Abstract
Habitual exercise training, including a high-intensity interval walking programme, improves cardiorespiratory fitness and alleviates lifestyle-related diseases, such as obesity, hypertension and dyslipidaemia. However, the extent of improvement has been shown to differ substantially among individuals for various exercise regimens. A body of literature has demonstrated that gene polymorphisms could account for the inter-individual variability in the improvement of risk factors for lifestyle-related diseases following exercise training. However, the fractions of the variability explained by the polymorphisms are small (∼5%). Also, it is likely that the effects of gene polymorphisms differ with exercise regimens and subject characteristics. These observations suggest the necessity for further studies to exhaustively identify such gene polymorphisms. More importantly, the physiological and molecular genetic mechanisms by which gene polymorphisms interact with exercise to influence the improvements of risk factors for lifestyle-related diseases differentially remain to be clarified. A better understanding of these issues should lead to more effective integration of exercise to optimize the treatment and management of individuals with lifestyle-related diseases.
Lifestyle-related diseases, which include obesity, diabetes, dyslipidaemia, hypertension and cardiovascular disease, represent the greatest global health threat. Epidemiological and clinical evidence indicates that poor cardiorespiratory fitness is a major risk factor for lifestyle-related diseases (Sawada et al. 1993, 2003; Wei et al. 1999; Lakka et al. 2001). Thus, the excess energy intake and adoption of a sedentary lifestyle by modern people can result in a decline in cardiorespiratory fitness, leading to the epidemic emergence of lifestyle-related diseases. In addition, cardiorespiratory fitness generally deteriorates with advancing age. In this regard, middle-aged and older individuals constitute another high-risk group for lifestyle-related diseases. Conversely, increasing cardiorespiratory fitness can be an effective measure in the prevention and alleviation of lifestyle-related diseases. One commonly recommended approach for increasing cardiorespiratory fitness and for decreasing the risks of, or alleviating the symptoms of lifestyle-related diseases is habitual exercise training, a low-cost, non-pharmacological intervention that is available to the vast majority of people (Kraus et al. 2002; Pescatello et al. 2004; O’Gorman & Krook, 2008). However, it has also become evident that the extent of improvement with exercise training differs substantially among individuals, irrespective of whether it is a standardized or controlled exercise-training programme (Bouchard & Rankinen, 2001). To appreciate the effects of exercise on prevention and alleviation of lifestyle-related diseases fully, it is indispensable to clarify the basis of the inter-individual variability.
Predisposition to lifestyle-related diseases has a genetic basis. Gene polymorphisms influence inter-individual variability in the predisposition to obesity (Rankinen et al. 2006) and hypertension (Levy et al. 2009; Newton-Cheh et al. 2009). Likewise, the inter-individual variability in the effects of exercise on alleviation of lifestyle-related diseases may be influenced by gene polymorphisms. Indeed, previous studies have consistently demonstrated involvement of genetic polymorphisms in the improvement of disease-related phenotypes for various exercise regimens. A genetic association study for the effects found a small collection of genes that influence improvement of diseases following habitual exercise training (Table 1). However, more studies are required to explore this hypothesis and establish a definitive gene–exercise relationship. Here, we briefly review the current status of the study of genetic associations of the effects of exercise on lifestyle-related diseases, including data obtained from our own study, and discuss a future perspective.
Table 1.
