Abstract
In humans, exercise training and moderate to high levels of physical activity are protective against cardiovascular disease. In fact they are ∼40% more protective than predicted based on the changes in traditional risk factors (blood lipids, hypertension, diabetes etc.) that they cause. In this review, we highlight the positive effects of exercise on endothelial function and the autonomic nervous system. We also ask if these effects alone, or in combination, might explain the protective effects of exercise against cardiovascular disease that appear to be independent of traditional risk factor modification. Our goal is to use selected data from our own work and that of others to stimulate debate on the nature and cause of the ‘risk factor gap’ associated with exercise and physical activity.
Exercise training and physical activity are protective against cardiovascular disease (Blair & Morris, 2009). In this paper, we raise ideas about why exercise training and physical activity are protective against cardiovascular disease. The main concept is that exercise (a term we will use generically to cover both exercise training and physical activity) does more than change traditional ‘risk factors’ (blood lipids, hypertension, diabetes, etc.; Mora et al. 2007; Green et al. 2008). In this context, there is a ‘risk factor gap’, and exercise appears to be far more protective than it should be based on changes in traditional risk factors alone. It should be noted at the outset that this paper is not a comprehensive review of ideas on this topic. It is also not designed to be a thorough weighing of the evidence pro and con for various mechanisms beyond traditional risk factors that might be protective against cardiovascular disease in exercising humans. Instead, this paper is designed to raise a few questions about the protective effects of exercise against cardiovascular disease. The main idea is that at least some of the protective effects of exercise are due largely to its impact on the autonomic nervous system. Additionally, autonomic dysfunction in combination with endothelial dysfunction may be an especially important combination that predisposes those with it to premature cardiovascular disease, disability and death.
Along the lines outlined above, we will briefly review the following topics.
We will discuss the impact of exercise on morbidity and mortality.
We will compare exercise with traditional drug-based and other interventions that alter risk factors and improve outcomes.
We will describe the risk factor gap.
We will then ask questions about what might explain the risk factor gap and make arguments that exercise and physical activity improve endothelial function and also preserve or improve autonomic function.
We will conclude by suggesting that a vicious cycle that promotes cardiovascular disease exists between inactivity and endothelial dysfunction that can be prevented or ameliorated by exercise.
Effects of exercise on cardiovascular mortality
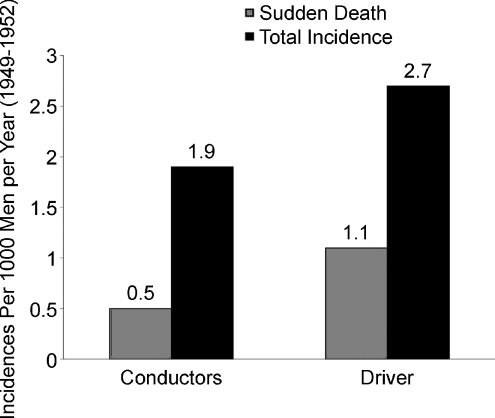
There is a vast literature on the effects of physical activity, exercise or both on all-cause and cardiovascular mortality that has recently been reviewed by Blair & Morris (2009). There are a number of landmark studies. For example, Morris reported in the 1950s that rates of cardiovascular disease were much lower in physically active London double-decker bus conductors than in sedentary bus drivers. He also showed similar differences between physically active postmen and civil servants employed as telephonists (Fig. 1). Other classic studies include the work of Paffenbarger on Harvard alumni and Blair and colleagues on the interactions of cardiorespiratory fitness with obesity in terms of both cardiovascular and all-cause mortality (Wei et al. 1999). In many studies, regular vigorous exercise or occupational physical activity has been shown to reduce the risk of cardiovascular disease by one-third to one-half. More importantly, very high levels of cardiorespiratory fitness appear to reduce these risks by up to 60–70%. These are remarkable data, and when large populations exercise regularly and follow other guidelines related to diet, smoking and obesity, there are profound effects on longevity and, most importantly, disability-free lifespan (Fraser & Shavlik, 2001).
