Abstract
Age is the major risk factor for cardiovascular diseases (CVD) and this is attributable in part to stiffening of large elastic arteries and development of vascular endothelial dysfunction (e.g. impaired endothelium-dependent dilatation, EDD). In contrast, regular aerobic exercise is associated with reduced risk of CVD. Endurance exercise-trained middle-aged/older adults demonstrate lower large elastic artery stiffness and greater EDD than their sedentary peers. With daily brisk walking, previously sedentary middle-aged/older adults show reduced stiffness and improved EDD. The mechanisms underlying the effects of regular aerobic exercise on large elastic artery stiffness with ageing are largely unknown, but are likely to include changes to the composition of the arterial wall. Enhanced EDD in older adults who exercise is mediated by increased nitric oxide (NO) bioavailability associated with reduced oxidative stress. Arteries from old rodents that perform regular aerobic exercise demonstrate increased expression and activity of endothelial NO synthase, reduced oxidative damage associated with reduced expression and activity of the oxidant enzyme NADPH oxidase, and increased activity of the antioxidant enzyme superoxide dismutase. Aerobic exercise also may protect arteries with ageing by increasing resistance to the effects of other CVD risk factors like LDL-cholesterol. Habitual aerobic exercise is an effective strategy to combat arterial ageing.
Cardiovascular diseases (CVD) remain the leading cause of mortality in modern societies. Approximately 80% of all CVD deaths are associated with dysfunction and disorders of arteries (Thom et al. 2006). That advancing age is the major risk factor for CVD (Lakatta & Levy, 2003) strongly suggests there is something about ageing that causes changes in arteries that lead to an increased risk of CVD.
It is likely that a wide array of adverse changes to arteries contribute to the increased risk of CVD with ageing. Two changes that are believed to be among the most important, however, are increased stiffness of large elastic arteries and dysfunction of the vascular endothelium (Lakatta & Levy, 2003). As such, factors that can delay, slow and/or prevent these changes hold promise for reducing age-associated CVD.
Regular aerobic exercise is associated with a reduced risk of CVD in middle-aged and older adults (Blair et al. 1989; Manson et al. 2002; Mora et al. 2007), although the mechanisms of action are incompletely understood. Here we review experimental evidence supporting the idea that this effect of habitual aerobic exercise may be mediated, at least in part, by reducing age-related increases in large elastic artery stiffness and the development of vascular endothelial dysfunction. We also discuss the integrative physiological mechanisms by which regular exercise may protect against these arterial changes with ageing. A broader treatment of the topic of habitual exercise and arterial ageing can be found elsewhere (Seals et al. 2008).
Large elastic artery stiffness
The large elastic arteries are responsible for ‘buffering’ the haemodynamic impact of the increase in left ventricular stroke volume that occurs with each contraction of the heart. These arteries receive the stroke volume by expanding and then recoiling, providing the energy to drive blood downstream and ensuring constant flow to the distal organs and tissues. Thus, their role is different from that of peripheral arteries, which are charged with the control of vascular conductance, distribution of blood flow, delivery of oxygen and nutrients, etc.
Changes with age and habitual exercise
Ageing, in the absence of disease, results in stiffening of large elastic arteries such as the aorta and carotid arteries in both humans and experimental animals (Avolio et al. 1985; Reddy et al. 2003). Aortic pulse wave velocity, the most common and, perhaps, clinically important measure of large elastic artery stiffness, increases progressively with age in adults without clinical CVD (Avolio et al. 1985; Mitchell et al. 2004). In turn, directly measured carotid artery compliance (assessed by simultaneous B-mode ultrasound imaging and applanation tonometry) decreases with age in healthy adults without major risk factors for CVD (Tanaka et al. 2000; Moreau et al. 2003) (Fig. 1). In contrast, peripheral arteries do not stiffen with ageing (Tanaka et al. 1998), indicating that increases in stiffness are specific to the large elastic arteries.
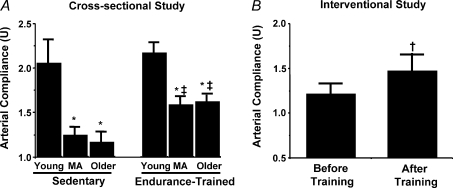
Figure 1. Carotid artery compliance, aging and habitual aerobic exercise.