Gene polymorphisms reported to be associated with inter-individual variability in responsiveness to exercise training
| Gene name | Gene symbol | dbSNP ID | Location of SNP | Effect of SNP | Phenotype | Selected reference |
|---|---|---|---|---|---|---|
| Fat mass and obesity associated gene | FTO | rs9939609* | intron 1 | — | BMI | Andreasen et al. (2008) |
| Insulin induced gene 2 | INSIG2 | rs7566605 | 5′ upstream (−10) | — | Fat volume | Orkunoglu-Suer et al. (2008) |
| Uncoupling protein 1 | UCP1 | rs1800592* | 5′ upstream (−3826) | — | Body weight | Kogure et al. (1998) |
| Uncoupling protein 3 | UCP3 | rs1800849* | 5′ upstream (−36) | — | BMI | Otabe et al. (2000) |
| Peroxisome proliferator-activated receptor α | PPARA | rs1800206 | exon 5 | L162V | Fat volume | Uthurralt et al. (2007) |
| Peroxisome proliferator-activated receptor δ | PPARD | rs2267668 | intron 2 | — | 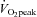 |
Stefan et al. (2007) |
| Peroxisome proliferator-activated receptor γ | PPARG | rs1805192 | exon 2 | A12P | Body weight | Lindi et al. (2002); Ostergard et al. (2005) |
| Cytochrome P450, family 19, subfamily A, polypeptide 1 | CYP19A1 | (TTTA)n repeat polymorphism | intron 4 | — | BMI, fat mass, % body fat | Tworoger et al. (2004) |
| Catechol-Omethyltransferase | COMT | rs4680 | exon 4 | V158M | % body fat | Tworoger et al. (2004) |
| Lipoprotein lipase | LPL | rs328* | exon 9 | S474X | BMI | Garenc et al. (2001) |
| Adrenergic receptor β2 | ADRB2 | rs1042713* | exon 1 | R16G | Body weight, BMI, % body fat | Sakane et al. (1999); Garenc et al. (2003) |
| rs1042714* | exon 1 | Q27E | % body fat | Meirhaeghe et al. (1999); Corbalán et al. (2002); Phares et al. (2004) | ||
| Adrenergic receptor β3 | ADRB3 | rs4994* | exon 1 | R64W | Body weight, % body fat | Yoshida et al. (1995); Phares et al. (2004) |
| Guanine nucleotide-binding protein β3 | GNB3 | rs5443 | exon 10 | aberrant splicing | Obesity, fat mass, % body fat | Rankinen et al. (2002); Grove et al. (2007) |
| Ectonucleotide pyrophosphatase/ phosphodiesterase | ENPP1 | rs1805101 | exon 4 | K171Q | BMI | Park et al. (2008) |
| Angiotensin I-converting enzyme | ACE | I/D polymorphism* | intron 16 | — | Diastolic blood pressure, fat mass | Hagberg et al. (1999); Montgomery et al. (1999) |
Polymorphisms examined in our study.
dbSNP ID: reference single nucleotide polymorphism identifier in SNPs database of NCBI (http://www.ncbi.nlm.nih.gov/SNP)
SNP: single nucleotide polymorphism
BMI: body mass index
VO2peak: peak aerobic capacity
Gene polymorphisms underlie the inter-individual variability in alleviation of lifestyle-related diseases following exercise training
There is a body of literature demonstrating associations between gene polymorphisms and exercise-training responsiveness of risk factors for lifestyle-related diseases (Table 1). Candidate genes come from a variety of functional categories. Several gene polymorphisms were reported to be associated with responsiveness of several risk factors. Angiotensin I-converting enzyme (ACE) is a dipeptidyl carboxypeptidase that plays an important role in blood pressure regulation and electrolyte balance. A polymorphism of the human ACE gene was identified in which the deletion rather than the insertion of a 287 bp fragment in intron 16 of the gene is associated with high tissue ACE activity (Danser et al. 1995). This insertion/deletion polymorphism influences not only the cardiovascular response (Hagberg et al. 1999), but also changes in body composition following exercise training (Montgomery et al. 1999). However, some gene–exercise interaction effects failed to be replicated in other studies. These facts imply a complex interrelationship among gene polymorphisms, exercise and lifestyle-related diseases. For more information, the interested reader can also refer to an excellent recent review on this topic (Bray et al. 2009).