Figure 1. Differences in sudden death cardiovascular mortality in physically active London bus conductors vs. sedentary bus drivers.
This was the first example showing that physical activity (exercise) was protective against cardiovascular disease. Data from Blair & Morris (2009).
It should be pointed out that for the studies highlighted above and those referenced below the issue of ‘type of exercise’ is challenging. In general, the interventional studies we cite all use aerobic or endurance training of varying intensities as the primary type of training. The cross-sectional studies are a mix between studies of trained athletic populations (almost exclusively endurance trained) and individuals who participate in either high levels of job-related physical activity or vigorous leisure-time activity. In this context, we do not cite any studies where the primary intervention has been strength training.
How does exercise compare with other interventions that reduce cardiovascular disease?
As is the case for exercise, there have been hundreds, if not thousands, of interventional trials (mostly with drugs or diet) that have attempted to control or modify one or more risk factors for cardiovascular disease. One recent notable example investigated the effects of moderate to high doses of statin treatment in a large cohort of patients who were judged at high risk for cardiovascular disease. Ridker et al. (2009) studied 17 800 patients with a low-denisity lipoprotein (LDL) value of >130 mg/dl and a C-reactive protein (CRP) value of >2 mg/L. They used a high dose of rosuvastatin to treat these patients. Using this approach, LDL dropped by about 50% and the risk of various cardiovascular events dropped between about one-third and one-half in this cohort. We chose this trial as an example because of the very large number of subjects and because the reduction in risk approximates, but does not exceed, that reported for exercise and physical activity studies. When these data are compared with Fig. 1, it appears that moderate to high levels of physical activity are as ‘protective’ or beneficial as a large drop in cholesterol is in high risk patients (Wilt et al. 2004).
However, it is interesting to note that various forms of exercise intervention typically cause only modest reductions in LDL or increases in high-density lipoproteins (HDL; Halverstadt et al. 2007; Green et al. 2008). This means that exercise is much less effective than statins in lowering LDL. Thus, it would seem that the beneficial effects of exercise on cardiovascular risk are not dependent on changes in blood lipids.
A similar story can be told for blood pressure, with exercise/physical activity having modest impact on blood pressure relative to medications in normotensive subjects and a relatively modest impact on subjects with hypertension (Green et al. 2003, 2008; Turnbull, 2003). The data are a little bit better for type 2 diabetes. There have been a number of recent intervention trials showing, for example, that 150 min per week of moderate or vigorous walking can reduce by about half the number of high-risk middle-aged subjects who develop full-blown type 2 diabetes over a 5–10 year period of follow-up (Lindstrom et al. 2006). Additionally, exercise seems better than drug-based interventions for the prevention of diabetes in high-risk patients (Tuomilehto et al. 2001; Knowler et al. 2002).
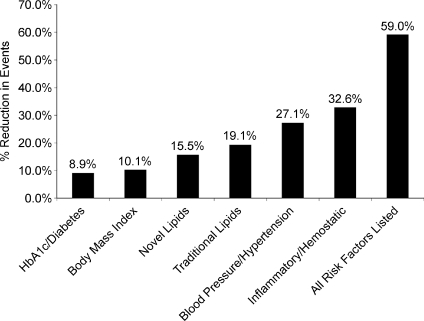
The question that arises from the data above is how much of the beneficial effect of exercise on cardiovascular risk is in fact due to its interaction with or effect on ‘traditional’ risk factors. In the nurses’ health study, Mora et al. (2007) evaluated ∼27 000 women. Those who had estimated physical activity or exercise levels >1500 calories per week in comparison to those with <200 calories per week had a ∼40% overall reduction in cardiovascular risk. However, less than half of the improvement in the risk for coronary heart disease could be attributed to improvements in traditional risk factors (Fig. 2). Additionally, only ∼59% of the risk reduction for all forms of cardiovascular disease could be attributed to the effects of exercise on traditional factors. This means that only ∼40–60% of the relative risk of coronary heart disease and cardiovascular disease in general can be explained on the basis of how exercise and physical activity modify traditional risk factors.