A, carotid artery compliance in sedentary and endurance exercise-trained healthy young, middle-aged (MA) and older men. *P < 0.05 vs. young, ‡P < 0.05 vs. sedentary of same age. B, carotid artery compliance before and after 3 months of moderate intensity aerobic exercise in healthy MA and older men. †P < 0.05 vs. before training. Values are means ±s.e.m. From Tanaka et al. (2000). Reproduced with permission from Journal of Applied Physiology.
Regular aerobic exercise is associated with less stiffening of large elastic arteries with ageing in healthy adults. Aortic pulse wave velocity is lower in middle-aged and older men (Vaitkevicius et al. 1993) and postmenopausal women (Tanaka et al. 1998) who regularly perform aerobic exercise compared with their healthy sedentary peers and, in some cases, is not different from young controls (Tanaka et al. 1998). Consistent with these observations, age-associated reductions in carotid artery compliance in endurance exercise-trained men (Tanaka et al. 2000) (Fig. 1) and women (Moreau et al. 2003) are only ∼50% as great as those observed in healthy non-exercising adults. The intensity, duration and frequency of aerobic exercise required for improving large elastic artery stiffness and other vascular functions (see below) have not been established. However, in previously sedentary, healthy middle-aged and older men (Tanaka et al. 2000) (Fig. 1) and estrogen-replaced postmenopousal women (Moreau et al. 2003), 3 months of daily brisk walking restores carotid artery compliance to levels observed in endurance exercise-trained adults of the same age and, in some cases, to levels of young adults (Moreau et al. 2003). It is unclear if aerobic exercise is as effective in adults with chronic elevations in systolic blood pressure (Seals et al. 2001).
Mechanisms
There is surprisingly little direct evidence as to the cellular and molecular mechanisms responsible for age-associated increases in large elastic artery stiffness, and even less information concerning the effects of habitual exercise. A major limitation in this regard is that these arteries are inaccessible in humans, both physically and with respect to experimental manipulation of potential signalling pathways.
It is generally believed that changes in the expression, architecture and/or bioactivity of structural proteins in the walls of large elastic arteries are major contributors to their stiffening with ageing. This is part of an overall remodelling of the extracellular matrix that includes increases in collagen and its cross-linking, fragmentation of elastin, and increases in fibronectin among other changes (Lakatta & Levy, 2003). Although the medial layer of arteries is thought to be the primary site for these changes, the potential role of the adventitia remains largely unexplored. Inflammation has been identified as a possible trigger for initiating these events (Vlachopoulos et al. 2005), but direct cause and effect evidence with ageing is lacking. In addition to such structural changes, increases in vascular smooth muscle tone and endothelial dysfunction (McEniery et al. 2006; Soucy et al. 2006) also may contribute to increases in large elastic artery stiffness with ageing. Oxidative stress could be involved, but evidence is inconsistent (Eskurza et al. 2004a; Moreau et al. 2005).
Little is known about the mechanisms by which regular aerobic exercise suppresses large elastic artery stiffening with ageing. Reduced vascular oxidative stress may explain the enhanced carotid artery compliance in exercising compared with sedentary postmenopausal women (Moreau et al. 2006). Increases in carotid artery compliance in response to aerobic exercise intervention in middle-aged and older men (Tanaka et al. 2000) and postmenopausal women (Moreau et al. 2003) are not related to improvements in conventional risk factors for CVD. Limited data from experimental animals do not show an association between habitual exercise-related reductions in large elastic artery stiffness and changes in total expression of major structural proteins (Nosaka et al. 2003).
Vascular endothelial dysfunction
Several expressions of arterial endothelial dysfunction develop with ageing, including impaired endothelium-dependent dilatation (EDD). EDD is evoked by both pharmacological (e.g. acetylcholine) and physiological (increases in blood flow or shear stress-mediated dilatation) stimuli that cause endothelial synthesis and release of dilators including nitric oxide (NO), vasodilatory prostaglandins and endothelial hyperpolarizing factors, with consequent relaxation of vascular smooth muscle.
