Abstract
Type 2 diabetes, cardiovascular diseases, colon cancer, breast cancer, dementia and depression constitute a cluster of diseases, which defines ‘a diseasome of physical inactivity’. Both physical inactivity and abdominal adiposity, reflecting accumulation of visceral fat mass, are associated with the occurrence of the diseases within the diseasome. Physical inactivity appears to be an independent and strong risk factor for accumulation of visceral fat, which again is a source of systemic inflammation. Chronic inflammation is involved in the pathogenesis of insulin resistance, atherosclerosis, neurodegeneration and tumour growth. Evidence suggests that the protective effect of exercise may to some extent be ascribed to the anti-inflammatory effect of regular exercise, which can be mediated via a reduction in visceral fat mass and/or by induction of an anti-inflammatory environment with each bout of exercise. The finding that muscles produce and release myokines provides a conceptual basis to understand the mechanisms whereby exercise influences metabolism and exerts anti-inflammatory effects. According to our theory, contracting skeletal muscles release myokines, which work in a hormone-like fashion, exerting specific endocrine effects on visceral fat. Other myokines work locally within the muscle via paracrine mechanisms, exerting their effects on signalling pathways involved in fat oxidation.
Introduction
Most human diseases are not independent of each other, although they are often treated individually.
To understand the mechanisms underlying these networks of diseases, it is obviously necessary to map out the detailed diagram of various cellular components that are influenced by specific genes and gene products. Such genetic network-based thinking has provided insights into the pathogenesis of several diseases (Barabasi, 2007).
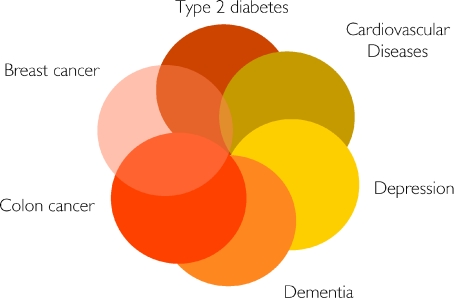
Another type of network in human disease is the so-called social network, which includes all human-to-human interactions (e.g. familial, friendship, sexual and proximity-based contacts) that play a role in the spread of pathogens (Barabasi, 2007). Interestingly, social networks have an important role also in the spread of obesity. Christakis and Fowler suggest that friends have an even more important effect on a person's risk of obesity than genes do (Christakis & Fowler, 2007). They showed that if one friend became obese during a given time interval, the other friend's chances of following suit increased by 171%. In contrast, if one sibling became obese, the chance that the other would become obese increased by 40%. The latter study underscores the role of social networks in the obesity epidemic. In continuation, I hereby suggest the existence of a ‘lifestyle network’, or more specifically a ‘physical inactivity network’ of key importance in understanding why several diseases appear in clusters, although they are highly different in terms of phenotypical presentation and required treatment. Type 2 diabetes, cardiovascular diseases, colon cancer, postmenopausal breast cancer, dementia and depression constitute a cluster of diseases, which I hereby identify as ‘the diseasome of physical inactivity’, as appears in Fig. 1.
Figure 1.
Type 2 diabetes, cardiovascular diseases, colon cancer, postmenopausal breast cancer, dementia and depression constitute a cluster of diseases, which can be identified as ‘the diseasome of physical inactivity’
It is well-established that physical inactivity increases the risk of type 2 diabetes (Tuomilehto et al. 2001), cardiovascular diseases (CVD) (Nocon et al. 2008), colon cancer (Wolin et al. 2009) and postmenopausal breast cancer (Monninkhof et al. 2007). In addition, physical inactivity may also play a role in the development of dementia (Rovio et al. 2005) and even depression (Paffenbarger et al. 1994). The latter diagnoses are not meant to represent an exclusive list of diseases or disorders related to physical inactivity. However, they are all frequent chronic diseases, associated with an enhanced risk of premature morbidity. Patients with type 2 diabetes have a markedly increased risk of CVD (Diamant & Tushuizen, 2006). In addition, type 2 diabetes is associated with impaired cognitive function as well as with both Alzheimer's disease and vascular dementia, and individuals with type 2 diabetes also have a high prevalence of affective illness, including major depression (reviewed in Komulainen et al. 2008). Other studies report that type 2 diabetes is associated with an elevated risk of developing colon and breast cancer, as well as pancreatic, liver and endometrial cancer (Richardson & Pollack, 2005). There is indeed also overlap between other diagnoses within the diseasome of physical inactivity; however, it appears that type 2 diabetes plays a central role.
It is interesting that the diseasome of physical inactivity represents highly different diseases, but they share important pathogenetic mechanisms. Clearly, independently of body mass index (BMI), physical inactivity is a risk factor for all-cause mortality (Pedersen, 2007). Moreover, chronic systemic inflammation is associated with physical inactivity independent of obesity (Fischer et al. 2007).
