Abstract
Once thought to be the consequence of oxygen lack in contracting skeletal muscle, the glycolytic product lactate is formed and utilized continuously in diverse cells under fully aerobic conditions. ‘Cell–cell’ and ‘intracellular lactate shuttle’ concepts describe the roles of lactate in delivery of oxidative and gluconeogenic substrates as well as in cell signalling. Examples of the cell–cell shuttles include lactate exchanges between between white-glycolytic and red-oxidative fibres within a working muscle bed, and between working skeletal muscle and heart, brain, liver and kidneys. Examples of intracellular lactate shuttles include lactate uptake by mitochondria and pyruvate for lactate exchange in peroxisomes. Lactate for pyruvate exchanges affect cell redox state, and by itself lactate is a ROS generator. In vivo, lactate is a preferred substrate and high blood lactate levels down-regulate the use of glucose and free fatty acids (FFA). As well, lactate binding may affect metabolic regulation, for instance binding to G-protein receptors in adipocytes inhibiting lipolysis, and thus decreasing plasma FFA availability. In vitro lactate accumulation upregulates expression of MCT1 and genes coding for other components of the mitochondrial reticulum in skeletal muscle. The mitochondrial reticulum in muscle and mitochondrial networks in other aerobic tissues function to establish concentration and proton gradients necessary for cells with high mitochondrial densities to oxidize lactate. The presence of lactate shuttles gives rise to the realization that glycolytic and oxidative pathways should be viewed as linked, as opposed to alternative, processes, because lactate, the product of one pathway, is the substrate for the other.
Introduction
The linkages between glycolytic and aerobic metabolism have long held interest in the fields of muscle physiology, biochemistry and metabolism. In fact key discoveries on the pathways and their linkages represent foundations upon which the fields are built (Meyerhof, 1920; Hill & Lupton, 1923). However, with newer technologies it has been possible to obtain unique data and interpret older data on lactate turnover in new ways. Hence, it is now possible to hypothesize that together with blood glucose, glycogen reserves in diverse tissues can be mobilized to provide lactate, a glycolytic product that can either be used within the cells of formation or transported through the interstitium and vasculature to adjacent and anatomically distributed cells for utilization. Subsequently, consistent with the lactate shuttle hypothesis, results from studies on laboratory rodents, dogs and humans have established that lactate is a quantitatively important oxidizable substrate and gluconeogenic precursor as well as a means by which metabolism in diverse tissues is coordinated, especially during physical exercise when sympathetic stimulation of muscle glycogenolysis and recruitment of fast-glycolytic muscle fibres cause lactate flux to be high and circulatory transit time to be low. Moreover, lactate functions as a regulator of cellular redox state by exchange and conversion to its more oxidized analogue, pyruvate, through actions of lactate dehydrogenase (LDH). Furthermore, when lactate is released into the systemic circulation and taken up by distal tissues and organs, lactate also affects redox state in the cells, tissues and organs of removal. In view of its purported autocrine-, paracrine- and endocrine-like actions, lactate may be an important signalling molecule, a ‘lactormone’.
Recognition that there exist both intra- and extracellular effects of lactate production and removal has led to renaming of the original ‘lactate shuttle’ hypothesis (Brooks, 1985) the ‘cell–cell lactate shuttle’ (Brooks, 1998). As well, rapid progress in ongoing research has led to an extension of the original hypothesis to include intracellular components. The ‘intracellular lactate shuttle’ hypothesis was articulated when it was realized that mitochondria isolated from rat heart, skeletal muscle and liver oxidize lactate directly. Subsequently, after learning that peroxisomes contain but a single glycolytic enzyme, LDH, peroxisomes were found to contain MCT1 and MCT2 (McClelland et al. 2003). Thus, lactate is exchanged on quantitative bases, both between and within cell compartments. Although controversial only a few years ago, the concept of lactate shuttles within and between cells has been confirmed by others who have observed lactate exchange between diverse cells and tissues including astrocytes and neurons (Pellerin et al. 1998; Hashimoto et al. 2008).