Effects of high-intensity interval walking training are also dependent on gene polymorphisms
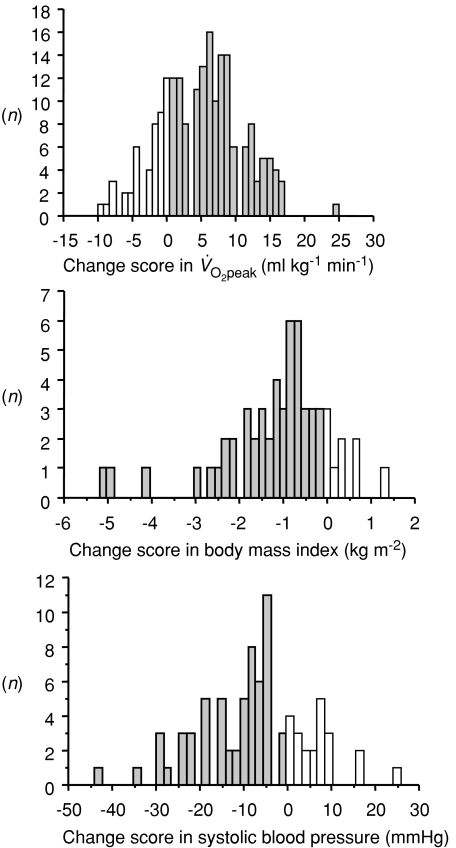
‘High-intensity interval walking’ is an aerobic exercise that improves cardiorespiratory fitness and alleviates lifestyle-related diseases in middle-aged and older individuals (Nemoto et al. 2007). We investigated the effects of a high-intensity interval walking training intervention in middle-aged and older Japanese females. Average initial values of this population (n = 217; 41–86 years of age; mean age, 63.3 years) for peak aerobic capacity 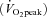 , body mass index (BMI) and systolic blood pressure (SBP) were 20.5 ml kg−1 min−1, 23.7 kg m−2 and 133.3 mmHg, respectively. After 10 months of high-intensity interval walking training, the parameters improved significantly to 25.6 ml kg−1 min−1
, body mass index (BMI) and systolic blood pressure (SBP) were 20.5 ml kg−1 min−1, 23.7 kg m−2 and 133.3 mmHg, respectively. After 10 months of high-intensity interval walking training, the parameters improved significantly to 25.6 ml kg−1 min−1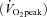 , 23.0 kg m−2 (BMI) and 130.3 mmHg (SBP). Among the 217 subjects, 57 had an initial BMI ≥ 25 kg m−2, which is the threshold value for the clinical diagnosis of obesity in Japan. Eighty-two had an initial SBP ≥ 140 mmHg, which is the threshold value for the clinical diagnosis of hypertension in Japan. Importantly, improvement was prominent for these subjects. In the obese subjects, BMI decreased significantly from 27.6 to 26.4 kg m−2. In the hypertensive subjects, SBP decreased significantly from 148.3 to 140.9 mmHg. However, the change scores in these parameters differed substantially among individuals (Fig. 1).
, 23.0 kg m−2 (BMI) and 130.3 mmHg (SBP). Among the 217 subjects, 57 had an initial BMI ≥ 25 kg m−2, which is the threshold value for the clinical diagnosis of obesity in Japan. Eighty-two had an initial SBP ≥ 140 mmHg, which is the threshold value for the clinical diagnosis of hypertension in Japan. Importantly, improvement was prominent for these subjects. In the obese subjects, BMI decreased significantly from 27.6 to 26.4 kg m−2. In the hypertensive subjects, SBP decreased significantly from 148.3 to 140.9 mmHg. However, the change scores in these parameters differed substantially among individuals (Fig. 1).
Figure 1. Distribution of change score in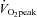 (n= 217), body mass index in obese subjects (BMI ≥ 25 kg m−2; n= 57) and systolic blood pressure in hypertensive subjects (SBP ≥ 140 mmHg; n= 82) after 10 months of high-intensity interval walking exercise training.
(n= 217), body mass index in obese subjects (BMI ≥ 25 kg m−2; n= 57) and systolic blood pressure in hypertensive subjects (SBP ≥ 140 mmHg; n= 82) after 10 months of high-intensity interval walking exercise training.
Grey bars represent subjects with improvement, whereas open bars represent subjects with no change or aggravation.
