, 0.58 to 0.76). Applying estimated total hospitalization costs (on/off cost ratio for hospitalizations, 0.98; 95% CI, 0.83 to 1.17) to patients identified as having inpatient admissions did not have a significant effect on the observed difference in total cost by Pathway status (on/off cost ratio, 0.71; 95% CI, 0.64 to 80).
Figure 1.
12-month cumulative cost by Pathway status.
We categorized costs to identify possible drivers of the observed cost differences and found that the majority of cost differences could be attributed to lower costs of chemotherapy and other infused medications among the on-Pathway cohort (Table 2). Chemotherapy costs were 37% lower (cost ratio, 0.63; 95% CI 0.55 to 0.76) for on-Pathway patients versus off-Pathway patients, and nonchemotherapy medications were 39% lower (cost ratio, 0.61; 95% CI, 0.52 to 0.74). For nonchemotherapy medications, we found that use of both erythropoietin stimulating agents (ESAs; cost ratio, 0.54; 95% CI, 0.42 to 0.69) and WBC growth factors (CSFs; cost ratio, 0.42; 95% CI, 0.32 to 0.63) was significantly lower in the on-Pathway cohort than in the off-Pathway cohort.
Table 2.
12-Month Average Cost by Pathway Status
| Charge Category | 12-Month Average Cost ($) |
Comparison of Cost |
|||
|---|---|---|---|---|---|
| On Pathway (n = 1,095) | Off Pathway (n = 314) | Cost Difference ($; on − off) | Cost Ratio (on/off) | 95% CI* for Cost Ratio | |
| Outpatient visits | 1,124 | 1,060 | 64 | 1.06 | 0.99 to 1.11 |
| Acute care visits | 437 | 364 | 73 | 1.20 | 0.95 to 1.46 |
| Chemotherapy | 11,839 | 18,762 | −6,923 | 0.63 | 0.55 to 0.76 |
| Other medication | 4,374 | 7,198 | −2,824 | 0.61 | 0.52 to 0.74 |
| ESAs | 1,011 | 1,867 | −856 | 0.54 | 0.42 to 0.69 |
| CSFs | 1,867 | 2,951 | −1,083 | 0.63 | 0.52 to 0.83 |
| Laboratory procedures | 223 | 295 | −73 | 0.75 | 0.67 to 0.84 |
| Minor procedures | 33 | 43 | −10 | 0.78 | 0.66 to 0.92 |
| Nursing care/hospice | 2.68 | 2.16 | 0.52 | 1.24 | 0.14 to 15.20 |
| Other | 1.25 | 0.99 | 0.25 | 1.25 | 0.39 to 9.04 |
| G codes | 7.86 | 11.94 | −4.08 | 0.66 | 0.40 to 1.27 |
| Total cost | 18,042 | 27,737 | −9,695 | 0.65 | 0.58 to 0.76 |
Abbreviations: ESA, erythropoietin stimulating agent; CSF, WBC growth factor.
95% CIs calculated using percentile method using 10,000 bootstrap samples.