Changes with age and habitual exercise
Ageing is associated with impaired EDD in response to both acetylcholine and increased shear in humans and experimental animals. In humans, the peak forearm blood flow response to acetylcholine is progressively lower in healthy men and women of increasing age (Taddei et al. 1995; DeSouza et al. 2000) (Fig. 2). Brachial artery flow-mediated dilatation becomes progressively lower after the ages of approximately 40 and 50 years, respectively, in men and women without CVD (Celermajer et al. 1994). EDD is reduced systemically, as well as in isolated conduit arteries and arterioles of old compared with young animals in the absence of atherosclerosis (Muller-Delp et al. 2002; Lesniewski et al. 2009).
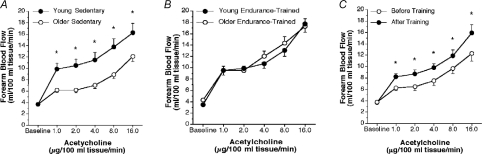
Figure 2. Endothelium-dependent dilation, aging and habitual aerobic exercise.
Forearm blood flow responses to brachial artery infusion of acetylcholine in healthy men. A, young and older sedentary; B, young and older endurance exercise-trained; C, before and after 3 months of daily walking in older men; *P < 0.05; values are mean ±s.e.m. From DeSouza et al. (2000). Reproduced with permission from Journal of Applied Physiology.
Habitual aerobic exercise is associated with enhanced EDD during ageing, at least in men. Middle-aged and older endurance exercise-trained men demonstrate greater EDD than their healthy, but less active peers (DeSouza et al. 2000; Taddei et al. 2000; Eskurza et al. 2004a) and, in some cases, similar to EDD observed in young men (DeSouza et al. 2000; Eskurza et al. 2004a) (Fig. 2). In previously sedentary middle-aged and older men, 3 months of daily moderate-intensity walking restores the forearm blood flow responses to acetylcholine to levels seen in age-matched endurance exercise-trained and in young men (DeSouza et al. 2000) (Fig. 2). Similar improvements have been observed with an exercise intervention in middle-aged adult males with the metabolic syndrome (Lavrencic et al. 2000). It is unknown if habitual aerobic exercise improves EDD in postmenopausal women and, if so, if this effect is similar to that observed in men.
Motorized treadmill exercise training improves EDD in isolated skeletal muscle arterioles from old rats (Spier et al. 2004, 2007). Recent work shows that in old mice, even modest volumes of spontaneous wheel running, designed to more closely mimic voluntary aerobic exercise in older humans, are sufficient to restore acetylcholine-mediated dilatation both systemically and in isolated conduit arteries to levels observed in young mice (Durrant et al. 2009).
Mechanisms
Evidence in both humans and experimental animals implicate increased NO bioavailability and reduced oxidative stress in the effects of regular exercise on EDD with aging (Taddei et al. 2000; Eskurza et al. 2004b; Spier et al. 2004; Durrant et al. 2009).
NO. Inhibitors of endothelial NO synthase (eNOS), the enzyme responsible for NO production in the vascular endothelium, have a much greater effect in suppressing resting blood flow (Seals et al. 2008) and EDD (Taddei et al. 2000; Spier et al. 2004; Durrant et al. 2009) in exercising older humans and animals than in sedentary controls of similar age, indicating augmented basal and stimulated NO bioavailability. Indeed, differences in EDD between sedentary and aerobically exercise-trained older humans and rodents are largely or completely explained by differences in NO bioavailability (Taddei et al. 2000; Spier et al. 2004; Durrant et al. 2009). Moreover, in previously sedentary healthy middle-aged and older men, daily brisk walking restores basal NO production to levels observed in young men (Seals et al. 2008).
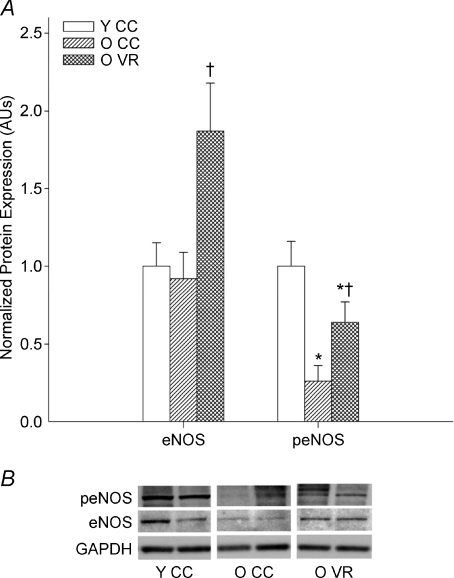
eNOS gene and protein expression (Spier et al. 2004; Durrant et al. 2009) and activation (Durrant et al. 2009) are increased in arteries of old exercising rodents compared with sedentary cage controls (Fig. 3), and in mammary arteries of patients with coronary artery disease who perform exercise training compared with untrained controls (Hambrecht et al. 2003). No information is currently available on the effects of habitual exercise on eNOS expression or activation in middle-aged and older men and women without clinical CVD.