Historical perspective
History of thinking on the causes and consequences of lactate production and removal has been reviewed previously (Brooks, 2002). The notion that lactic acid is formed as the result of oxygen lack can be traced to work of Louis Pasteur in the nineteenth century (Pasteur, 1863). Then at the beginning of the twentieth century, studies on isolated frog muscles produced results that caused investigators to find common threads in the metabolism of yeast and muscles of lower vertebrates (Hochachka, 1980). In 1920, using non-perfused and non-oxygenated frog muscle preparations, Otto Meyerhof identified glycogen as the precursor of lactic acid. He also provided evidence strongly linking contraction to lactate formation and loss of excitability.
Following the 1920 paper of Krogh and Lindhard who first reported the exponential decline in O2 consumption in men after exercise, Hill and associates turned their attention to studies of humans in an attempt to unite the new knowledge of muscle biochemistry and human metabolism. In 1923 Hill and Lupton defined ‘O2 debt’ as the ‘total amount of oxygen used, after cessation of exercise in recovery there from.’ Recognizing that during exercise onset and maximal exercise conditions there was a ‘deficit’ in oxygen consumption, Hill and associates sought to measure the O2 debt to obtain an energy equivalent of the anaerobic lactate producing work done during exercise.
In 1933 Margaria, Edwards and Dill reinterpreted the biphasic curve describing whole-body  during recovery from exercise. They concluded that the rapid O2 debt phase was a result of the restoration of phosphagen in recovering muscle and named the phase ‘alactacid’ (not having to do with lactic acid removal). The investigators also termed the second, slow O2 debt phase that coincided with the decline in blood [lactate] as the ‘lactacid’ O2 debt.
during recovery from exercise. They concluded that the rapid O2 debt phase was a result of the restoration of phosphagen in recovering muscle and named the phase ‘alactacid’ (not having to do with lactic acid removal). The investigators also termed the second, slow O2 debt phase that coincided with the decline in blood [lactate] as the ‘lactacid’ O2 debt.
In the early twentieth century great physiologists who worked on issues relating lactate metabolism and muscle energetics achieved positions of eminence. Krogh, Hill and Meyerhof became Nobel Laureates. D. B. Dill became Director of the Harvard Fatigue Laboratory and eventually served as President of the American Physiological Society, and Rudolfo Margaria was appointed Professor of Physiology in Milan. Thus, in the 1920s and 1930s the luminaries in science had adopted a Pasteur effect–O2 debt model of interpreting data on glycolytic metabolism. World events and science moved on and the O2 debt model was immortalized in textbooks of physiology and biochemistry. Obviously, traditional and contemporary lactate shuttle concepts are very different in terms of how biochemical and physiological processes are organized. Still, however, from standpoints of metabolic integration and muscle energetics, interest persists in the linkages between glycolytic and oxidative metabolism.
The cell–cell lactate shuttle
The initial lactate shuttle concept (Brooks, 1985) relied heavily on the use of isotope tracers, and subsequently we progressed to using combinations of techniques: tracers, net exchange measurements, and muscle biopsies (Bergman et al. 1999, 2000; Brooks, 2002).
Blood lactate kinetics in laboratory rats during exercise
We developed treadmill calorimetry and [14C]lactate tracer infusion and blood sampling techniques to simultaneously determine lactate flux and oxidation rates on resting and exercising rats. Parallel experiments were conducted using [3H]- and [14C]glucose. These techniques allowed us to compare and contrast the effects of training on glucose–lactate interactions. We observed that lactate was always produced and turned over rapidly, even in resting animals. Oxidation accounted for approximately half of lactate disposal rate (Rd) at rest, and the fraction of lactate removed by oxidation increased to 75–80% during sustained treadmill running. Training did little to affect lactate production (assessed from rate of appearance, Ra) in exercising rats; the major effect of training was to improve lactate (La−) clearance rate (=Rd/[La−]) especially during exercise when metabolic rate was high. And, by measuring 14C-from infused tracer lactate into blood glucose, as well as by [3H]- and [14C]glucose disposal rates, we established that lactate was the major gluconeogenic (GNG) precursor in exercising rats (Brooks & Donovan, 1983; Donovan & Brooks, 1983).
Blood lactate kinetics in humans during exercise
When stable, non-radioactive tracer technology became available, we moved to the study of glucose–lactate interactions in resting and exercising men and women on whom we conducted both cross-sectional and longitudinal training studies. First, we again demonstrated lactate turnover and oxidation in resting, postabsorptive men (Mazzeo et al. 1986) at sea level and high altitude (Brooks et al. 1991). Again, approximately half the lactate production was disposed of via oxidation at rest, and 75–80% during exercise in the range of 50–75% (Fig. 1).