Figure 2. Effects of >1500 versus <200 calories per week of exercise/physical activity on risk factors and their impact on coronary heart disease and cardiovascular disease.
The filled bars show the risk reduction due to high levels of physical activity for all traditional risk factors in combination and for specific individual risk factors. The effects of exercise on traditional risk factors explained less than half the risk of coronary heart disease and ∼60% of cardiovascular disease. Data from Mora et al. (2007). HbA1c = elevated glycosylated haemoglobin.
What about NO and endothelial function?
Over the last 25–30 years, the vascular endothelium has emerged as a key site of cardiovascular control. In this context, blunted endothelial function is emerging as a risk factor for cardiovascular disease, and normal or enhanced endothelial function appears to be protective against cardiovascular disease (Green et al. 2003, 2004, 2008). For example, endothelial function is blunted in patients with a number of traditional cardiovascular risk factors, and treatment of those risk factors tends to improve or restore endothelial function. Statin therapy that lowers LDL cholesterol by about one-third tends to improve endothelial function dramatically (O’Driscoll et al. 1997; Perticone et al. 2000). Importantly, so does exercise training (DeSouza et al. 2000; Walsh et al. 2003; Green et al. 2004). There are numerous examples of the beneficial effects of exercise on endothelial function. For example, DeSouza and colleagues (2000) showed that young sedentary subjects had much better endothelial function as measured by acetylcholine-mediated forearm vasodilatation than older sedentary subjects. By contrast, there was no difference between young endurance-trained and older endurance-trained subjects. Additionally, the ‘restoring’ effects of exercise on acetylcholine-mediated dilatation in the forearm appear at least as good as or better than those of statins.
Therefore, what can we say about the exercise risk factor gap?
Exercise is roughly as effective as statins in preventing cardiovascular disease.
The changes in traditional risk factors associated with exercise training are modest compared with the impact of medications, with the possible exception of (pre-)diabetes.
Physical activity and exercise can have a profound influence on either preserving endothelial function in the face of various risk factors and aging or improving it when used as a therapeutic intervention.
However, is the improvement in endothelial function alone enough to explain the exercise effect? Or is there something else?
Does the autonomic nervous system contribute to the risk factor gap?
There is a variety of emerging evidence that altered autonomic function can have a profound effect on cardiovascular disease (Seals & Bell, 2004; Seals & Dinenno, 2004). There are various indices of autonomic function; however, none of them is perfect, and none is as simple as obtaining a blood test for cholesterol or glucose or simply measuring blood pressure with a cuff. Additionally, many widely studied or used markers of autonomic function are subject to some debate regarding their underlying mechanisms (Eckberg, 2009; Karemaker, 2009). In some cases, there are also questions about how generalizable autonomic outflow to one organ or vascular bed is to other organs or vascular beds (Wallin & Charkoudian, 2007), but in general the resting sympathetic outflow to one vascular bed is related to outflow in other vascular beds. For example, renal noradrenaline spillover correlates well with muscle sympathetic nerve activity in resting humans (Wallin et al. 1996). In spite of some of these limitations, we believe that enough information is available to begin to ask whether autonomic dysfunction is in fact the missing risk factor that is altered by exercise, and how it might interact with other risk factors.