Figure 3. Endothelial nitric oxide synthase (eNOS), aging and habitual aerobic exercise.
Aortic expression of total and phosphorylated (p-) eNOS in young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice. A, summary graph (protein expression normalized to GAPDH). *P < 0.05 vs. Y CC, †P < 0.05 vs. O CC; values are mean ±s.e.m. B, representative blot. From Durrant et al. (2009).
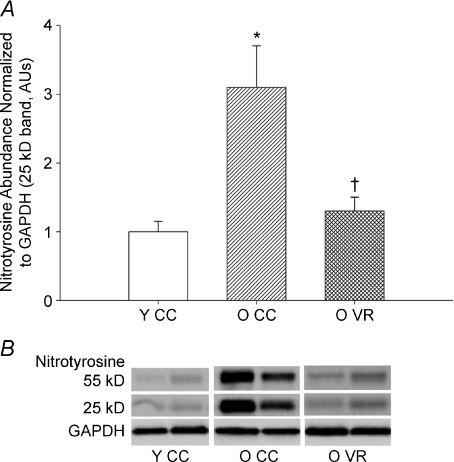
Oxidative stress. Acute administration of antioxidants to middle-aged and older humans (Taddei et al. 2000; Eskurza et al. 2004b) and isolated conduit arteries of old mice (Durrant et al. 2009) improve EDD in the sedentary, but not in the exercise-trained state, consistent with the idea that exercise exerts its effects by reducing oxidative stress-related suppression of EDD. Direct evidence that voluntary aerobic exercise reduces vascular oxidative stress with ageing comes from recent work showing that in old mice given access to running wheels, aortic staining for nitrotyrosine, a cellular marker of oxidative modification of proteins, is markedly lower than in old cage controls and similar to young mice (Durrant et al. 2009) (Fig. 4). Nitrotyrosine staining increases with ageing in vascular endothelial cells obtained from healthy sedentary humans (Donato et al. 2007), but it is not known if older adults who exercise regularly demonstrate less endothelial cell oxidative stress than their sedentary peers.
Figure 4. Nitrotyrosine, aging and habitual aerobic exercise.
Aortic nitrotyrosine abundance in young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice. A, summary graphs (protein expression normalized to GAPDH). *P < 0.05 vs. Y CC, †P < 0.05 vs. O CC; values are mean ±s.e.m. B, representative blot. From Durrant et al. (2009).
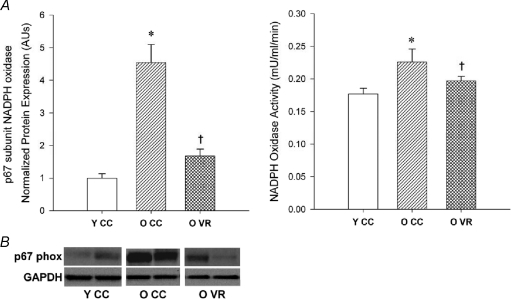
The mechanisms by which habitual aerobic exercise may suppress the development of vascular oxidative stress with ageing are incompletely understood. Aerobic exercise-trained patients with coronary disease have lower mammary artery production of reactive oxygen species and expression of the oxidant enzyme NADPH oxidase than patients who do not undergo training (Adams et al. 2005). With regard to ageing, recent findings in mice demonstrate that voluntary wheel running in old animals is associated with reduced protein expression of the p67phox subunit of NADPH oxidase and reduced NADPH oxidase activity in the absence of changes in the other major oxidant enzyme found in arteries, xanthine oxidase (Durrant et al. 2009) (Fig. 5). Consistent with this, inhibition of NADPH oxidase activity with apocynin improves isolated conduit artery dilatation in response to acetylcholine in old sedentary mice by increasing NO bioavailability, but has no effect on old mice after voluntary wheel running (Durrant et al. 2009). Although NADPH oxidase expression increases with age in healthy sedentary adults (Donato et al. 2007), it is unknown if older humans who exercise have reduced vascular expression of NADPH oxidase compared with their less active peers.