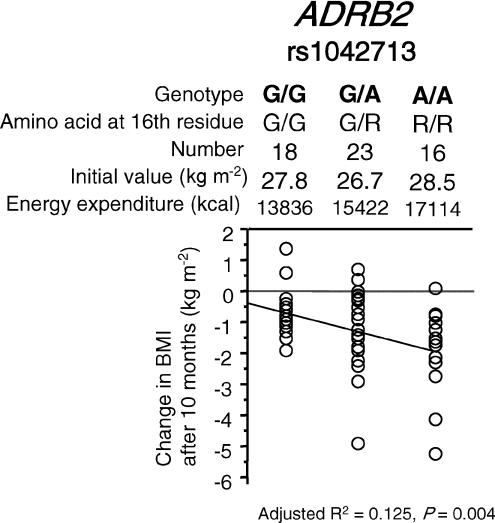
Next, a study was performed to determine the association between the change score and gene polymorphisms. Most of these polymorphisms were reported to be associated with inter-individual variability in the effects of exercise on the improvement of obesity or hypertension (Table 1). Our results, however, failed to replicate the gene–exercise interaction effects or pre- and post-training values for most polymorphisms. This discrepancy may be partly explained by differences in the exercise regimen, such as type (e.g. aerobic or endurance), strength, frequency and duration. The gene–exercise interaction would also be influenced by subject characteristics, such as ethnicity, age, sex, energy intake and baseline physical activity. A single nucleotide polymorphism (SNP), rs1042713, in the adrenergic receptor β2 (ADRB2) gene (also known as a Gly16Arg polymorphism) was found to be associated with the change score in BMI in obese subjects (Fig. 2). The Arg allele was associated with a greater reduction of BMI following exercise training. This polymorphism explained 12.5% of the inter-individual variability in change scores following exercise training. This result was consistent with one of the previous reports (Garenc et al. 2003), but inconsistent with the other report (Sakane et al. 1999), in which the Gly allele was associated with a greater reduction in body weight, implying intricate gene–exercise interaction.
Figure 2. Association of a SNP rs1042713 in the ADRB2 genes and change in BMI in obese subjects (BMI ≥ 25 kg m−2; n = 57) after 10 months of high-intensity interval walking training.
Stepwise multiple regression analysis was employed. This figure shows the result drawn by a simple linear regression analysis. Average initial values for BMI and energy expenditure from high-intensity walking were not statistically different between genotypes. The change score in BMI was not correlated with age or initial BMI value.
Towards comprehensive identification of polymorphisms for effects of exercise training
In order to attain full comprehension of intricate gene–exercise interaction in alleviation of lifestyle-related diseases, two major subjects need to be achieved in the future. Firstly, it is necessary to exhaustively identify candidate gene polymorphisms associated with alleviation of lifestyle-related diseases following exercise training. So far, most of the studies have employed a candidate gene approach, in which only one or a few specific genes of interest were examined. The choice of candidate polymorphisms has primarily been based on the hypothesis that the polymorphisms, which determine the predisposition to lifestyle-related diseases, would also be a determinant of recovery feasibility from the diseases following exercise. This hypothesis was true for several genes, such as ACE (Rush & Aultman, 2008) and fat mass and obesity associated gene (FTO) (Frayling et al. 2007). However, it might not always be the case. Furthermore, the sample sizes in previous studies were generally too small (∼1000) to provide adequate statistical power. Currently, a genome-wide association study, which allows simultaneous examination of over 50 000 polymorphisms without accompanying hypothesis in thousands of subjects, is becoming the main strategy to analyse the genetic basis of predisposition to lifestyle-related diseases (The Wellcome Trust Genome Case Control Consortium, 2007). This approach confirmed the results obtained by the candidate gene approach with more strict statistical conditions. More importantly, it resulted in successful discoveries of hundreds of new SNPs for lifestyle-related diseases (Thorleifsson et al. 2008; Willer et al. 2009; Levy et al. 2009; Newton-Cheh et al. 2009), implying the greatest promise also for comprehensive identification of polymorphisms for even more intricate gene–exercise interaction in alleviation of the diseases.