(Fig. 1).
Figure 1. Lactate disposal (Ri) and oxidation (Rox) rates plotted as functions of oxygen consumption rate ( ) in 6 men at rest and exercise power outputs eliciting 50 and 75% of
) in 6 men at rest and exercise power outputs eliciting 50 and 75% of .
.
Values are means ±s.e.m. Reprinted from Mazzeo et al. (1986) with permission of the American Physiological Society.
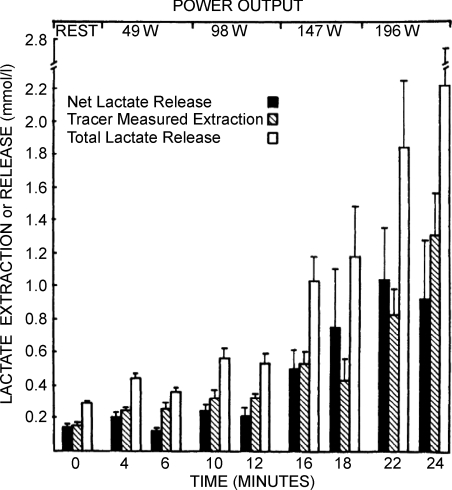
With combinations of 2H, 13C and 14C tracers we compared glucose and lactate fluxes and GNG from lactate in men during rest and exercise. This body of work (Stanley et al. 1986, 1988; Trimmer et al. 2002) is distinguished by virtue of the simultaneous comparisons afforded. For example, we reported comparisons of glucose and lactate flux rates in men during rest and easy (40% ) exercise (Stanley et al. 1986). During post-absorptive rest, glucose flux is twice as great as lactate flux; however at during even mild exercise lactate flux equals or exceeds glucose flux. During harder exercises, lactate Ra is far greater than glucose Rd (Bergman et al. 1999; 2000;). Because tracers as well as limb arteriovenous (a–v) differences for metabolite concentrations and isotopic differences as well as blood flow measurements could be obtained, simultaneous lactate uptake (extraction) and release by working human limb muscles was demonstrated (Fig. 2), with production being equal to the total of extraction and net release. In the same set of studies, coronary sinus catheterization allowed measurements of cardiac metabolism. In terms of lactate shuttling, it was clear that lactate released from working muscle and other tissue beds was the primary fuel for the heart during exercise (Gertz et al. 1988).
) exercise (Stanley et al. 1986). During post-absorptive rest, glucose flux is twice as great as lactate flux; however at during even mild exercise lactate flux equals or exceeds glucose flux. During harder exercises, lactate Ra is far greater than glucose Rd (Bergman et al. 1999; 2000;). Because tracers as well as limb arteriovenous (a–v) differences for metabolite concentrations and isotopic differences as well as blood flow measurements could be obtained, simultaneous lactate uptake (extraction) and release by working human limb muscles was demonstrated (Fig. 2), with production being equal to the total of extraction and net release. In the same set of studies, coronary sinus catheterization allowed measurements of cardiac metabolism. In terms of lactate shuttling, it was clear that lactate released from working muscle and other tissue beds was the primary fuel for the heart during exercise (Gertz et al. 1988).
Figure 2. Net lactate release, tracer-measured lactate extraction and total lactate release (extraction + net release) in working leg muscles as a function of time.
Net lactate release underestimates total intramuscular turnover at all times. Values are means ±s.e.m., n= 6 for all but the last sample when n= 3. Reprinted from Stanley et al. (1986) with permission of the American Physiological Society.
In our earlier studies we could see little difference in lactate production rates in trained and untrained men exercising at equivalent power outputs; main differences were in clearance rates. Subsequently, in a longitudinal training study we (Bergman et al. 1999) confirmed that training lowers arterial [La−] by increasing lactate clearance (Fig. 3).
Figure 3. Effects of exercise intensity and training on lactate on lactate metabolic clearance rate (MCR).
Values are means ±s.e.m. for 8–9 subjects. Reprinted from Bergman et al. (1999) with permission of the American Physiological Society.