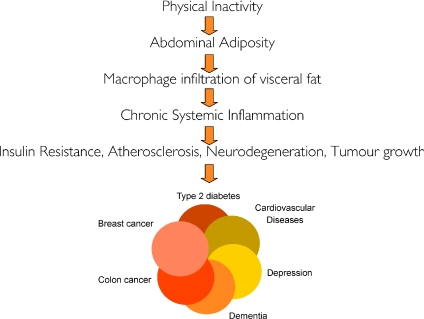
I herein put forward the hypothesis that physical inactivity leads to accumulation of visceral fat and consequently the activation of a network of inflammatory pathways, which promote development of insulin resistance, atherosclerosis, neurodegenation and tumour growth, and thereby the development of the diseases belonging to the ‘diseasome of physical inactivity’. The hypothesis is illustrated in Fig. 2.
Figure 2.
Hypothesis: Physical inactivity leads to accumulation of visceral fat and consequently to the activation of a network of inflammatory pathways, which promotes development of insulin resistance, atherosclerosis, neurodegeneration, and tumour growth, leading to the development of ‘the diseasome of physical inactivity’
Physical inactivity and abdominal adiposity
A relatively large amount of subcutaneous adipose tissue has little or no damaging effect and may even offer protection against chronic diseases, whereas strong evidence exists for the detrimental effects of visceral fat and fat in the liver and in muscle (Pischon et al. 2008).
In this context, fat is not just fat and ectopic fat accumulation may be regarded as fat in ‘the wrong places’. Abdominal adiposity is associated with cardiovascular disease (CVD) (Haffner, 2007), type 2 diabetes (Bays, 2009), dementia (Whitmer et al. 2008), colon cancer (Giovannucci, 2007) and breast cancer (Xue & Michels, 2007) as well as all-cause mortality independent of BMI, even in people with a normal body weight (Pischon et al. 2008). Thus, the health consequences of abdominal adiposity and physical inactivity are similar. Moreover, both physical inactivity (Pedersen & Febbraio, 2008) and abdominal adiposity (Yudkin, 2007) are associated with persistent systemic low-grade inflammation (Festa et al. 2002; Handschin & Spiegelman, 2008).
We recently conducted a model of physical inactivity that certainly points at a direct link between physical inactivity and accumulation of visceral fat. A group of young healthy men decreased their daily stepping for 2 weeks to 1500 steps from the range recommended for adults of around 10 000. During this time, they developed a markedly impaired glucose tolerance as well as attenuation of postprandial lipid metabolism. The intervention was associated with a 7% increase in intra-abdominal fat mass, measured by MR scanning, without a change in total fat mass while total fat-free mass and BMI decreased (Olsen et al. 2008). Thus, the striking consequences of physical inactivity included accumulation of visceral fat mass and impaired glucose and lipid metabolism.
Visceral fat as a cause of low-grade systemic inflammation
Models of lipodystrophy suggest that if the subcutaneous fat becomes inflamed and adipocytes undergo apoptosis/necrosis, the fat storing capacity is impaired; hence, fat is deposited as ectopic fat. Given the anti-inflammatory effects of regular exercise (Petersen & Pedersen, 2005), physical inactivity may lead to inflammation of subcutaneous adipose tissue and impaired ability to store fat also in people who do not fulfil the criteria for lipodystrophy.
Although speculative, one explanation of the differential outcome of accumulating fat subcutaneously or as ectopic fat could be that when fat is stored in ‘the wrong places’, it will stimulate an inflammatory response. Evidence exists that visceral fat is more inflamed than subcutaneous fat and constitutes an important source of systemic inflammation (Yudkin, 2007).
Thus, although 2 weeks of inactivity did not provoke an increase in circulating levels of cytokines, evidence exists of an association between physical inactivity and low-grade systemic inflammation in healthy, young individuals (Petersen & Pedersen, 2005). These findings would be compatible with the notion that accumulation of visceral fat precedes chronic systemic inflammation.
Inflammation as a cause of chronic diseases
Chronic inflammation promotes development of insulin resistance, atherosclerosis, neurodegenation and tumour growth (Handschin & Spiegelman, 2008), and thereby the development of the diseases belonging to the ‘diseasome of physical inactivity’.
Mounting evidence suggests that TNF-α plays a direct role in the metabolic syndrome (recently reviewed in Pedersen & Febbraio, 2008). In short, patients with diabetes demonstrate high protein expression of TNF-α in skeletal muscle and increased TNF-α levels in plasma, and it is likely that adipose tissue, which produces TNF-α, is the main source of the circulating TNF-α. In vitro studies demonstrate that TNF-α has direct inhibitory effects on insulin signalling. Recently it was demonstrated that TNF-α infusion in healthy humans induces insulin resistance in skeletal muscle, without an effect on endo-genous glucose production. It has also been proposed that TNF-α causes insulin resistance indirectly in vivo by increasing the release of free fatty acids (FFA) from adipose tissue. TNF-α increases lipolysis in human and 3T3-L1 adipocytes. However, TNF-α has no effect on muscle fatty acid oxidation, but increases fatty acid incorporation into diacylglycerol, which may be involved in the development of the TNF-α-induced insulin resistance in skeletal muscle. Moreover, evidence suggests that TNF-α plays a direct role in linking insulin resistance to vascular disease (Yudkin et al. 2005; Plomgaard et al. 2005). In cardiovascular diseases, activated immune cells also play major roles, particularly in the aetiology of atherosclerosis (Matter & Handschin, 2007).