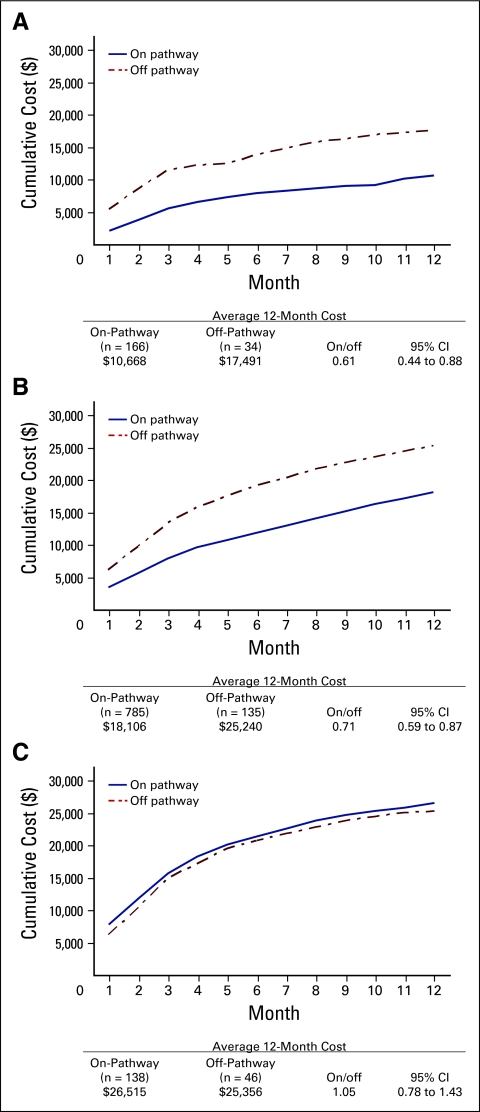
Because of the significant difference in line of therapy for patients on Pathway versus off Pathway, we stratified the cost analysis by line of therapy. Figure 2 shows 12-month total costs by Pathway status for patients receiving adjuvant, first-line, and second-line care. We found that costs remained significantly lower for patients treated on Pathway versus off Pathway in the adjuvant and first-line settings, whereas no difference in overall cost was observed in patients receiving second-line care. Among patients in the adjuvant setting, we found that those treated on Pathway had a reduced cost of 39% compared with patients treated off Pathway (cost ratio, 0.61; 95% CI, 0.44 to 0.88); a 29% decreased overall cost was associated with on-Pathway patients receiving first-line care compared with off-Pathway patients receiving first-line care (cost ratio, 0.71; 95% CI, 0.59 to 0.87). Use of ESAs and CSFs was significantly less frequent among on-Pathway patients in adjuvant, first-line, and second-line settings. Because of the insufficient number of on-Pathway patients, we were unable to evaluate differences in cost by Pathway status among patients receiving third-line care.
Figure 2.
12-month cumulative cost by Pathway status and line of therapy for patients receiving (A) adjuvant, (B) first-line, and (C) second-line care.
Overall Survival
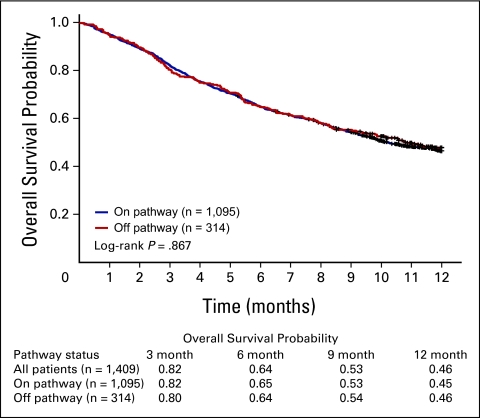
No difference in 12-month overall survival was observed by Pathway status (Fig 3; 12-month survival probability, 0.45 v 0.46 for on v off Pathway; log-rank P = .867). In a multivariable Cox regression model (Table 3), we found that Pathway status was not associated with 12-month risk of mortality after adjusting for age, sex, stage, performance status, and line of therapy (multivariable hazard ratio [HR], 0.95; 95% CI, 0.77 to 1.16). Significant independent predictors of overall survival included stage at diagnosis (HR, 1.41; 95% CI, 1.10 to 1.80), lower performance status (HR, 1.78; 95% CI, 1.50 to 2.11 for performance status of 1; HR, 3.46; 95% CI, 2.63 to 4.57 for performance status of 2 to 3; P trend < .0001), and current treatment for metastatic disease (HR, 2.78; 95% CI, 1.83 to 4.23).
Figure 3.
Overall survival by Pathway status.
Table 3.