Facilitated lactate exchange transport across membranes
Early on, and subsequently, we could see that whole body and working muscle oxidation rates appeared to follow Michaelis–Menten kinetics, so we moved to determine if lactate exchange was mediated by lactate transport proteins. Through our efforts and those of others we now know that facilitated transport of lactate across membranes is accomplished by a family of monocarboxylate transport proteins (MCTs) that are differentially expressed in cells and tissues. Initial evidence for a carrier-mediated muscle cell membrane lactate transporter obtained on rat sarcolemmal vesicles (Roth & Brooks, 1990a,b;) was followed by cloning and sequencing of the first lactate (monocarboxylate) transport protein (MCT) (Garcia et al. 1994). That discovery was soon followed by cloning and sequencing of several additional isoforms that are differentially expressed in mammalian tissues (Price et al. 1998). In the study of Bergman et al. (1999), muscle biopsies were taken and Western blots showed training effects on expression of muscle sarcolemmal and mitochondrial MCT1, but not MCT4 (Dubouchaud et al. 2000). Training-induced changes in expression of sarcolemmal MCT1 and mitochondrial proteins resulted in an increase in working muscle lactate clearance during exercise.
Lactate as a gluconeogenic precursor
Not all lactate produced in muscle is disposed of immediately by oxidation, so during exercise arterial [lactate] rises when there is net release from working muscle beds (Brooks et al. 1991). But this too provides utility, allowing working muscle and other tissues to fuel the heart (Gertz et al. 1988), as well as to serve as a gluconeogenic precursor. We have studied gluconeogenesis during exercise by various means, including dual isotope labelling involving combinations of D2- and 13C-labelled glucose (Friedlander et al. 1998), and the secondary labelling of glucose from infused [13C]lactate (Bergman et al. 2000). Additionally, most recently we have developed and employed the lactate clamp technique that involves the combination of exogenous lactate and isotope tracer infusion (Miller et al. 2002a,b;). Moreover, we have compared gluconeogenesis from lactate with other precursors (e.g. glycerol, Trimmer et al. 2002), and the predominant role of lactate is always evident, not just during exercise (Stanley et al. 1988; Brooks et al. 1991; Bergman et al. 2000; Trimmer et al. 2002). It is remarkable that while lactate is disposed of mainly through oxidation and only a minor fraction supports GNG, lactate is the main gluconeogenic precursor during sustained exercise.
The intracellular lactate shuttle
To oxidize lactate it was hypothesized that mitochondria needed a symport such as an MCT, and LDH, both of which were subsequently identified (Brooks et al. 1999a,b;). Though controversial because of deficiencies in methodology by some other investigators, using contemporary techniques Pagliarini et al. (2008) developed the MitoCarta identifying over 1000 proteins in the mitochondrial proteome; among those are LDH and MCT1. Historically, the histochemical localization of LDH in mitochondria of rat heart and skeletal muscle is attributable to efforts of Baba and Sharma (1971) who used electron microscope histochemistry and showed LDH to be associated with the inner membrane and matrix of rat pectoralis and cardiac muscle mitochondria. They were probably the first to speculate on the presence of a ‘lactate shuttle’, but in the absence of physiological or biochemical data they were unable to expand on the significance of their observation.
Perhaps the first depiction of an intracellular lactate shuttle was by Hochachka (1980) who linked the presence of a unique LDH isoform (LDH-C) to the ability of sperm mitochondria to oxidize lactate. Hochachka fully recognized the physiological and evolutionary significance of lactate oxidation by sperm mitochondria, but he did not have the opportunity to extend his findings to other cell systems.