Towards elucidation of the mechanisms for the genotype-dependent effects of exercise training
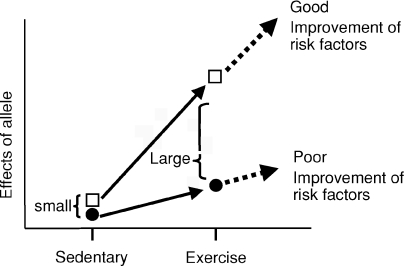
Secondly, the physiological and molecular genetic mechanisms by which the variations in the genes exert genotype-dependent differential effects on alleviation of lifestyle-related diseases following exercise training remain to be clarified. Habitual exercise training induces multiple adaptations within skeletal muscle. Also, exercise training elicits improvements in endothelium-dependent dilatation or reduces sympathetic activity. Thus, exercise training is considered to elicit metabolic as well as physiological reprogramming systemically, which contributes to alleviation of lifestyle-related diseases. Alterations in actions of plenty of genes through epigenetic modification, changes in expression level and stability of transcripts, post-translational modification of gene products and other mechanisms undoubtedly underlie this reprogramming process. Common gene polymorphisms may have only a negligible or subtle influence on gene functions in sedentary conditions. Exercise training may amplify the differential effects between polymorphic alleles, which then manifest as differences in responsiveness to exercise between individuals (Fig. 3). Indeed, a few studies have demonstrated that nucleotide polymorphisms caused differences in gene expression level after exercise training (Prior et al. 2006; Oberbach et al. 2008). This, in addition to other possibilities, should be studied in the future. This would be achieved by integration of data obtained from two different approaches. Firstly, responses of each candidate polymorphic gene to exercise training should be carefully examined at various levels from DNA to a mature protein product. A second useful approach is transcriptome, proteome and physiome analysis, which would give a comprehensive picture of physiological dynamics occurring after exercise training.
Figure 3.
Proposed model of allele–exercise interaction for alleviation of lifestyle-related diseases
Conclusion
It is evident that gene polymorphisms play a crucial role in determination of the improvement of risk factors for lifestyle-related diseases following exercise training. Full comprehension of gene polymorphism–exercise interaction in alleviation of the diseases should help in the development of individualized training programmes to optimize the treatment and management of subjects with lifestyle-related diseases. It should also provide clues as to which pathways to target with agents that mimic or potentiate the effects of exercise for the treatment of lifestyle-related diseases (Narkar et al. 2008; Hawley & Holloszy, 2009).
Acknowledgments
This work was supported in part by the Shinshu University Partnership Project between Shinshu University, Jukunen Taiiku Daigaku Research Center (JTRC), the Ministry of Education, Culture, Sports, Science and Technology of Japan, Matsumoto City, Sanyo Electric Co., Japan. This work was also supported also by a Grant-in-Aid for Scientific Research from the Ministry of Health, Labor and Welfare of Japan.
Glossary
Abbreviations
- ACE
angiotensin I-converting enzyme
- ADRB2
adrenergic receptor β2
- BMI
body mass index
- FTO
, fat mass and obesity associated gene
- SBP
systolic blood pressure
-
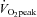 , peak aerobic capacity.
, peak aerobic capacity.
Author contributions
All authors contributed to the conception and design of the study, interpretation of data and drafting and revising the manuscript. M.M. performed experiments and analysed the data. K.H. performed experiments. A.S. analysed the data. Y.T. performed experiments. T.M. performed experiments. H.N. performed experiments. All authors approved the published version of the manuscript. All the experiments were done at the Institute on Aging and Adaptation, Shinshu University Graduate School of Medicine.
Author addresses
Shinshu University Genetic Research Consortium: Hiroshi Nose1, Shizue Masuki1, Keiichi Higuchi2, Masayuki Mori2, Jinko Sawashita2, Shun’ichiro Taniguchi3, Michiko Takeoka3, Koki Nakajima3, Yoshimitsu Fukushima4, Akihiro Sakurai4, Shin-ichi Usami5, Shigenari Hashimoto5,6 and Kenji Sano7: 1Department of Sports Medical Sciences, Institute on Aging and Adaptation, 2Department of Aging Biology, Institute on Aging and Adaptation, 3Department of Molecular Oncology, Institute on Aging and Adaptation, 4Department of Medical Genetics, 5Department of Otorhinolaryngology, 6Preventive Medical Center and 7Department of Laboratory Medicine.