Figure 5. NADPH oxidase, aging and habitual aerobic exercise.
Aortic NADPH oxidase (p67 subunit) in young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice. A, summary graphs for protein expression (left panel) and enzyme activity (right panel) (protein expression normalized to GAPDH). *P < 0.05 vs. Y CC, †P < 0.05 vs. O CC; values are mean ±s.e.m. B, representative blots. From Durrant et al. (2009).
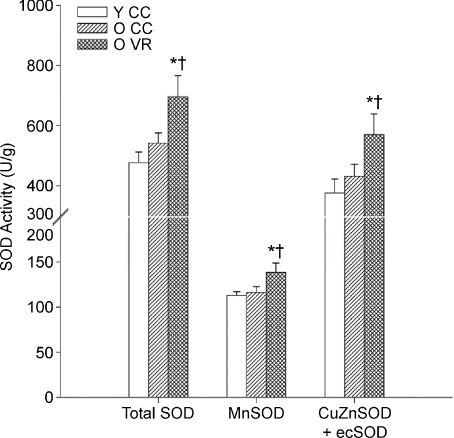
Recent data in mice indicate that the antioxidant enzyme, superoxide dismutase (SOD), may be a key mechanism by which regular aerobic exercise suppresses the development of vascular oxidative stress with ageing. Total SOD, manganese (mitochondrial) SOD and copper–zinc (cytosolic) + extracellular SOD activities are greater in aorta of old mice that perform voluntary wheel running compared with old sedentary animals (Durrant et al. 2009). Arterial expression of extracellular SOD also tends to be greater in old rodents that exercise (Durrant et al. 2009; Trott et al. 2009) (Fig. 6). Consistent with these findings, treatment of isolated conduit arteries with Tempol, a SOD mimetic, restores dilatation to acetylcholine in sedentary old mice by restoring the NO component of EDD, but has no effect in old exercising mice (Durrant et al. 2009). It is unknown if older adult humans who exercise have greater vascular expression or circulating activity of SOD compared with sedentary older adults.
Figure 6. Superoxide disumutase (SOD) activity, aging and habitual aerobic exercise.
Aortic SOD activity in young (Y) and old (O) cage control (CC) and voluntary wheel running (VR) mice. *P < 0.05 vs. Y CC, †P < 0.05 vs. O CC; values are mean ±s.e.m. Mn: manganese, CuZn: copper zinc, ec: extracellular; From Durrant et al. (2009).
Tetrahydrobiopterin. Tetrahydrobiopterin (BH4) is an essential co-factor for eNOS production of NO. BH4 is readily oxidized to its biologically inactive form, BH2, by peroxynitrite formed by the reaction of NO with the reactive oxygen species molecule superoxide (Milstien & Katusic, 1999). Findings in both humans (Higashi et al. 2006) and rodents (Delp et al. 2008) implicate reduced BH4 concentrations or bioactivity in impaired NO-mediated EDD with sedentary ageing. Acute administration of BH4 improves EDD in healthy older sedentary adults, but has no effect on EDD in young controls or in endurance exercise-trained older adults (Eskurza et al. 2005). The latter findings support the idea that preserved bioactivity of BH4 may be an important mechanism by which habitual exercise enhances EDD in older adults. This could be mediated via reduced oxidative stress, which would preserve BH4 bioactivity, thus maintaining NO production and EDD.
Endothelin-1. Endothelin-1 (ET-1) is the most potent vasoconstrictor molecule produced by the vascular endothelium and opposes NO-mediated vasodilatation (Van Guilder et al. 2007; Donato et al. 2009). Expression of ET-1 in vascular endothelial cells increases with age in sedentary healthy men (Donato et al. 2009). Sedentary older adults demonstrate tonic ETA receptor-mediated vasoconstriction, which is abolished by aerobic exercise training (Van Guilder et al. 2007). In old mice, carotid artery EDD is improved during ETA receptor blockade, indicating that ET-1 signalling tonically suppresses EDD with ageing (Donato et al. 2009). However, it is unknown if lower vascular endothelial ET-1 expression and/or bioactivity contribute to the enhanced EDD of older adults who exercise compared with their sedentary peers.