Cox Regression Analysis: Overall Survival
| Characteristic | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Pathway status | ||||||
| On | 1.00 | 1.00 | ||||
| Off | 0.99 | 0.83 to 1.17 | .87 | 0.95 | 0.77 to 1.16 | .58 |
| Sex | ||||||
| Male | 1.00 | — | — | |||
| Female | 0.91 | 0.79 to 1.05 | .20 | — | — | — |
| Age (continuous) | 1.01 | 1.002 to 1.02 | .01 | 1.01 | 1.001 to 1.02 | .03 |
| Stage IIIB to IV at diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.13 | 1.75 to 2.58 | < .001 | 1.41 | 1.10 to 1.80 | .002 |
| ECOG performance status | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.94 | 1.64 to 2.30 | < .001 | 1.78 | 1.50 to 2.11 | < .001 |
| 2-3 | 3.95 | 2.99 to 5.21 | < .001 | 3.46 | 2.61 to 4.57 | < .001 |
| Line of therapy | ||||||
| Adjuvant | 1.00 | 1.00 | ||||
| Metastatic | 3.79 | 2.77 to 5.20 | < .001 | 2.78 | 1.83 to 4.23 | < .001 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Again, because of differences in line of therapy by Pathway status, we evaluated the association between overall survival and Pathway status by line of therapy. No significant differences were observed in survival for patients on and off Pathway in adjuvant, first-line, and second-line settings. Furthermore, because patients receiving therapy beyond third line were considered to be off Pathway, and because there were few patients treated on Pathway in the third-line setting, we compared survival among patients receiving adjuvant and second line care combined and found no difference in survival by Pathway status (data not shown).
Cost Effectiveness
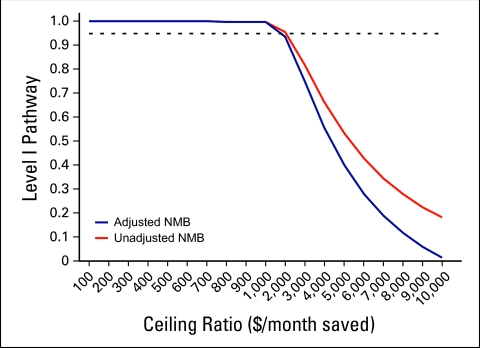
Finally, to estimate the overall cost effectiveness of Level I Pathways, we modeled the joint density of cost and effect (as measured by overall survival) by calculating the net monetary benefit approach of Pathways across a range of willingness to pay ceiling ratios (Fig 4, online only). We found that treating patients on Pathway remained cost effective across the plausible range of society's (or payers') willingness to pay, even after adjusting for potential confounders (age, stage, line of therapy, and performance status).
Figure 4.
Net monetary benefit (NMB) and cost-effectiveness acceptability curves.
Conclusion
The findings of this study reinforce the concepts introduced in the landmark studies of Fisher et al17–19 and Wennberg et al,20 which convincingly demonstrated that high quality was not necessarily associated with high cost. Assuming that value is directly proportional to outcomes and inversely proportional to costs, quality would be improved by either improved outcomes and/or lower costs. These studies left oncologists with the task of determining the behaviors that lead to cost-effective oncology care.21,22
To our knowledge, this is the first study to empirically measure the cost effectiveness of evidence-based treatment guidelines for cancer therapy. Results suggest that treating patients according to evidence-based guidelines is a cost-effective strategy for delivering care to patients with NSCLC, especially in the adjuvant and first-line settings. Over 12 months, average cost of care for patients on Pathway was 35% less compared with patients treated off Pathway. This cost difference was driven predominately by significantly lower costs for chemotherapy and other medications. It is worth pointing out that Pathways includes costly cancer chemotherapeutics like bevacizumab and erlotinib, albeit restricted to lines of therapy in which evidence is sound, so this is not simply a cost comparison between one group receiving expensive drugs and another receiving cheaper ones. Patients treated on Pathway also did not receive chemotherapy beyond third line, which may be another reason why costs were lower in this cohort. We also found that there were no observed differences in 12-month overall survival by Pathway status, suggesting that the added cost associated with treating patients off Pathways (including therapy beyond third line) did not translate to improved outcomes.
One question that arises is the extent to which differences in cost by Pathway status are driven simply by use of less expensive drugs or a decrease in use of therapy overall. We found that it is likely a combination of both. Although costs were demonstrably lower among patients treated on Pathway, we also found that patients treated on Pathway had a 22% lower frequency (P < .001) of chemotherapy infusion visits and 23% lower frequency (P < .001) of administrations of nonchemotherapy agents, including supportive care agents (data not shown). One specific difference is that on-Pathway regimens have standard order sets that define dosing strengths and number of cycles. Off-Pathway regimens have much more likelihood of being administered for additional cycles by the treating physician.
