Abstract
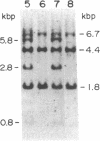
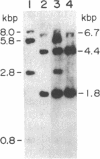
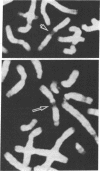
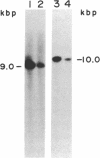
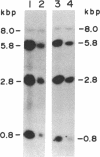
The human HST1 gene, previously designated the hst gene, and now assigned the name HSTF1 for heparin-binding secretory transforming factor in human gene nomenclature, was originally identified as a transforming gene in DNAs from human stomach cancers by transfection assay with mouse NIH 3T3 cells. The amino acid sequence of the product deduced from DNA sequences of the HST1 cDNA and genomic clones had approximately 40% homology to human basic and acidic fibroblast growth factors and mouse Int-2-encoded protein. We have mapped the human HST1 gene to chromosome 11 at band q13.3 by Southern blot hybridization analysis of a panel of human and mouse somatic cell hybrids and in situ hybridization with an HST1 cDNA probe. The HST1 gene was found to be amplified in DNAs obtained from a stomach cancer and a vulvar carcinoma cell line, A431. In all of these samples of DNA, the INT2 gene, previously mapped to human chromosome 11q13, was also amplified to the same degree as the HST1 gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Whang J. L., Tumolo A., Mergia A., Friedman J., Gospodarowicz D., Fiddes J. C. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986 Oct;5(10):2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey G., Smith R., McGillivray D., Peters G., Dickson C. Characterization and chromosome assignment of the human homolog of int-2, a potential proto-oncogene. Mol Cell Biol. 1986 Feb;6(2):502–510. doi: 10.1128/mcb.6.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., Basilico C. Isolation of a rearranged human transforming gene following transfection of Kaposi sarcoma DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5660–5664. doi: 10.1073/pnas.84.16.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Koda T., Sasaki A., Matsushima S., Kakinuma M. A transforming gene, hst, found in NIH 3T3 cells transformed with DNA from three stomach cancers and a colon cancer. Jpn J Cancer Res. 1987 Apr;78(4):325–328. [PubMed] [Google Scholar]
- Marx J. L. Oncogene action probed. Science. 1987 Aug 7;237(4815):602–603. doi: 10.1126/science.3603042. [DOI] [PubMed] [Google Scholar]
- Moore R., Casey G., Brookes S., Dixon M., Peters G., Dickson C. Sequence, topography and protein coding potential of mouse int-2: a putative oncogene activated by mouse mammary tumour virus. EMBO J. 1986 May;5(5):919–924. doi: 10.1002/j.1460-2075.1986.tb04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagama H., Ohnishi S., Imawari M., Hirai H., Takaku F., Sakamoto H., Terada M., Nagao M., Sugimura T. Identification of transforming genes as hst in DNA samples from two human hepatocellular carcinomas. Jpn J Cancer Res. 1987 Jul;78(7):651–654. [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983 Jun;33(2):369–377. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- Rogelj S., Weinberg R. A., Fanning P., Klagsbrun M. Basic fibroblast growth factor fused to a signal peptide transforms cells. Nature. 1988 Jan 14;331(6152):173–175. doi: 10.1038/331173a0. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Mori M., Taira M., Yoshida T., Matsukawa S., Shimizu K., Sekiguchi M., Terada M., Sugimura T. Transforming gene from human stomach cancers and a noncancerous portion of stomach mucosa. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3997–4001. doi: 10.1073/pnas.83.11.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Yoshida T., Nakakuki M., Odagiri H., Miyagawa K., Sugimura T., Terada M. Cloned hst gene from normal human leukocyte DNA transforms NIH3T3 cells. Biochem Biophys Res Commun. 1988 Mar 30;151(3):965–972. doi: 10.1016/s0006-291x(88)80460-1. [DOI] [PubMed] [Google Scholar]
- Satoh H., Yoshida M. C., Matsushime H., Shibuya M., Sasaki M. Regional localization of the human c-ros-1 on 6q22 and flt on 13q12. Jpn J Cancer Res. 1987 Aug;78(8):772–775. [PubMed] [Google Scholar]
- Semba K., Yamanashi Y., Nishizawa M., Sukegawa J., Yoshida M., Sasaki M., Yamamoto T., Toyoshima K. Location of the c-yes gene on the human chromosome and its expression in various tissues. Science. 1985 Mar 1;227(4690):1038–1040. doi: 10.1126/science.2983418. [DOI] [PubMed] [Google Scholar]
- Semple T. U., Quinn L. A., Woods L. K., Moore G. E. Tumor and lymphoid cell lines from a patient with carcinoma of the colon for a cytotoxicity model. Cancer Res. 1978 May;38(5):1345–1355. [PubMed] [Google Scholar]
- Taira M., Yoshida T., Miyagawa K., Sakamoto H., Terada M., Sugimura T. cDNA sequence of human transforming gene hst and identification of the coding sequence required for transforming activity. Proc Natl Acad Sci U S A. 1987 May;84(9):2980–2984. doi: 10.1073/pnas.84.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J., Wada M., Shimosato Y., Terada M., Sugimura T. Loss of heterozygosity on chromosomes 3, 13, and 17 in small-cell carcinoma and on chromosome 3 in adenocarcinoma of the lung. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9252–9256. doi: 10.1073/pnas.84.24.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Miyagawa K., Odagiri H., Sakamoto H., Little P. F., Terada M., Sugimura T. Genomic sequence of hst, a transforming gene encoding a protein homologous to fibroblast growth factors and the int-2-encoded protein. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7305–7309. doi: 10.1073/pnas.84.20.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Sakamoto H., Miyagawa K., Little P. F., Terada M., Sugimura T. Genomic clone of hst with transforming activity from a patient with acute leukemia. Biochem Biophys Res Commun. 1987 Feb 13;142(3):1019–1024. doi: 10.1016/0006-291x(87)91516-6. [DOI] [PubMed] [Google Scholar]
- Yuasa Y., Sudo K. Transforming genes in human hepatomas detected by a tumorigenicity assay. Jpn J Cancer Res. 1987 Oct;78(10):1036–1040. [PubMed] [Google Scholar]