Several downstream mediators and signalling pathways seem to provide the cross talk between inflammatory and metabolic signalling. These include the discovery of c-Jun N-terminal kinase (JNK) and Iκβ kinase (IκβK) as critical regulators of insulin action activated by TNF-α (Hotamisligil, 2003). In human TNF-α infusion studies, TNF-α increases phosphorylation of p70 S6 kinase, extracellular signal-regulated kinase-1/2 and c-Jun NH2-terminal kinase, concomitant with increased serine and reduced tyrosine phosphorylation of insulin receptor substrate-1. These signalling effects are associated with impaired phosphorylation of Akt substrate 160, the most proximal step identified in the insulin signalling cascade regulating GLUT4 translocation and glucose uptake (Plomgaard et al. 2005).
With regard to IL-6, its role in insulin resistance is highly controversial (as reviewed in Pedersen & Febbraio, 2008). Infusion of recombinant human (rh) IL-6 into resting healthy humans does not impair whole body, lower limb, or subcutaneous adipose tissue glucose uptake or endogenous glucose production (EGP), although IL-6 contributes to the contraction-induced increase in endo-genous glucose production. When diabetes patients were given rhIL-6-infusion, plasma concentrations of insulin declined to levels comparable with that in age- and BMI-matched healthy controls, indicating that the IL-6 enhanced insulin sensitivity.
A number of studies indicate that IL-6 enhances lipolysis, as well as fat oxidation, via an activation of AMPK (reviewed in Pedersen & Febbraio, 2008). Consistent with this idea, Wallenius et al. (2002) demonstrated that IL-6 deficient mice developed mature-onset obesity and insulin resistance. In addition, when the mice were treated with IL-6, there was a significant decrease in body fat mass in the IL-6 knock-out, but not in the wild-type, mice. To determine whether physiological concentrations of IL-6 affected lipid metabolism, our group administered physiological concentrations of rhIL-6 to healthy young and elderly humans as well as patients with type 2 diabetes (Petersen et al. 2005; van Hall et al. 2003). The latter studies identified IL-6 as a potent modulator of fat metabolism in humans, increasing lipolysis as well as fat oxidation without causing hypertriacylglycerolaemia.
Of note, whereas it is known that both TNF-α and IL-6 induce lipolysis, only IL-6 appears to induce fat oxidation (van Hall et al. 2003; Plomgaard et al. 2007). Given the different biological profiles of TNF-α and IL-6 and given that TNF-α may trigger an IL-6 release, one theory holds that it is TNF-α derived from adipose tissue that is actually the major ‘driver’ behind inflammation-induced insulin resistance and atherosclerosis. Importantly, also tumour initiation, promotion and progression are stimulated by systemic elevation of pro-inflammatory cytokines (Lin & Karin, 2007; Handschin & Spiegelman, 2008).
A chronic inflammatory environment will lead to a state of insulin resistance with hyperinsulinaemia. The so-called hyperinsulinaemia hypothesis goes hand in hand with the inflammation hypothesis.
The hyperinsulinaemia hypothesis suggests that elevated levels of insulin and free IGF-1 promote proliferation of colon cells and lead to a survival benefit of transformed cells, ultimately resulting in colorectal cancer (Berster & Goke, 2008). In addition, a number of neurodegenerative diseases are linked to a local inflammatory response in the brain (neuroinflammation), e.g. interleukin-1β (IL-1β), TNF-related apoptosis-inducing ligand (TRAIL) and other cytokines have been postulated to be involved in the aetiology of Alzheimer's disease (Zipp & Aktas, 2006). Moreover, in addition to the neuroinflammation found in many neurodegenerative disorders, systemic inflammation may further exacerbate the progression of neurodegeneration (Perry et al. 2007).
The myokine concept
The protective effect of exercise against diseases associated with chronic inflammation may to some extent be ascribed to an anti-inflammatory effect of regular exercise. I hereby suggest that the long-term anti-inflammatory effects of exercise may be mediated via effects of exercise leading to a reduction in visceral fat mass.
In line with the acceptance of adipose tissue as an endocrine organ, we came up with the innovative idea that also skeletal muscle should be viewed as an endocrine organ (Pedersen & Febbraio, 2008). In continuation, we have suggested that cytokines and other peptides that are produced, expressed and released by muscle fibres and exert paracrine or endocrine effects should be classified as ‘myokines’ (Pedersen & Febbraio, 2008). Given that skeletal muscle is the largest organ in the human body, our discovery of contracting muscle as a cytokine producing organ opens up a whole new paradigm: through evolution muscle has had a central role in orchestrating metabolism and function of other organs. This paradigm provides a conceptual basis explaining the multiple consequences of a physically inactive life style. If the endocrine function of the muscle is not stimulated through contractions, this will cause malfunction of several organs and tissues of the body as well as an increased risk of cardiovascular disease, cancer and dementia (Pedersen & Febbraio, 2008).