References
- Andreasen CH, Stender-Petersen KL, Mogensen MS, Torekov SS, Wegner L, Andersen G, Nielsen AL, Albrechtsen A, Borch-Johnsen K, Rasmussen SS, Clausen JO, Sandbaek A, Lauritzen T, Hansen L, Jørgensen T, Pedersen O, Hansen T. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc. 2001;33:S446–S451. doi: 10.1097/00005768-200106001-00013. [DOI] [PubMed] [Google Scholar]
- Bray MS, Hagberg JM, Perusse L, Rankinen T, Roth SM, Wolfarth B, Bouchard C. The human gene map for performance and health-related fitness phenotypes: the 2006–2007 update. Med Sci Sports Exrc. 2009;41:35–73. doi: 10.1249/mss.0b013e3181844179. [DOI] [PubMed] [Google Scholar]
- Corbalán MS, Marti A, Forga L, Martínez-González MA, Martínez JA. The 27Glu polymorphism of the β2-adrenergic receptor gene interacts with physical activity influencing obesity risk among female subjects. Clin Genet. 2002;61:305–307. doi: 10.1034/j.1399-0004.2002.610411.x. [DOI] [PubMed] [Google Scholar]
- Danser AH, Schalekamp MA, Bax WA, Van Den Brink AM, Saxena PR, Riegger GA, Schunkert H. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation. 1995;92:1387–1388. doi: 10.1161/01.cir.92.6.1387. [DOI] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garenc C, Pérusse L, Bergeron J, Gagnon J, Chagnon YC, Borecki IB, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Evidence of LPL gene-exercise interaction for body fat and LPL activity: the HERITAGE family study. J Appl Physiol. 2001;91:1334–1340. doi: 10.1152/jappl.2001.91.3.1334. [DOI] [PubMed] [Google Scholar]
- Garenc C, Pérusse L, Chagnon YC, Rankinen T, Gagnon J, Borecki IB, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Effects of β2-adrenergic receptor gene variants on adiposity: the HERITAGE Family Study. Obes Res. 2003;11:612–618. doi: 10.1038/oby.2003.88. [DOI] [PubMed] [Google Scholar]
- Grove ML, Morrison A, Folsom AR, Boerwinkle E, Hoelscher DM, Bray MS. Gene–environment interaction and the GNB3 gene in the Atherosclerosis Risk in Communities study. Int J Obes. 2007;31:919–926. doi: 10.1038/sj.ijo.0803545. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Ferrell RE, Dengel DR, Wilund KR. Exercise training-induced blood pressure and plasma lipid improvements in hypertensives may be genotype dependent. Hypertension. 1999;34:18–23. doi: 10.1161/01.hyp.34.1.18. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Holloszy JO. Exercise: it's the real thing! Nutr Rev. 2009;67:172–178. doi: 10.1111/j.1753-4887.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- Kogure A, Yoshida T, Sakane N, Umekawa T, Takakura Y, Kondo M. Synergic effect of polymorphisms in uncoupling protein 1 and β3-adrenergic receptor genes on weight loss in obese Japanese. Diabetologia. 1998;41:1399. doi: 10.1007/s001250051084. [DOI] [PubMed] [Google Scholar]
- Kraus WE, Housmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Eng J Med. 2002;347:1438–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- Lakka TA, Laukkanen JA, Rauramaa R, Salonen R, Lakka HM, Kaplan GA, Salonen JT. Cardiorespiratory fitness and the progression of carotid atherosclerosis in middle-aged men. Ann Intern Med. 2001;134:12–20. doi: 10.7326/0003-4819-134-1-200101020-00008. [DOI] [PubMed] [Google Scholar]
- Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindi VI, Uusitupa MI, Lindström J, Louheranta A, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Tuomilehto J, Finnish Diabetes Prevention Study Association of the Pro12Ala polymorphism in the PPAR-γ2 gene with 3-year incidence of type 2 diabetes and body weight change in the Finnish Diabetes Prevention Study. Diabetes. 2002;51:2581–2586. doi: 10.2337/diabetes.51.8.2581. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Helbecque N, Cottel D, Amouyel P. Beta2-adrenoceptor gene polymorphism, body weight, and physical activity. Lancet. 1999;353:896. doi: 10.1016/S0140-6736(99)00251-2. [DOI] [PubMed] [Google Scholar]