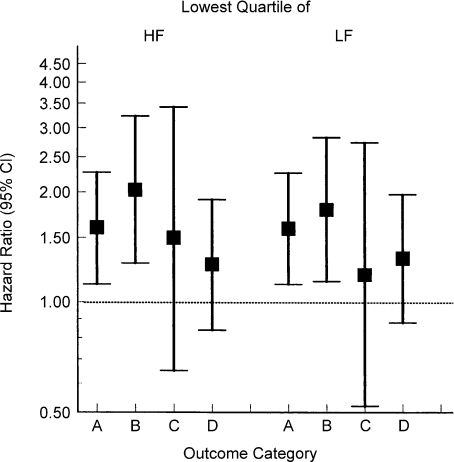
For example, in the Atherosclerosis Risk in Communities (ARIC) study of 16 000 middle-aged subjects (Fig. 3), reduced heart rate variability was associated with a marked increase in the incidence of coronary heart disease, myocardial infarction, fatal coronary heart disease and total mortality in a subset of diabetic patients (Liao et al. 2002). This shows that reduced heart rate variability is a marker of poor outcomes in middle-aged patients at risk for cardiovascular disease. In subsequent sections, we will argue that poor heart rate variability is related to increased vascular stiffness as a result of poor endothelial function and reduced baroreflex function.
Figure 3. Effects of reduced (lowest quartile) high-frequency (HF) or low-frequency (LF) heart rate variability on morbidity and mortality in diabetic patients form the ARIC study.
A, B, C and D reflect specific outcomes as follows: A, coronary heart disease; B, myocardial infarction; C, fatal coronary heart disease; and D, total mortality. Diabetic subjects with any marker of reduced heart rate variability had increased morbidity and mortality. Data from Liao et al. (2002).
In the Studies of left ventricular dysfunction (SOLVD) trial of asymptomatic subjects with left ventricular dysfunction, individuals with resting noradrenaline levels above the median had double or triple the all-cause mortality, cardiovascular mortality, hospitalization for development of congestive heart failure and development of myocardial infarction or angina (Benedict et al. 1996). Thus, evidence of sympathetic activation in these patients appeared to be at best a poor prognostic sign and was probably a major contributor to their poor outcomes. In the Coronary artery risk development in young adults (CARDIA) study, young healthy subjects were followed for 15 years in an effort to understand factors that might predispose some individuals to develop hypertension. One of the most powerful predictors of who became hypertensive was the blood pressure response to acute sympathoexcitatory stress at baseline (Matthews et al. 2004). Individuals in the quartile with the largest increase in blood pressure developed hypertension at about five times the rate of individuals in the lowest quartile. This again suggests that there is an important link between the autonomic nervous system and cardiovascular risk factors in humans.
However, when measurements of baseline muscle sympathetic nerve activity (MSNA) are made in healthy humans using microneurography, there appears to be no interaction between baseline levels of sympathetic activity and blood pressure (Wallin & Charkoudian, 2007). Less is known about how baseline levels of sympathetic activity interact with lipid and metabolic risk factors. Given the potent vasoconstricting effects of increased sympathetic traffic, how is normotension maintained in subjects with high sympathetic activity? We have recently demonstrated that, at least in young healthy men, individuals with high levels of baseline sympathetic activity have lower cardiac outputs and that their blood vessels are less sensitive to α-adrenergic vasoconstricting effects of noradrenaline (Wallin & Charkoudian, 2007; Joyner et al. 2008). These two factors limit the blood pressure-raising effects of the high sympathetic activity in these subjects. In young women, the story appears more complex, but one idea is that high levels of sympathetic traffic in women have little impact on vascular resistance owing to interactions between female reproductive hormones and vasodilating β2-receptors in the vasculature (Hart et al. 2009).
In terms of hypertensive subjects, there is evidence both for and against a relationship between sympathetic activity and blood pressure. However, even in studies that show a relationship between sympathetic activity and blood pressure, the differences between normotensive and hypertensive subject groups are not dramatic (Wallin & Charkoudian, 2007). By contrast, blood pressure typically rises with age and becomes more closely linked to sympathetic activity after the age of 40 (Narkiewicz et al. 2005). This is particularly apparent in women and consistent with the idea expressed above that in young women β2-mediated vasodilatation, driven in part by female reproductive hormones, limits the vasoconstrictor effects of noradrenaline. The hypothesis is that in older women β2-mediated vasodilator effects are lost, permitting a fuller expression of the vasoconstricting effects of high sympathetic activity. Therefore, we have at best a modest relationship between increased baseline sympathetic activity and blood pressure along with limited data on how the sympathetic nervous system interacts with metabolic risk factors such as lipids and diabetes. However, there is at least a hint of what might be happening during ageing, especially in female subjects.