Today, it appears that skeletal muscle has the capacity to express several myokines. The list includes interleukin IL-6, IL-8, IL-15, brain-derived neurotrophic factor (BDNF) and leukaemia inhibitory factor (LIF) (Broholm et al. 2008). Through other strategies, Kenneth Walsh, Boston, has recently identified the myokines FGF21 and follistatin-like-1 (Izumiya et al. 2008; Ouchi et al. 2008). Thus, although the idea of an ‘exercise factor’ can be traced back many years, our recent identification of muscle as a myokine-producing organ opens up a whole new field of research. In this review, I briefly highlight some myokines with specific effect on fat oxidation.
IL-6: the myokine prototype
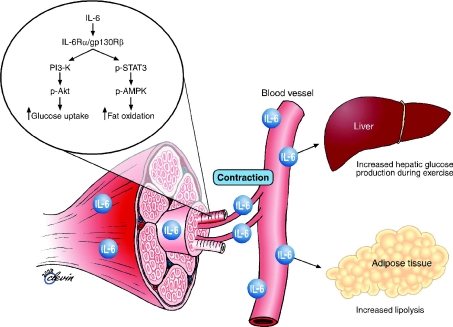
The first identified and most studied myokine is the gp130 receptor cytokine interleukin-6 (IL-6) (Pedersen & Febbraio, 2008). IL-6 was discovered as a myokine because of the observation that it increases up to 100-fold in the circulation during physical exercise. Identification of IL-6 production by skeletal muscle during physical activity generated renewed interest in the metabolic role of IL-6 because it created a paradox. On one hand, IL-6 is markedly produced and released in the post-exercise period when insulin action is enhanced, but on the other hand, IL-6 has also been associated with obesity and reduced insulin action (Pedersen & Febbraio, 2008). However, a number of studies during the past decade have revealed that in response to muscle contractions, both type I and type II muscle fibres express the myokine IL-6, which subsequently exerts its effects both locally within the muscle (e.g. through activation of AMPK) and – when released into the circulation – peripherally in several organs in a hormone-like fashion. Within skeletal muscle, IL-6 acts locally to signal through a gp130Rβ/IL-6Rα homodimer, resulting in activation of AMP kinase and/or PI3 kinase to increase glucose uptake and fat oxidation. IL-6 may also work in an endocrine fashion to increase hepatic glucose production during exercise or lipolysis in adipose tissue (reviewed in Pedersen & Febbraio, 2008). Although it has not been demonstrated that IL-6 has specific effects on visceral fat mass, it appears to play an important role in lipid metabolism as is illustrated in Fig. 3.
Figure 3. Biological role of contraction-induced IL-6.
Skeletal muscle expresses and releases myokines into the circulation. In response to muscle contractions, both type I and type II muscle fibres express the myokine IL-6, which subsequently exerts its effects both locally within the muscle (e.g. through activation of AMPK) and – when released into the circulation – peripherally in several organs in a hormone-like fashion. Specifically, in skeletal muscle, IL-6 acts in an autocrine or paracrine manner to signal through a gp130Rβ/IL-6Rα homodimer, resulting in activation of AMP kinase and/or PI3 kinase to increase glucose uptake and fat oxidation. IL-6 is also known to increase hepatic glucose production during exercise or lipolysis in adipose tissue. Modified from Pedersen & Febbraio (2008) with permission from the American Physiological Society and from Pedersen and Fischer (2007) with permission from Elsevier.
IL-15 – a role in muscle–fat cross talk?
IL-15 is expressed in human skeletal muscle and has been identified as an anabolic factor in muscle growth. In addition to its anabolic effects on skeletal muscle in vitro and in vivo, IL-15 appears to play a role in lipid metabolism (Nielsen & Pedersen, 2007). Therefore, IL-15 was suggested to be involved in muscle–fat cross talk.
Recently, we demonstrated that IL-15 mRNA levels were upregulated in human skeletal muscle following a bout of strength training (Nielsen et al. 2007) suggesting that IL-15 may accumulate within the muscle as a consequence of regular training. Interestingly, we further demonstrated a negative association between plasma IL-15 concentration and trunk fat mass, but not limb fat mass, in humans. In support of the human data, we demonstrated a decrease in visceral fat mass, but not subcutaneous fat mass, when IL-15 was overexpressed in murine muscle (Nielsen et al. 2008). Quinn et al. (2009) found that elevated circulating levels of IL-15 resulted in significant reductions in body fat and increased bone mineral content, without appreciably affecting lean body mass or levels of other cytokines. Although, the latter model represented an artificial system, the findings lend some support to the idea that IL-15 secretion from muscle tissue may modulate visceral fat mass in particular via an endocrine mechanism.