Possible explanations for the observed difference in cost of nonchemotherapy medications by Pathway status is that by definition, on-Pathway regimens are typically lower in toxicity, so fewer antiemetics and growth factors are required, and doctors who choose to adhere to Pathways tend to be less likely to prescribe more expensive supportive care drugs unless there is to strong evidence to validate use.
The limitations of this study include the fact that costs were estimated using outpatient claims, and we were therefore unable to directly calculate total cost related to hospitalizations. To address this limitation, we identified events using evaluation and management charges in the CDW data related to inpatient admissions/consultations and applied a total hospitalization cost estimate to our overall outpatient cost. Results of the cost analyses were robust to the application of these quasi-theoretical inpatient cost estimates. Furthermore, although there is always the possibility of misclassification as a result of missing hospitalization events in the CDW claims data, the probability of this occurring would likely be balanced across comparison groups, in which case any bias introduced would be toward the null hypothesis leading to an underestimation of the true differences in cost.
The strength of this study lies in the clinically rich EMR data used in conjunction with claims data to evaluate the impact of Level I Pathways on costs and survival of patients with NSCLC receiving care in a large, geographically dispersed network of community-based oncology practices. Given the extensive use and range of chemotherapy use for NSCLC without strong evidence regarding therapeutic superiority of any given regimen, it is important to consider the economic impact treatment may have on patients and society.
Given an absence of difference in survival by treatment strategy, as demonstrated in this article, it becomes increasingly important to focus on delivering consistent, high-value care that maximizes patient quality of life while at the same time minimizing the economic burden of care. Results of this study indicate that Level I Pathways is a cost-effective treatment strategy for NSCLC in the community setting, and future studies should evaluate these evidence-based guidelines for other cancer sites.
It is important to ask whether there are attributes to the Level I Pathways program that contributed to the enhanced value realized. Grilli et al23 suggested critical components to guidelines: types of professionals involved in guideline development are specified, strategy used to identify primary evidence is specified, and recommendations are clearly graded. To these we add the following characteristics to the Pathways process used in this study: ready access and mandatory participation in the Pathways program and a prospective review process, in which Pathways exceptions are reviewed before the administration of treatment. Satisfying these five criteria has been critical to the success of the program.
Supplementary Material
Acknowledgment
We thank Claire Spettell, PhD, and Kirsten Anderson, MD, for their assistance in preparing this manuscript; Stephanie Dutton, MPA, and her team of registered nurses (Joan Fleming, Kim Henry, Joleen Nachbar, and Sandi Leshikar) for assisting with data collection; and Joe Ensor, PhD, for his guidance in the design of this study.
Appendix
Table A1.
Non Small-Cell Lung Cancer Level I Pathways for Study Timeframe
| Pathway |
|---|
| Adjuvant, stage IB to IIIA |
| Paclitaxel/carboplatin |
| Cisplatin/VP-16 |
| Unresected, stage IIIA to B |
| Paclitaxel/carboplatin |
| Cisplatin/VP-16 |
| Best supportive care |
| Metastatic first line |
| Paclitaxel/carboplatin |
| Paclitaxel/carboplatin + bevacizumab (if nonsquamous, no brain metastases or hemoptysis, no history of bleeding, no anticoagulants) |
| Vinorelbine (if poor performance status) |
| Best supportive care |
| Metastatic second line |
| Pemetrexed |
| Docetaxel |
| Erlotinib |
| Best supportive care |
| Metastatic third line* |
| Erlotinib |
| Best supportive care |
Chemotherapy beyond third line considered off Pathway.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Marcus A. Neubauer, US Oncology; J. Russell Hoverman, US Oncology; Michael Kolodziej, US Oncology; Stephen K. Gruschkus, US Oncology; Susan Hoang, US Oncology; Marilyn McArthur, Aetna; Michael Forsyth, US Oncology; Todd Rothermel, Aetna Consultant or Advisory Role: Marcus A. Neubauer, Amgen, Veridex Stock Ownership: J. Russell Hoverman, US Oncology; Marilyn McArthur, Aetna; Todd Rothermel, Aetna Honoraria: Marcus A. Neubauer, Amgen Research Funding: None Expert Testimony: None Other Remuneration: None
References
- 1.American Cancer Society. Statistics for 2009: Cancer Facts & Figures 2009. http://www.cancer.org/docroot/STT/STT_0.asp.