In the late 1980s Kline et al. (1986) and Brandt et al. (1987) demonstrated the presence of LDH in rat liver, kidney and heart mitochondria. Further, they showed that isolated liver mitochondria were capable of oxidizing lactate at least as fast as pyruvate (Kline et al. 1986). They interpreted their results as permitting the lactate shuttle (Brandt et al. 1987). Subsequently, the ability of isolated muscle mitochondria to oxidize lactate has been confirmed (Brooks et al. 1999b), as has the intra-mitochondrial localization of LDH (Dubouchaud et al. 2000; Hashimoto et al. 2005, 2006, 2008; Pagliarini et al. 2008; Passarella et al. 2008). Additionally, the mitochondrial lactate/pyruvate transporter in muscle has been identified as MCT1 (Brooks et al. 1999a; Dubouchaud et al. 2000; Hashimoto et al. 2005, 2006, 2008; Pagliarini et al. 2008). Because proton and concentration gradients are necessary for lactate flux via diffusion and facilitated transport, and because removal of lactate is via oxidation and gluconeogenesis, actively respiring mitochondria are essential for lactate shuttles to operate (Brooks, 2002). The role of mitochondrial LDH in gluconeogenesis has been emphasized by Passarella and colleagues (2008).
Beyond a cytosol to mitochondria lactate shuttle, other intercellular lactate shuttles are likely to exist, for instance between cytosol and peroxisomes where it is known that a system for the reoxidation of NADH is essential for the functioning of β-oxidation. In this context it is noteworthy that to control peroxisomal redox balance, lactate for pyruvate exchange across peroxisomal membranes must be accomplished (McClelland et al. 2003).
Results of studies using proton and 13C-NMR support the contention of lactate shuttles in vivo, but the data suggest that our knowledge of cell–cell and intracellular lactate exchange and metabolism are in their infancy. For instance, while results from NMR spectroscopy show preferential lactate oxidation in skeletal muscle (Bertocci & Lujan, 1999) and heart (Laughlin et al. 1993; Chatham et al. 2001), the pathways are not necessarily as expected. Pyruvate tracer given into the circulation is rapidly converted to lactate, likely to occur through uptake via the action of LDH in erythrocytes and cell lactate/pyruvate exchange mediated by MCT1. With tracers injected directly into the myocardial circulation, [13C]pyruvate exchanges with lactate and alanine in cytosol, all three peaks being visualized in spectra (Chatham et al. 2001). However, when [13C]lactate is injected, cytosolic pyruvate is not visualized (Laughlin et al. 1993). Most recently, Chatham et al. (2001) have elaborated on this apparent compartmentation of lactate metabolism and results of their studies indicate preferential oxidation of exogenous lactate in heart with glycolytically derived lactate exported from heart.
Is lactate a signalling molecule (a ‘lactormone’)?
Redox signalling
Lactate is more reduced than its complimentary keto-acid, pyruvate. Consequently, whenever lactate is oxidized to pyruvate, or exchanged for pyruvate, and subsequently oxidized, cell redox balance is changed. Hence, lactate production in one cell compartment and its removal in another, whether the compartments are adjacent or anatomically removed, represents a major signalling mechanism because redox changes occur at the millimolar as opposed to the micro- or nanomolar level.
Examples of lactate substituting for and down-regulating the use of energy substrates abound. When arterial lactate rises during exercise, it becomes the predominate fuel for the heart, decreasing relative use of other energy substrates (Gertz et al. 1988). With regard to glucose, we (Miller et al. 2002a,b;) found that when the arterial [La−] was clamped (raised) at 4 mm by exogenous infusion in resting or exercising men, lactate disposal and oxidation increase with a stoichiometric decrease in glucose disposal and oxidation.
With regard to FFA mobilization, in the field of exercise physiology, the effect of acidosis inhibiting lipolysis has long been recognized as plasma [FFA] falls during hard exercise when arterial [La−]a rises (Brooks, 1998). More recently, Liu et al. (2009) showed that lactate inhibits lipolysis in fat cells through activation of an orphan G-protein coupled receptor (GPR81) that acts as a lactate sensor, the response of which is to inhibit lipolysis.
With regard to FFA oxidation, it is widely recognized that when exercise is hard and arterial [La−] rises, FFA oxidation decreases because of mass action and redox control (Brooks, 1998, 2002). When glycolysis is accelerated during muscle contraction, concentrations of the glycolytic products lactate and pyruvate (Pyr−) rise as does the lactate/pyruvate ratio ([La−]/[Pyr−]). At rest, the [La−]/[Pyr−] in muscle and venous effluent from a muscle bed approximates 10, but the ratio rises an order of magnitude or more during moderate intensity exercise (Henderson et al. 2004). The monocarboxylate pair dominates substrate entry into the mitochondrial matrix (Saddik et al. 1993; Brooks, 1998; Friedlander et al. 1998), giving rise to acetyl-CoA and, thereby, malonyl-CoA formation. The rise in malonyl-CoA inhibits the entry of activated FFA into the mitochondrial matrix by inhibiting carnitine–palmitoyl transferase-1 (CPT1) (Saddik et al. 1993). As well, the accumulation of acetyl-CoA down-regulates β-ketothiolase, the terminal and rate-limiting enzyme of the mitochondrial β-oxidation pathway, which is sensitive to redox and substrate inhibition.