BDNF – a role in neurobiology and peripheral metabolism
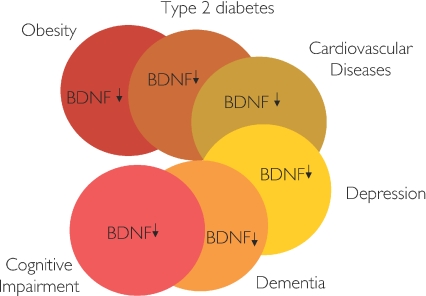
It was by realizing the disease cluster of physical inactivity that we recently became interested in brain-derived neurotrophic factor (BDNF), which is a member of the neurotrophic factor family. BDNF is recognized as playing a key role in regulating survival, growth and maintenance of neurons (Mattson et al. 2004), and it plays a role in learning and memory (Tyler et al. 2002). Hippocampal samples from Alzheimer's disease donors show decreased BDNF expression (Connor et al. 1997) and individuals with Alzheimer's disease have low plasma levels of BDNF (Laske et al. 2005). Also, patients with major depression have lower levels of serum BDNF than normal control subjects (Karege et al. 2002). Other studies suggest that plasma BDNF is a biomarker of impaired memory and general cognitive function in ageing women (Komulainen et al. 2008) and a low circulating BDNF level was recently shown to be an independent and robust biomarker of mortality risk in old women (Krabbe et al. 2009). In addition, it has previously been described that patients with acute coronary syndromes have reduced levels of BDNF in plasma (Manni et al. 2005). Interestingly, we found low levels of circulating BDNF also in individuals with both obesity and type 2 diabetes (Krabbe et al. 2007). Thus, BNDF is low in people with Alzheimer's disease, major depression, impaired cognitive function, CVD, type 2 diabetes and obesity, and constitutes a diseasome of low BDNF as illustrated in Fig. 4.
Figure 4.
The diseasome of low circulating levels of brain derived neurotropic factor (BDNF) has a major overlap with the diseases included in the diseasome of physical inactivity
The finding of low plasma levels of BDNF in patients with type 2 diabetes stimulated us to study if the brain could potentially contribute to the systemic levels of BDNF. In a human in vivo model, we demonstrated that there is a cerebral output of BDNF at basal condition, and that cerebral output of BDNF is inhibited during hyperglycaemic clamp conditions in humans. The latter finding may explain the concomitant finding of low circulating levels of BDNF in individuals with type 2 diabetes, and the association between low plasma BDNF and the severity of insulin resistance (Krabbe et al. 2007). The latter human data are in accordance with reports from animal models suggesting that BDNF also plays a role in insulin resistance and in energy balance. Thus, BDNF reduces food intake and lowers blood glucose in genetically modified (db/db) obese mice; however, the hypoglycaemic effect of BDNF cannot be ascribed solely to the hypophagic effect of BDNF, because BDNF administration has beneficial effects on glucose homeostasis and improves insulin resistance in db/db mice even when food-intake is controlled (Ono et al. 1997; Tonra et al. 1999; Nakagawa et al. 2000), suggesting that BDNF may also have peripheral metabolic effects.
We studied whether skeletal muscle would produce BDNF in response to exercise (Matthews et al. 2009). It was found that BDNF mRNA and protein expression were increased in human skeletal muscle after exercise; however, muscle-derived BDNF appeared not to be released into the circulation. BDNF mRNA and protein expression were increased in muscle cells that were electrically stimulated. Interestingly, BDNF increased phosphorylation of AMPK and Acetyl CoA Carboxylase (ACC) and enhanced fat oxidation both in vitro and ex vivo. Thus, we have been able to identify BDNF as a novel contraction-induced muscle cell-derived protein that may increase fat oxidation in skeletal muscle in an AMPK-dependent fashion, Fig. 2. The possibility exists that BDNF may be classified as a myokine, which works in an autocrine or paracrine fashion with strong effects on peripheral metabolism, including fat oxidation with a subsequent effect on the size of adipose tissue.
Is EPO a new myokine?
Most recent findings suggest that even erythropoietin (EPO) should be classified as a myokine (Hojman et al. 2009). Thus, we over-expressed EPO in murine skeletal muscles by gene electrotransfer, resulting in 100-fold increase in serum EPO and significant increases in haemoglobin levels. At 12 weeks, EPO expression resulted in a 23% weight reduction in EPO transfected obese mice. Correspondingly, dual energy x-ray absorptiometry (DXA) scanning revealed that this was due to a 28% reduction in adipose tissue mass. The decrease in adipose tissue mass was accompanied by a complete normalisation of fasting insulin levels and glucose tolerance in the high-fat fed mice. EPO expression also induced a 14% increase in muscle volume and a 25% increase in vascularisation of the EPO transfected muscle. In addition, muscular fat oxidation was increased 1.8-fold in both the EPO transfected and the contralateral muscle. Thus, although we were not able to draw conclusions with regard to the clinical relevance of these data, it appears that at least supra-physiological levels of EPO have substantial metabolic effects (Hojman et al. 2009). In the latter study, EPO mimics a myokine in that it is produced and secreted from skeletal muscles and exhibits paracrine and endocrine effects on other muscles. However, the DNA electrotransfer method represents an artificial model to over-express and secrete EPO from muscles and our findings do not prove that EPO is indeed secreted natively from muscles. However, most recently Rundqvist et al. reported that EPO is released from the exercising leg to the circulation, possibly corresponding to an increased bioavailability of EPO. Although the release of EPO could only be verified in the initial period of an exercise bout, it suggests that EPO may be a true myokine (Rundqvist et al. 2009). Interestingly, the latter study reports that the EPO receptor (EPOR) mRNA and EPOR-associated Janus kinase (JAK) 2 phosphorylation were increased, suggesting that signalling through EPOR is involved in exercise-induced skeletal muscle adaptation.