Protection from adverse effects of risk factors
Up to 60% of the CVD risk-lowering influence of regular aerobic exercise can be explained by its favourable effects on conventional (e.g. body fatness, plasma LDL cholesterol, blood pressure and blood glucose) and non-conventional (e.g. inflammatory/haemostatic markers) risk factors (Mora et al. 2007). A novel, complementary hypothesis is that habitual exercise not only improves CVD risk factors, but also protects arteries from the levels of risk factors to which they are exposed. In a broader context, it is possible that sedentary ageing increases our risk of developing CVD by lessening our ‘resistance’ to these factors, whereas physically active ageing preserves such resistance.
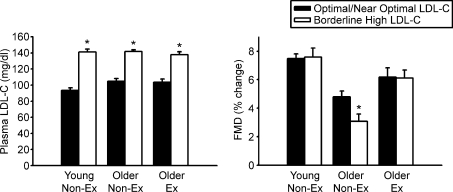
Recent evidence in humans provides support for this hypothesis (Walker et al. 2009). Among older sedentary healthy men, those with borderline high plasma LDL-cholesterol demonstrated impaired EDD compared with men who had optimal/near optimal LDL-cholesterol (Fig. 7). In contrast, similar differences in plasma LDL-cholesterol were not associated with differences in EDD among older endurance exercise-trained men (or young untrained men). Thus, older men who regularly performed aerobic exercise appeared to be ‘protected’ from the adverse effects of plasma LDL-cholesterol on EDD. It is possible that habitual exercise protects arteries against the effects of other risk factors with ageing and/or atherogenic physiological states (e.g. high fat diets, postprandial lipaemia), and that this is an important mechanism by which vascular function is preserved and the risk of CVD is reduced in middle-aged and older adults who exercise.
Figure 7. Plasma LDL-cholesterol, aging, endothelium-dependent dilation and habitual aerobic exercise.
Plasma low-density lipoprotein cholesterol (LDL-C) (left) and brachial artery flow-mediated dilation (FMD) (right) in young and older non-exercising (Non-Ex) and exercising (Ex) healthy men with either optimal/near optimal (filled bars) or borderline high (open bars) LDL-C; *P < 0.05 vs. optimal/near optimal group of same age and exercise status; values are mean ±s.e.m. Reprinted by permission from MacMillan Publishers Ltd from American Journal of Hypertension (Walker et al. 2009).
Summary and conclusions
Ageing is the major risk factor for CVD. Stiffening of large elastic arteries and the development of vascular endothelial dysfunction, most commonly measured as impaired EDD, are key contributors to the increased risk of CVD with ageing. Adults who regularly perform aerobic exercise have less large elastic artery stiffness and largely preserved vascular endothelial function with ageing. A moderate-intensity aerobic exercise intervention can improve large elastic artery stiffness in middle-aged men and women and improve/restore EDD in men. It is unknown if regular exercise improves EDD in postmenopausal women.
The mechanisms by which habitual aerobic exercise favourably influences large elastic artery stiffness are unknown, but do not obviously involve modification of conventional CVD risk factors. Current evidence indicates that regular aerobic exercise preserves/restores EDD with ageing by increasing NO bioavailability as a result of increasing the expression and activation of endothelial NO synthase, reducing vascular oxidative stress and increasing the bioactivity of BH4. Habitual aerobic exercise lowers oxidative stress in old mice by reducing NADPH oxidase expression/activity-associated superoxide production and increasing SOD activity. Regular exercise also may act to preserve vascular function with advancing age by protecting arteries from the adverse effects of conventional risk factors for CVD.
Taken together, these effects may explain, at least in part, why middle-aged and older adults who exercise demonstrate a higher functional capacity and a lower prevalence of CVD compared with their sedentary peers. Given the increased numbers of older adults today and predicted in the future, regular aerobic exercise can play a major role in the prevention of premature CVD-related morbidity and mortality and the promotion of healthy vascular ageing.