Gene expression
In addition to short-term effects on cell metabolism via redox modulation, lactate has the potential to produce long-term changes via effects on gene expression. It has long been known that endurance training stimulates mitochondrial biogenesis (27). As well, we (6) observed that endurance training increases MCT1 expression, and also that changes in MCT1 expression correlated with levels of mitochondrial proteins. Those observations on laboratory rats and humans led us to suspect that lactate was a signalling molecule that affected its own metabolism.
Monocarboxylate transporter isoforms (e.g. MCT1) are members of a gene super family coding for solute transporters. The first protein identified was termed MCT1 by the discoverers, Garcia et al. (1994). Of the family, the first four isoforms are lactate/pyruvate transporters. Of those, MCT1 (SLC16A1) is most widespread, being expressed in diverse cells and tissues from neurons to erythrocytes and sperm (Price et al. 1998). As well, MCT1 is expressed in various cell domains; in muscle those include the plasma (sarcolemmal) (Garcia et al. 1994), mitochondrial (Brooks et al. 1999a) and peroxisomal (McClelland et al. 2003) membranes. Because of our interest in understanding muscle lactate metabolism, to date we have focused on the regulation of MCT1 expression.
The art of cell culture traditionally involves cell incubation in high glucose media under aerobic conditions. Basic techniques have been in existence for nearly a century. Initial studies with L6 cells produced perplexing results on the effects of the above-identified putative physiological signals of MCT1 expression (e.g. H2O2, lactate, H+, Ca2+) until we understood our cell system and believed our hypothesis that lactate was a physiological signal. Then, remembering the Warburg effect, we realized that the cells glycolyse rapidly and hence produce lactate continuously. So, if lactate were a physiological signal, in high glucose medium cells would produce lactate continuously, and the expression of MCT1 would rise correspondingly. In fact, continuous lactate production under fully aerobic conditions and rising MCT1 protein levels were features of our initial studies. Therefore, we found it necessary to control incubation [lactate] because changing lactate was accompanied by changing MCT1 protein level.
After realizing the problem of baseline drift in MCT1 expression due to endogenous lactate production, we could describe ordered changes in MCT1 mRNA and protein levels in response to [lactate] (Fig. 4). These data can be interpreted to indicate regulation at the level of transcription (Hashimoto et al. 2007).
Figure 4. MCT1 message (A), and protein levels (B) in L6 cells after 1 h incubation at the indicated [lactate] levels.
A good correlation between message and protein levels is apparent. Protein levels determined from Western blotting and mRNA from RT-PCR. Data from Hashimoto et al. (2007).
With regard to the mechanism of lactate signalling in cultured L6 cells, we pursued the hypothesis of reactive oxygen species (ROS). This suspicion arose from studies of the role of lactate in wound healing, and was reinforced based on our analysis of the MCT1 gene promoter region which suggested the presence of multiple potential binding sites for known ROS-responsive transcription factors such as cAMP response element-binding protein (CREB), nuclear factor-κB (NF-κB), activated protein-a (AP-1), stimulating protein-1 (SP-1), and nuclear factor erythroid 2 (NF-E2, or Neff) (Hashimoto et al. 2007.
Accordingly, to confirm the idea of lactate as a ROS generator, we determined hydrogen peroxide (H2O2) production in L6 cells exposed to 20 mm lactate. Both high glucose and lactate supplemented peroxide production (Hashimoto et al. 2007). The results obtained by us are consistent with those of others showing high glucose to affect ROS production. However, our results are novel for the effect of lactate as a ROS generator in cultured myocytes.