Summary on Myokines
In the context of specific myokines, I suggest that physical inactivity is an independent cause of fat accumulation in ‘the wrong places’. Accordingly, contracting skeletal muscles release myokines, which work in a hormone-like fashion, exerting specific endocrine effects on visceral fat and other ectopic fat deposits. Other myokines will work locally within the muscle via paracrine mechanisms, exerting their effects on signalling pathways involved in fat oxidation.
The anti-inflammatory effects of an acute bout of exercise
Regular exercise appears to induce anti-inflammatory effects, suggesting that physical activity per se may suppress systemic low-grade inflammation. And several studies show that markers of inflammation are reduced following longer-term behavioural changes involving both reduced energy intake and increased physical activity (reviewed in Petersen & Pedersen, 2005). However, the mediators of this effect are unresolved. A number of mechanisms have been identified. Exercise increases the release of adrenaline, cortisol, growth hormone, prolactin and other factors that have immunomodulatory effects (Nieman, 2003; Handschin & Spiegelman, 2008).
To study whether acute exercise induces a true anti-inflammatory response, a model of ‘low grade inflammation’ was established in which a low dose of E. coli endotoxin was administered to healthy volunteers, who had been randomised to either rest or exercise prior to endotoxin administration. In resting subjects, endotoxin induced a 2- to 3-fold increase in circulating levels of TNF-α. In contrast, when the subjects performed 3 h of ergometer cycling and received the endotoxin bolus at 2.5 h, the TNF-α response was totally blunted (Starkie et al. 2003). This study provides some evidence that acute exercise may inhibit TNF production.
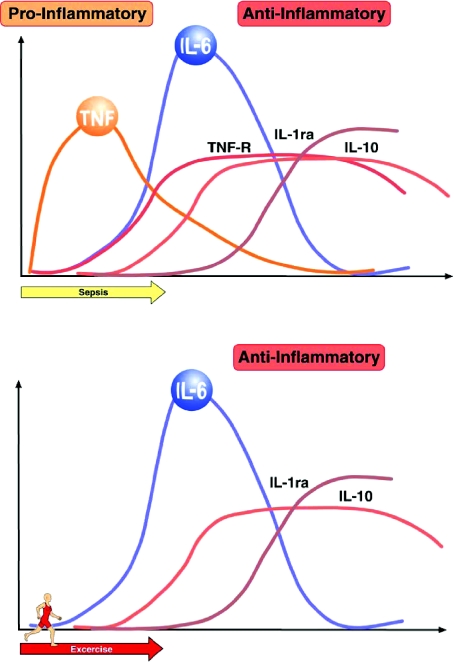
The cytokine response to exercise differs from that elicited by severe infections (see Fig. 5). The fact that the classical pro-inflammatory cytokines, TNF-α and IL-1β, in general do not increase with exercise indicates that the cytokine cascade induced by exercise markedly differs from the cytokine cascade induced by infections (reviewed in Pedersen & Febbraio, 2008).
Figure 5. Comparison of sepsis-induced versus exercise-induced increases in circulating cytokines.
During sepsis, there is a marked and rapid increase in circulating TNF-α, which is followed by an increase in IL-6. In contrast during exercise the marked increase in IL-6 is not preceded by elevated TNF-α. From Pedersen & Febbraio (2008) with permission from the American Physiological Society.
Typically, IL-6 is the first cytokine released into the circulation during exercise. The level of circulating IL-6 increases in an exponential fashion (up to 100-fold) in response to exercise and declines in the post-exercise period. The circulating levels of well-known anti-inflammatory cytokines such as IL-1ra and IL-10 also increase after exercise. Taken together, exercise provokes an increase primarily in IL-6, followed by an increase in IL-1ra and IL-10. The appearance of IL-6 in the circulation is by far the most marked and its appearance precedes that of the other cytokines, and a number of studies have demonstrated that contracting skeletal muscle fibres per se produce and release IL-6. Moreover, it appears that muscle-derived IL-6 may account for most of the systemic IL-6 response to exercise.
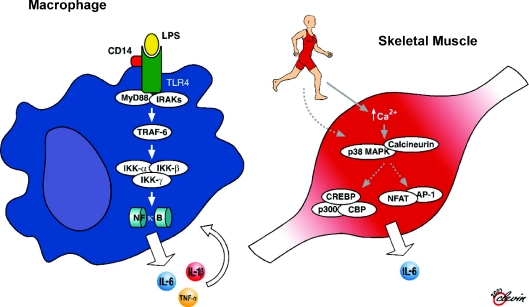
Recent work has shown that both up-stream and down-stream signalling pathways for IL-6 differ markedly between myocytes and macrophages (see also Fig. 6). It appears that unlike IL-6 signalling in macrophages, which is dependent upon activation of the NFκB signalling pathway, intramuscular IL-6 expression is regulated by a network of signalling cascades that among other pathways is likely to involve cross talk between the Ca2+ Nuclear Factor of activated T cells (NFAT) and glycogen/p38 MAPK pathways. Thus, when IL-6 is signalling in monocytes or macrophages, it creates a proinflammatory response, whereas IL-6 activation and signalling in muscle is totally independent of a preceding TNF response or NFκB activation (Pedersen & Febbraio, 2008).