- 2.National Cancer Institute. Cancer Trends Progress Report—2007 Update. http://progressreport.cancer.gov.
- 3.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Piana R. Cancer drug pipeline is healthy, says head of oncologic drug approval for FDA. Oncology News International. http://www.cancernetwork.com/display/article/10165/1263819?verify=0.
- 5.Kritz FL. Cancer patients facing costly treatments can benefit from frank talks with doctors. Los Angeles Times. 2009 Feb 16; [Google Scholar]
- 6.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 7.Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non–small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 8.Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18:317–323. doi: 10.1093/annonc/mdl377. [DOI] [PubMed] [Google Scholar]
- 9.Carlson JJ, Veenstra DL, Ramsey SD. Pharmacoeconomic evaluations in the treatment of non- small cell lung cancer. Drugs. 2008;68:1105–1113. doi: 10.2165/00003495-200868080-00007. [DOI] [PubMed] [Google Scholar]
- 10.Lin DY, Feuer EJ, Etzioni R, et al. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53:419–434. [PubMed] [Google Scholar]
- 11.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall; 1993. [Google Scholar]
- 12.Mitra N, Indurkhya A. A propensity score approach to estimating the cost-effectiveness of medical therapies from observational data. Health Econ. 2005;14:805–815. doi: 10.1002/hec.987. [DOI] [PubMed] [Google Scholar]
- 13.Indurkhya A, Mitra N, Schrag D. Using propensity scores to estimate the cost-effectiveness of medical therapies. Stat Med. 2006;25:1561–176. doi: 10.1002/sim.2267. [DOI] [PubMed] [Google Scholar]
- 14.Stinnett AA, Mullahy J. Net health benefits: A new framework for the analysis of uncertainty in costeffectiveness analysis. Med Dec Mak. 1998;18(suppl 2):S65–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 15.van Hout BA, Al MJ, Gordon GS, et al. Costs, effects and C:E-ratios alongside a clinical trial. Health Econ. 1994;3:309–319. doi: 10.1002/hec.4730030505. [DOI] [PubMed] [Google Scholar]
- 16.Willan AR, Lin DY, Manca A. Regression methods for cost-effectiveness analysis with censored data. Stat Med. 2005;24:131–145. doi: 10.1002/sim.1794. [DOI] [PubMed] [Google Scholar]
- 17.Fisher ES, Wennberg JE. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med. 2003;46:69–79. doi: 10.1353/pbm.2003.0004. [DOI] [PubMed] [Google Scholar]
- 18.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending: Part 1—The content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 19.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending: Part 2—Health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 20.Wennberg JE, Fisher ES, Baker L, et al. Evaluating the efficiency of California providers in caring for patients with chronic illnesses. Health Aff (Millwood) 2005;W5(suppl):526–543. doi: 10.1377/hlthaff.w5.526. [DOI] [PubMed] [Google Scholar]
- 21.Hoverman JR, Robertson SM. Lung cancer: A cost and outcome study based on physician practice patterns. Dis Manag. 2004;7:112–123. doi: 10.1089/1093507041253262. [DOI] [PubMed] [Google Scholar]
- 22.Coukell AJ, Noble S, Faulds D. Vinorelbine in advanced non-small cell lung cancer: A pharmacoeconomic review. Pharmacoeconomics. 1999;15:405–417. doi: 10.2165/00019053-199915040-00008. [DOI] [PubMed] [Google Scholar]
- 23.Grilli R, Magrini N, Penna A, et al. Practice guidelines developed by specialty societies: The need for a critical appraisal. Lancet. 2000;355:103–106. doi: 10.1016/S0140-6736(99)02171-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.