High sympathetic activity and reduced endothelial function: a bad combination?
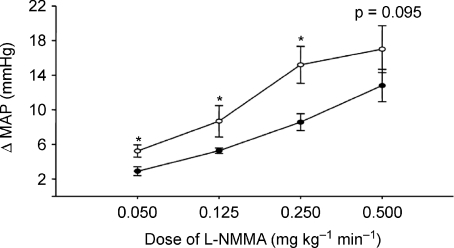
In an effort to understand how reduced endothelial function in combination with high MSNA might influence the cardiovascular system, we performed a systemic infusion of the nitric oxide synthase inhibitor NG-monomethyl-l-arginine (l-NMMA) and measured the effects on blood pressure and systemic haemodynamics in a group of healthy young men with widely varying baseline MSNA (Charkoudian et al. 2006). The idea was that there was at least some evidence that NO levels are higher in individuals with high baseline MSNA and that this NO buffers the blood pressure-raising effects of the high sympathetic activity. Additionally, because so many risk factors for cardiovascular disease, including hypertension, diabetes, hyperlipidaemia, inactivity and ageing, are all associated with ‘endothelial dysfunction’, we felt this approach would provide important insights into how sympathetic outflow and endothelial function interact. We found that in subjects with high resting muscle MSNA, very modest doses of systemic l-NMMA caused significantly greater increases in mean arterial pressure in comparison to subjects with low resting levels of MSNA (Fig. 4). This observation supports our contention that high levels of baseline sympathetic outflow are not ‘dangerous’per se, but that high levels of sympathetic outflow in conjunction with endothelial dysfunction may have a synergistic and detrimental effect in terms of cardiovascular risk. While the 2–7 mmHg differences noted in the dose–response curves in Fig. 4 may not seem impressive from a physiological perspective, when translated into population-based differences in risk over time they would be likely to have a dramatic effect on outcomes.
Figure 4. Effects of systemic nitric oxide synthase inhibition with l-NMMA on blood pressure in healthy male subjects with high vs. low levels of baseline muscle sympathetic nerve activity.
Low- and moderate-dose nitric oxide synthase inhibition causes larger increases in blood pressure in the subset of subjects with high baseline levels of MSNA. *P < 0.05, differences between the groups. Data from Charkoudian et al. (2006). Open circles = high MSNA subjects; closed circles = low MSNA subjects.
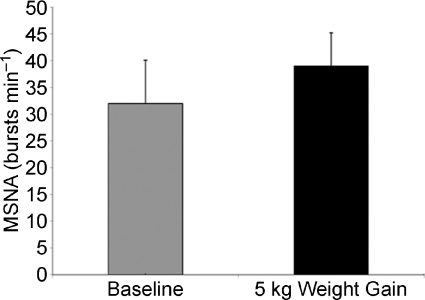
Based on epidemiological evidence, and observations similar to those outlined above, we propose that high levels of sympathetic activity will interact negatively with traditional risk factors in middle-aged subjects. This is likely to be made worse by weight gain, which has been shown to dramatically increase baseline levels of MSNA (Fig. 5; Seals & Bell, 2004; Gentile et al. 2007). This means that as individuals enter their 40s and 50s, those with high MSNA or who those gain weight (especially visceral fat) may be at increased risk. These individuals will also be likely to have reduced vasodilator function as a result of endothelial dysfunction associated with the metabolic syndrome, sedentary lifestyle and high levels of oxidative stress that limit the ability of NO to cause vasodilatation. As noted previously, almost all of the major cardiovascular risk factors are associated with reduced endothelial function. In addition to reduced endothelial function, there might be increased circulating vasoconstrictor substances and increased vasoconstrictor responsiveness that will further contribute to vascular dysfunction as middle-aged individuals drift into the cardiovascular disease phenotype (Nielsen et al. 2004).