- Montgomery H, Clarkson P, Barnard M, Bell J, Brynes A, Dollery C, Hajnal J, Hemingway H, Mercer D, Jarman P, Marshall R, Prasad K, Rayson M, Saeed N, Talmud P, Thomas L, Jubb M, World M, Humphries S. Angiotensin-converting-enzyme gene insertion/deletion polymorphism and response to physical training. Lancet. 1999;353:541–545. doi: 10.1016/S0140-6736(98)07131-1. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARδ agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto K, Gen-no H, Masuki S, Okazaki K, Nose H. Effects of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clin Proc. 2007;82:803–811. doi: 10.4065/82.7.803. [DOI] [PubMed] [Google Scholar]
- Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, Van Der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Wellcome Trust Case Control Consortium. Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Döring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O’Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O’Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvänen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dörr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Völker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Völzke H, Uiterwaal CS, Van Der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberbach A, Lehmann S, Kirsch K, Krist J, Sonnabend M, Linke A, Tonjes A, Stumvoll M, Bluher M, Kovacs P. Long-term exercise training decreases interleukin-6 (IL-6) serum levels in subjects with impaired glucose tolerance: effect of the −174G/C variant in IL-6 gene. Eur J Endocrinol. 2008;159:129–136. doi: 10.1530/EJE-08-0220. [DOI] [PubMed] [Google Scholar]
- O’Gorman DJ, Krook A. Exercise and the treatment of diabetes and obesity. Endocrinol Metab Clin North Am. 2008;37:887–903. doi: 10.1016/j.ecl.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Orkunoglu-Suer FE, Gordish-Dressman H, Clarkson PM, Thompson PD, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Harmon B, Seip RL, Hoffman EP, Devaney JM. INSIG2 gene polymorphism is associated with increased subcutaneous fat in women and poor response to resistance training in men. BMC Med Genet. 2008;9:117–124. doi: 10.1186/1471-2350-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergard T, Ek J, Hamid Y, Saltin B, Pedersen OB, Hansen T, Schmitz O. Influence of the PPAR-γ2 Pro12Ala and ACE I/D polymorphisms on insulin sensitivity and training effects in healthy offspring of type 2 diabetic subjects. Horm Metab Res. 2005;37:99–105. doi: 10.1055/s-2005-861174. [DOI] [PubMed] [Google Scholar]
- Otabe S, Clement K, Dina C, Pelloux V, Guy-Grand B, Froguel P, Vasseur F. A genetic variation in the 5′ flanking region of the UCP3 gene is associated with body mass index in humans in interaction with physical activity. Diabetologia. 2000;43:245–249. doi: 10.1007/s001250050037. [DOI] [PubMed] [Google Scholar]
- Park S, Han T, Son T, Kang HS. PC-1 genotype and IRS response to exercise training. Int J Sports Med. 2008;29:294–249. doi: 10.1055/s-2007-965352. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA, American College of Sports Medicine American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- Phares DA, Halverstadt AA, Shuldiner AR, Ferrell RE, Douglass LW, Ryan AS, Goldberg AP, Hagberg JM. Association between body fat response to exercise training and multilocus ADR genotypes. Obes Res. 2004;12:807–815. doi: 10.1038/oby.2004.97. [DOI] [PubMed] [Google Scholar]
- Prior SJ, Hagberg JM, Paton CM, Douglass LW, Brown MD, McLenithan JC, Roth SM. DNA sequence variation in the promoter region of the VEGF gene impacts VEGF gene expression and maximal oxygen consumption. Am J Physiol Heart Circ Physiol. 2006;290:H1848–H1855. doi: 10.1152/ajpheart.01033.2005. [DOI] [PubMed] [Google Scholar]