Figure 6. The proposed cytokine signalling pathways for macrophages and contracting skeletal muscle.
While it is well known that transcription of IL-6 and other pro-inflammatory cytokines such as TNF-α and IL-β is principally regulated by the TLR receptor signalling cascade that results in nuclear translocation and activation of NFκB, evidence in contracting skeletal muscle suggests that contraction leads to increased cytosolic Ca2+ and activation of p38 MAPK and/or calcineurin, which leads to activation of transcription factors depending upon these upstream events. From Pedersen & Febbraio (2008) with permission from the American Physiological Society.
The possibility exists that with regular exercise, the anti-inflammatory effects of an acute bout of exercise will protect against chronic systemic low-grade inflammation, but such a direct link between the acute effects of exercise and the long-term benefits has yet to be established.
Conclusion and perspective
Much focus has been on the detrimental effects of abdominal adiposity. However, following adjustment for sex, age and hormonal levels, it remains a mystery why some people accumulate fat in ‘the wrong places’ (e.g. as visceral fat) and why others do not. In this review, physical inactivity is given the central role as an independent and strong risk factor for accumulation of visceral fat and other ectopic fat deposits. The finding that muscles produce and release myokines provides a conceptual basis for understanding some of the molecular mechanisms underlying organ cross talk, including muscle–fat cross talk. Accumulating data suggest that contracting skeletal muscles release myokines, which work in a hormone-like fashion, exerting specific endocrine effects on visceral fat and other ectopic fat deposits. Other myokines work locally within the muscle via paracrine mechanisms, exerting their effects on signalling pathways involved in fat oxidation. However, physical inactivity and muscle disuse lead to metabolic deterioration.
Acknowledgments
I wish to gratefully acknowledge my collaborators, post-doctoral fellows, students and technicians who have contributed much of the work reported in this review. The Centre of Inflammation and Metabolism is supported by a grant from the Danish National Research Foundation (DG 02-512-555). In addition, support was obtained from the Danish Medical Research Council and the Commission of the European Communities (contract no. LSHM-CT-2004-005272 EXGENESIS). The Capital Region of Copenhagen and the Copenhagen University support the Copenhagen Muscle Research Centre.
References
- Barabasi AL. Network medicine – from obesity to the ‘diseasome’. N Engl J Med. 2007;357:404–407. doi: 10.1056/NEJMe078114. [DOI] [PubMed] [Google Scholar]
- Bays HE. ‘Sick fat,’ metabolic disease, and atherosclerosis. Am J Med. 2009;122:S26–S37. doi: 10.1016/j.amjmed.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Berster JM, Goke B. Type 2 diabetes mellitus as risk factor for colorectal cancer. Arch Physiol Biochem. 2008;114:84–98. doi: 10.1080/13813450802008455. [DOI] [PubMed] [Google Scholar]
- Broholm C, Mortensen OH, Nielsen S, Akerstrom T, Zankari A, Dahl B, Pedersen BK. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J Physiol. 2008;586:2195–2201. doi: 10.1113/jphysiol.2007.149781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Brain Res Mol Brain Res. 1997;49:71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- Diamant M, Tushuizen ME. The metabolic syndrome and endothelial dysfunction: common highway to type 2 diabetes and CVD. Curr Diab Rep. 2006;6:279–286. doi: 10.1007/s11892-006-0061-4. [DOI] [PubMed] [Google Scholar]
- Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- Fischer CP, Berntsen A, Perstrup LB, Eskildsen P, Pedersen BK. Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scand J Med Sci Sports. 2007;17:580–587. doi: 10.1111/j.1600-0838.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–s842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120:S10–S16. doi: 10.1016/j.amjmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. The role of exercise and PGC1α in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojman P, Brolin C, Gissel H, Brandt C, Zerahn B, Pedersen BK, Gehl J. Erythropoietin over-expression protects against diet-induced obesity in mice through increased fat oxidation in muscles. PLoS One. 2009;4:e5894. doi: 10.1371/journal.pone.0005894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008;582:3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Komulainen P, Pedersen M, Hanninen T, Bruunsgaard H, Lakka TA, Kivipelto M, Hassinen M, Rauramaa TH, Pedersen BK, Rauramaa R. BDNF is a novel marker of cognitive function in ageing women: The DR's EXTRA Study. Neurobiol Learn Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Mortensen EL, Avlund K, Pedersen AN, Pedersen BK, Jorgensen T, Bruunsgaard H. Brain-derived neurotrophic factor predicts mortality risk in older women. J Am Geriatr Soc. 2009;57:1447–1452. doi: 10.1111/j.1532-5415.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, Bartels M, Buchkremer G, Schott K. Stage-dependent BDNF serum concentrations in Alzheimer's disease. J Neural Transm. 2005;113:1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni L, Nikolova V, Vyagova D, Chaldakov GN, Aloe L. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int J Cardiol. 2005;102:169–171. doi: 10.1016/j.ijcard.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Matter CM, Handschin C. RANTES (regulated on activation, normal T cell expressed and secreted), inflammation, obesity, and the metabolic syndrome. Circulation. 2007;115:946–948. doi: 10.1161/CIRCULATIONAHA.106.685230. [DOI] [PubMed] [Google Scholar]