Figure 5. A 5 kg weight gain causes a marked increase in muscle sympathetic nerve activity in young subjects.
Data from Gentile et al. (2007).
These factors are also likely to operate to make the large vessels stiffer, and stiffer large vessels play a role in age- and risk factor-associated baroreflex dysfunction, which would tend to reinforce the high sympathetic outflow state and potentially contribute to reduced heart rate variability (Seals & Dinenno, 2004; Joyner et al. 2008). All of these factors together then contribute to a vicious cycle of high sympathetic outflow, reduced vasodilator function and cardiovascular disease that would self amplify over time (Fig. 6).
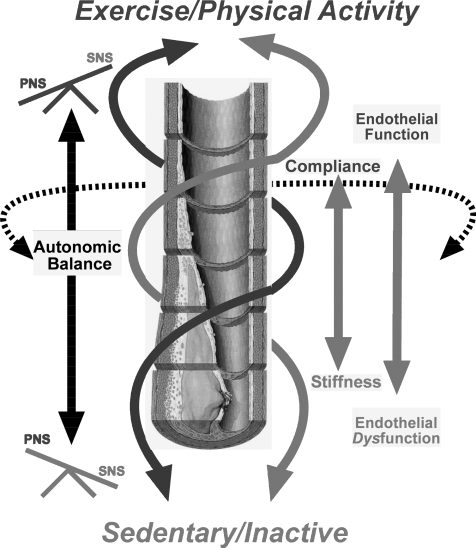
Figure 6. Schematic diagram of the interactions proposed in this paper.
In the endurance exercise-trained state or with high levels of physical activity, endothelial function and parasympathetic tone (augmented heart rate variability) are enhanced. Large conducting vessels remain compliant, and the effects of high sympathetic outflow, when present, are buffered. These positive interactions may account for observations showing that exercise is more protective against cardiovascular risk than predicted by its effects on traditional risk factors. With physical inactivity, there is a loss of endothelial function during middle age, a potential accumulation of risk factors, and increased vessel stiffness. These effects of physical inactivity permit the effects of high sympathetic tone to be more fully expressed while parasympathetic tone is progressively lost. These negative interactions may account for observations showing that physical inactivity is a more potent cardiovascular risk factor than widely appreciated.
Can exercise keep the autonomic nervous system healthy?
There is strong evidence to suggest that exercise training can keep the autonomic nervous system healthy. For example, exercise is protective against age-related reductions in baroreflex function in humans (Monahan et al. 2000). Additionally, there appears to be an exercise ‘dose–response’ relationship so that in both middle-aged and older subjects the baroreflex function is worse in the most sedentary subjects, better in those who participate in moderate exercise or physical activity programmes, and better still in those who perform regular endurance exercise training. In this context, endurance-trained older subjects have baroreflex function that is similar to moderately active young subjects.
This improvement in baroreflex function, which on a population basis is likely to manifest as improved heart rate variability, could be the result of both greater blood vessel distensibility and better signal transduction in barosensitive areas of the carotid sinus and aortic arch, or it could also represent improved or maintained central integration in the brainstem cardiovascular centres. However, the key point is that moderate exercise, endurance training and high levels of physical activity are highly protective against age-associated baroreflex dysfunction. Along similar lines, literally hundreds of studies over the last 10–20 years have demonstrated that physically active and/or exercise-trained humans, in general, have improved heart rate variability in comparison to control groups (Davy et al. 1996) This appears to be the case in young and old subjects, in subjects with and without cardiovascular risk factors, and also in subjects with frank cardiovascular disease. Finally, exercise training can clearly reduce muscle sympathetic nerve activity in patients with congestive heart failure (Fraga et al. 2007). Since exercise is also protective against weight gain and visceral obesity, it is likely to blunt the age-associated rise in MSNA.