- Rankinen T, Rice T, Leon AS, Skinner JS, Wilmore JH, Rao DC. G protein beta 3 polymorphism and hemodynamic and body composition phenotypes in the HERITAGE Family Study. Physiol Genomics. 2002;8:151–157. doi: 10.1152/physiolgenomics.00102.2001. [DOI] [PubMed] [Google Scholar]
- Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Pérusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity. 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- Rush JW, Aultman CD. Vascular biology of angiotensin and the impact of physical activity. Appl Physiol Nutr Metab. 2008;33:162–172. doi: 10.1139/H07-147. [DOI] [PubMed] [Google Scholar]
- Sakane N, Yoshida T, Umekawa T, Kogure A, Kondo M. β2-adrenoreceptor gene polymorphism and obesity. Lancet. 1999;353:1976. doi: 10.1016/S0140-6736(05)77192-0. [DOI] [PubMed] [Google Scholar]
- Sawada S, Tanaka H, Funakoshi M, Shindo M, Kono S, Ishiko T. Five year prospective study on blood pressure and maximal oxygen uptake. Clin Exp Pharmacol Physiol. 1993;20:483–487. doi: 10.1111/j.1440-1681.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- Sawada SS, Lee IM, Muto T, Matuszaki K, Blair SN. Cardiorespiratory fitness and the incidence of type 2 diabetes: prospective study of Japanese men. Diabetes Care. 2003;26:2918–2922. doi: 10.2337/diacare.26.10.2918. [DOI] [PubMed] [Google Scholar]
- Stefan N, Thamer C, Staiger H, Machicao F, Machann J, Schick F, Venter C, Niess A, Laakso M, Fritsche A, Häring HU. Genetic variations in PPARD and PPARGCIA determine mitochondrial function and change in aerobic physical fitness and insulin sensitivity during lifestyle intervention. J Clin Endocrinol Metab. 2007;92:1827–1833. doi: 10.1210/jc.2006-1785. [DOI] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, Jonsdottir T, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Jonsson F, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Lauritzen T, Aben KK, Verbeek AL, Roeleveld N, Kampman E, Yanek LR, Becker LC, Tryggvadottir L, Rafnar T, Becker DM, Gulcher J, Kiemeney LA, Pedersen O, Kong A, Thorsteinsdottir U, Stefansson K. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2008;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Chubak J, Aiello EJ, Yasui Y, Ulrich CM, Farin FM, Stapleton PL, Irwin ML, Potter JD, Schwartz RS, McTiernan A. The effect of CYP19 and COMT polymorphisms on exercise-induced fat loss in postmenopausal women. Obes Res. 2004;12:972–981. doi: 10.1038/oby.2004.119. [DOI] [PubMed] [Google Scholar]
- Uthurralt J, Gordish-Dressman H, Bradbury M, Tesi-Rocha C, Devaney J, Harmon B, Reeves EK, Brandoli C, Hansen BC, Seip RL, Thompson PD, Price TB, Angelopoulos TJ, Clarkson PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Gordon PM, Hoffman EP. PPARα L162V underlies variation in serum triglycerides and subcutaneous fat volume in young males. BMC Med Genet. 2007;8:55. doi: 10.1186/1471-2350-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130:89–96. doi: 10.7326/0003-4819-130-2-199901190-00002. [DOI] [PubMed] [Google Scholar]
- The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O’Rahilly S, Purmann C, Rees MG, Ridderstråle M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, Waeber G, Wallace C, Watanabe RM, Waterworth DM, Watkins N, Wellcome Trust Case Control Consortium. Witteman JC, Zeggini E, Zhai G, Zillikens MC, Altshuler D, Caulfield MJ, Chanock SJ, Farooqi IS, Ferrucci L, Guralnik JM, Hattersley AT, Hu FB, Jarvelin MR, Laakso M, Mooser V, Ong KK, Ouwehand WH, Salomaa V, Samani NJ, Spector TD, Tuomi T, Tuomilehto J, Uda M, Uitterlinden AG, Wareham NJ, Deloukas P, Frayling TM, Groop LC, Hayes RB, Hunter DJ, Mohlke KL, Peltonen L, Schlessinger D, Strachan DP, Wichmann HE, McCarthy MI, Boehnke M, Barroso I, Abecasis GR, Hirschhorn JN, for GIANT Consortium Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Sakane N, Umekawa T, Sakai M, Takahashi T, Kondo M. Mutation of β3-adrenergic-receptor gene and resonse to treatment of obesity. Lancet. 1995;346:1433–1434. doi: 10.1016/s0140-6736(95)92452-3. [DOI] [PubMed] [Google Scholar]