- Matthews VB, Åström M-B, Chan MHS, Bruce CR, Prelovsek O, Åkerström T, Yfanti C, Broholm C, Mortensen OH, Penkowa M, Hojman P, Zankari A, Watt MJ, Pedersen BK, Febbraio MA. Brain derived neutrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMPK. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, van Leeuwen FE. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436–444. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- Nielsen AR, Hojman P, Erikstrup C, Fischer CP, Plomgaard P, Mounier R, Mortensen OH, Broholm C, Taudorf S, Krogh-Madsen R, Lindegaard B, Petersen AM, Gehl J, Pedersen BK. Association between IL-15 and obesity: IL-15 as a potential regulator of fat mass. J Clin Endocrinol Metab. 2008;93:4486–4493. doi: 10.1210/jc.2007-2561. [DOI] [PubMed] [Google Scholar]
- Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK. Expression of interleukin-15 in human skeletal muscle – effect of exercise and muscle fibre type composition. J Physiol. 2007;584:305–312. doi: 10.1113/jphysiol.2007.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen AR, Pedersen BK. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl Physiol Nutr Metab. 2007;32:833–839. doi: 10.1139/H07-054. [DOI] [PubMed] [Google Scholar]
- Nieman DC. Current perspective on exercise immunology. Curr Sports Med Rep. 2003;2:239–242. doi: 10.1249/00149619-200310000-00001. [DOI] [PubMed] [Google Scholar]
- Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA. 2008;299:1261–1263. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- Ono M, Ichihara J, Nonomura T, Itakura Y, Taiji M, Nakayama C, Noguchi H. Brain-derived neurotrophic factor reduces blood glucose level in obese diabetic mice but not in normal mice. Biochem Biophys Res Commun. 1997;238:633–637. doi: 10.1006/bbrc.1997.7220. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Lee IM, Leung R. Physical activity and personal characteristics associated with depression and suicide in American college men. Acta Psychiatr Scand Suppl. 1994;377:16–22. doi: 10.1111/j.1600-0447.1994.tb05796.x. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Body mass index-independent effect of fitness and physical activity for all-cause mortality. Scand J Med Sci Sports. 2007;17:196–204. doi: 10.1111/j.1600-0838.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Petersen EW, Carey AL, Sacchetti M, Steinberg GR, Macaulay SL, Febbraio MA, Pedersen BK. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro: evidence that IL-6 acts independently of lipolytic hormones. Am J Physiol Endocrinol Metab. 2005;288:E155–E162. doi: 10.1152/ajpendo.00257.2004. [DOI] [PubMed] [Google Scholar]
- Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, Van Der Schouw YT, Spencer E, Moons KGM, Tjonneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PHM, May AM, Bueno-De-Mesquita HB, van Duijnhoven FJB, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quiros JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. New Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- Plomgaard P, Fischer CP, Ibfelt T, Pedersen BK, van Hall G. TNF-α modulates human in vivo lipolysis. J Clin Endocrinol Metab. 2007;93:543–549. doi: 10.1210/jc.2007-1761. [DOI] [PubMed] [Google Scholar]
- Plomgaard P, Keller P, Keller C, Pedersen BK. TNF-α, but not IL-6, stimulates plasminogen activator inhibitor-1 expression in human subcutaneous adipose tissue. J Appl Physiol. 2005;98:2019–2023. doi: 10.1152/japplphysiol.01220.2004. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argiles JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab. 2009;296:E191–E202. doi: 10.1152/ajpendo.90506.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson LC, Pollack LA. Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005;2:48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Rundqvist H, Rullman E, Sundberg CJ, Fischer H, Eisleitner K, Stahlberg M, Sundblad P, Jansson E, Gustafsson T. Activation of the erythropoietin receptor in human skeletal muscle. Eur J Endocrinol. 2009;161:427–434. doi: 10.1530/EJE-09-0342. [DOI] [PubMed] [Google Scholar]
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. FASEB J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- Tonra JR, Ono M, Liu X, Garcia K, Jackson C, Yancopoulos GD, Wiegand SJ, Wong V. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes. 1999;48:588–594. doi: 10.2337/diabetes.48.3.588. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signalling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, Pedersen BK. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88:3005–3010. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007;86:s823–s835. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- Yudkin JS. Inflammation, obesity, and the metabolic syndrome. Horm Metab Res. 2007;39:707–709. doi: 10.1055/s-2007-985898. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Eringa E, Stehouwer CD. ‘Vasocrine’ signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006;29:518–527. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]