There are also important confirmatory and mechanistic data from animal studies to show that exercise training limits sympathoexcitation and favours sympathoinhibition in the brainstem cardiovascular centres. For example, Mueller (2007) has shown that the increases in arterial pressure and lumbar sympathetic nerve activity are blunted when bicuculline is injected into the rostral ventrolateral medulla. Additionally, the reductions in heart rate during the pressor response are greater in exercise-trained animals. These data imply strong central nervous system effects of exercise on the autonomic nervous system that favour sympathoinhibition and enhanced vagal outflow.
Conclusion
In summary, we have tried to be provocative in this paper and used an impressionistic approach to integrate key data about exercise and the risk factor gap in cardiovascular disease. In this context, autonomic dysfunction is clearly a marker of poor outcomes in large populations of middle-aged humans. Autonomic dysfunction, including sympathetic activation, is also a marker of bad outcomes in patients with various risk factors for cardiovascular disease. These observations suggest that autonomic dysfunction and/or sympathoexcitation predispose humans to cardiovascular disease. We also argue that any negative effects of autonomic dysfunction are amplified by concurrent endothelial dysfunction and, indeed, there is some evidence for direct and deleterious interactions between elevated sympathetic nervous system activity and NO function (Hijmering et al. 2002). In the context of the above ideas, we believe there is a vicious cycle between autonomic dysfunction and endothelial dysfunction that can largely be prevented or ameliorated by exercise training. We also believe that this vicious cycle explains why exercise is more protective than it should be based on its effects on traditional risk factors for cardiovascular disease. Therefore, our global hypothesis is that the risk factor gap can be explained by the following effects of exercise and physical activity on the cardiovascular system.
Enhanced vagal tone via improved peripheral baroreflex function and CNS cardiovascular regulation. In populations, this will be protective and be seen as improved or maintained heart rate variability.
Enhanced or maintained endothelial function that will both favour vasodilatation and contribute to enhanced peripheral baroreflex function by limiting age- or risk factor-associated increases in vascular stiffness.
Positive interactions between enhanced endothelial function and sympathetic outflow that limit the effects of high levels of baseline sympathetic outflow on blood pressure.
In closing, the novel concept raised in our review is that these factors operating in concert explain the ‘missing’ protective effects of exercise and physical activity on the cardiovascular system.
Acknowledgments
Dr Joyner was supported by NIH HL-46493, HL83947, RR4150 and the Caywood Professorship. Dr Green was supported by National Heart Foundation of Australia.