Future directions
Much remains to be understood about habitual exercise and these arterial changes with ageing. A major void is the absence of information on the mechanisms by which regular aerobic exercise lessens/improves large elastic artery stiffness with ageing. Given the inherent limitations in human investigations, such mechanisms will need to be identified by in vivo and ex vivo investigations using animal models, and perhaps, cell culture. Studies aimed at identifying the cellular and molecular events involved and the sites of those events (e.g. medial vs. adventitial layers of arteries) are needed.
In this context, the possible role of reduced oxidative stress in habitual exercise-associated attenuation of large elastic stiffening with ageing is of interest (Moreau et al. 2006). The potential involvement of vascular inflammation in the development of arterial stiffness and endothelial dysfunction with ageing, and the intriguing possibility that the anti-vascular ageing effects of regular aerobic exercise may be mediated in part by suppressing the development of inflammation in arteries also merit investigation.
To complement and extend observations from animal models and cell culture, more work is needed on the effects of ageing and habitual exercise on vascular cells and tissue obtained from humans, particularly for studies of endothelial function in which sampling from peripheral sites is both feasible and useful (because dysfunction is observed in peripheral arteries). Novel translational techniques (Rizzoni et al. 2001; Colombo et al. 2002) are available to pursue such investigations.
Finally, clinical studies focused on optimal types and ‘doses’ (intensity, duration and frequency) of exercise for preventing and treating these and other expressions of arterial ageing are needed. At present, we have no definative information as to whether habitual aerobic exercise improves EDD in postmenopausal women, a population at increased risk of CVD as a result of the combination of age and oestrogen deficiency. Investigations aimed at determining the efficacy of regular aerobic exercise in treating large elastic artery stiffness and vascular endothelial dysfunction in middle-aged and older adults with CVD risk factors and/or clinical CVD also are warranted.
Acknowledgments
This work was supported by NIH AG006537, AG013038, AG022241, AG031141, AG015897, AG000279, AG031617 and RR00051 and AHA 0715735Z.
Glossary
Abbreviations
- BH4
tetrahydrobiopterin
- CVD
cardiovascular diseases
- EDD
endothelium-dependent dilatation
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- NO
nitric oxide
- SOD
superoxide dismutase
Author contributions
All authors contributed to drafting and revision of the article and provided final approval of the version to be published.
References
- Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O’Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202–210. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortalityA prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- Celermajer D, Sorensen K, Bull C, Robinson J, Deanfield J. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2008;586:1161–1168. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol. 2004a;286:H1528–1534. doi: 10.1152/ajpheart.00879.2003. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004b;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–395. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lavrencic A, Salobir BG, Keber I. Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Arterioscler Thromb Vasc Biol. 2000;20:551–555. doi: 10.1161/01.atv.20.2.551. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, Cockcroft JR, Wilkinson IB. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003;57:861–868. doi: 10.1016/s0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45:1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Gavin KM, Plum AE, Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause. 2006;13:951–958. doi: 10.1097/01.gme.0000243575.09065.48. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Nosaka T, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol. 2003;28:204–212. doi: 10.1139/h03-016. [DOI] [PubMed] [Google Scholar]
- Reddy AK, Li YH, Pham TT, Ochoa LN, Trevino MT, Hartley CJ, Michael LH, Entman ML, Taffet GE. Measurement of aortic input impedance in mice: effects of age on aortic stiffness. Am J Physiol Heart Circ Physiol. 2003;285:H1464–1470. doi: 10.1152/ajpheart.00004.2003. [DOI] [PubMed] [Google Scholar]
- Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, Girelli A, Rodella L, Bianchi R, Sleiman I, Rosei EA. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation. 2001;103:1238–1244. doi: 10.1161/01.cir.103.9.1238. [DOI] [PubMed] [Google Scholar]
- Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–513. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol. 2006;101:1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Stallone JN, Dominguez JM, 2nd, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2007;292:H3119–3127. doi: 10.1152/ajpheart.00588.2006. [DOI] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–132. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics – 2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2009;106:1925–1934. doi: 10.1152/japplphysiol.91232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension. 2007;50:403–409. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–2200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens. 2009;22:250–256. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]