References
- Benedict CR, Shelton B, Johnstone DE, Francis G, Greenberg B, Konstam M, Probstfield JL, Yusuf S. Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventricular dysfunction. Circulation. 1996;94:690–697. doi: 10.1161/01.cir.94.4.690. [DOI] [PubMed] [Google Scholar]
- Blair SN, Morris JN. Healthy hearts—and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19:253–256. doi: 10.1016/j.annepidem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol. 2006;291:H1378–H1383. doi: 10.1152/ajpheart.00234.2006. [DOI] [PubMed] [Google Scholar]
- Davy KP, Miniclier NL, Taylor JA, Stevenson ET, Seals DR. Elevated heart rate variability in physically active postmenopausal women: a cardioprotective effect? Am J Physiol Heart Circ Physiol. 1996;271:H455–H460. doi: 10.1152/ajpheart.1996.271.2.H455. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Point: counterpoint: Respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol. 2009;106:1740–1742. doi: 10.1152/japplphysiol.91107.2008. [DOI] [PubMed] [Google Scholar]
- Fraga R, Franco FG, Roveda F, de Matos LNJ, Braga AMFW, Rondon MUPB, Rotta DR, Brum PC, Barretto ACP, Middlekauff HR, Negrao CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail. 2007;9:630–636. doi: 10.1016/j.ejheart.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Fraser GE, Shavlik DJ. Ten years of life: is it a matter of choice? Arch Intern Med. 2001;161:1645–1652. doi: 10.1001/archinte.161.13.1645. [DOI] [PubMed] [Google Scholar]
- Gentile CL, Orr JS, Davy BM, Davy KP. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1834–R1838. doi: 10.1152/ajpregu.00876.2006. [DOI] [PubMed] [Google Scholar]
- Green DJ, Majorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, O’Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol. 2008;105:766–768. doi: 10.1152/japplphysiol.01028.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Walsh JH, Maiorana A, Best MJ, Taylor RR, O’Driscoll JG. Exercise-induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: pooled analysis of diverse patient populations. Am J Physiol Heart Circ Physiol. 2003;285:H2679–H2687. doi: 10.1152/ajpheart.00519.2003. [DOI] [PubMed] [Google Scholar]
- Halverstadt A, Phares DA, Wilund KR, Goldberg AP, Hagberg JM. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism. 2007;56:444–450. doi: 10.1016/j.metabol.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmering ML, Stroes ESG, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol. 2008;93:715–724. doi: 10.1113/expphysiol.2007.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karemaker JM. Counterpoint: Respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol. 2009;106:1742–1743. doi: 10.1152/japplphysiol.91107.2008a. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes. The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes. 2002;51:3524–3531. doi: 10.2337/diabetes.51.12.3524. [DOI] [PubMed] [Google Scholar]
- Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M, Tuomilehto J, Finnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol. 2000;529:263–271. doi: 10.1111/j.1469-7793.2000.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Cook N, Buring JE, Ridker PM, Lee I-M. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PJ. Exercise training attenuates increases in lumbar sympathetic nerve activity produced by stimulation of the rostral ventrolateral medulla. J Appl Physiol. 2007;102:803–813. doi: 10.1152/japplphysiol.00498.2006. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Halliwill JR, Joyner MJ, Jensen MD. Vascular response to angiotensin II in upper body obesity. Hypertension. 2004;44:435–441. doi: 10.1161/01.HYP.0000142111.67601.6b. [DOI] [PubMed] [Google Scholar]
- O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Maio R, Cloro C, Candigliota M, Scozzafava A, Mongiardo A, Mastroroberto P, Chello M, Mattioli PL. Effects of atorvastatin and vitamin C on endothelial function of hypercholesterolemic patients. Atherosclerosis. 2000;152:511–518. doi: 10.1016/s0021-9150(00)00370-1. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, JUPITER Trial Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Eng J Med. 2009;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Seals DR, Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes. 2004;53:276–284. doi: 10.2337/diabetes.53.2.276. [DOI] [PubMed] [Google Scholar]
- Seals DR, Dinenno FA. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol. 2004;287:H1895–H1905. doi: 10.1152/ajpheart.00486.2004. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Turnbull F, Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491:881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JH, Yong G, Cheetham C, Watts GF, O’Driscoll GJ, Taylor RR, Green DJ. Effects of exercise training on conduit and resistance vessel function in treated and untreated hypercholesterolaemic subjects. Eur Heart J. 2003;24:1681–1689. doi: 10.1016/s0195-668x(03)00384-1. [DOI] [PubMed] [Google Scholar]
- Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS, Jr, Blair SN. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese man. JAMA. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- Wilt TJ, Bloomfield HE, MacDonald R, Nelson D, Rutks I, Ho M, Larsen G, McCall A, Pineros S, Sales A. Effectiveness of statin therapy in adults with coronary heart disease. Arch Intern Med. 2004;164:1427–1436. doi: 10.1001/archinte.164.13.1427. [DOI] [PubMed] [Google Scholar]