In order to examine whether increased MCT1 gene expression in L6 cells after lactate incubation involved activation of ROS-sensitive transcription factors, we assessed DNA binding using EMSA after 20 mm lactate treatments ranging from 10 min to 24 h. Increased NF-κB DNA binding was detected from 10 min to 3 h. As well, lactate incubation increased NF-E2 DNA binding activity, but no changes in binding activities of AP-1 and SP-1 were detected. Hence, it appeared that NF-κB and NF-E2 DNA binding are responsive to lactate-induced oxidative stress.
Because the terminal electron transport chain element cytochrome oxidase (COX) as well as lactate dehydrogenase (LDH) and MCT1 may constitute a mitochondrial lactate oxidation (LOX) Complex (20), we determined the effect of 20 mm lactate incubation on COX and peroxisome proliferator activated receptor γ coactivator-1α (PGC1α) protein levels. PGC1α is of interest because it is considered to be a master coordinator of mitochondrial biogenesis. Consistent with the purported role of PGC1α, we observed elevated PGC1α mRNA and protein levels in L6 cells after incubation in 20 mm. As well, lactate incubation increased L6 cell COX mRNA and protein levels (22).
In its role as a master coordinator of mitochondrial biogenesis, PGC1α interacts with transcription factors for mitochondrial gene expression, including those for COX, such as CREB, nuclear respiratory factor (NRF)-1 and NRF-2. Accordingly, we determined the effect of L6 cell incubation on NRF-1, NRF-2 and CREB binding to DNA. Lactate incubation did not affect DNA binding to NRF-1, but binding activity for NRF-2 was increased after 1 h incubation in 20 mm lactate, and CREB binding was increased within 30 min incubation (Hashimoto et al. 2007). In the aggregate these findings can be interpreted to mean that lactate exposure increases MCT1 expression and mitochondrial biogenesis, the latter by binding to NRF-2 and CREB to DNA.
As exercise physiologists interested in advancing knowledge of the linkages between glycolytic and oxidative metabolism, we were excited about the prospects for attributing relevance to the above described studies using contemporary molecular and biochemical techniques. Hence, we sought means to conduct studies at several levels of physiological organization, and with access to confocal laser scanning microscopy, we sought to visualize the mitochondrial reticulum as well as protein components of the mitochondrial lactate oxidation complex proteins in cultured myocytes (Fig. 5). Using combinations of primary and fluorescently labelled secondary antibodies plus MitoTracker Red and dual-wavelength scanning confocal microscopy, colocalization of MCT1, COX and LDH is seen in mitochondria (Hashimoto et al. 2005, 2006, 2008). These results were confirmed by two independent techniques, immunocoprecipitation and Western blotting of isolated cell fractions (Hashimoto et al. 2006). To date, work related to visualizing mitochondrial dynamics has involved MCT1 and other LOX proteins (Hashimoto et al. 2005, 2006, 2008). Work on determining effects of physiological signals affecting expression of the four GTPases (Mfn1, Mfn2, OPA1, Drp1) and Fis1 are in progress.
Figure 5. Immunohistochemical images demonstrating some components of the lactate oxidation complex in cultured L6 muscle cells.
This complex involves the mitochondrial constituent cytochrome oxidase (COX), the lactate–pyruvate transport protein (MCT1), lactate dehydrogenase (LDH) and other constituents. A, co-localization of MCT1 and the mitochondrial reticulum. MCT1 was detected at both sarcolemmal and intracellular domains (A1). Using MitoTracker the mitochondrial reticulum was extensively elaborated and detected at intracellular domains throughout L6 cells (A2). When signals from probes for the lactate transporter (MCT1, green, A1) and mitochondria (red, A2) were merged, superposition of the signals (yellow) showed co-localization of MCT1 and components of the mitochondrial reticulum, particularly at perinuclear cell domains (A3). B, lactate dehydrogenase (LDH) (B1) and mitochondrial cytochrome oxidase (COX) (B2) are imaged. Superposition of signals for LDH (red, B1) and COX (green, B2) shows co-localization of LDH in the mitochondrial reticulum (yellow) of cultured L6 rat muscle cells (D3). Depth of field ∼1 μm, scale bar = 10 μm. Reprinted from Hashimoto et al. (2006) with permission of the American Physiological Society.
Concern about results obtained on an immortalized cell line are mitigated by observations that similar results were obtained on adult rat plantaris, a mixed fibre type skeletal muscle (Fig. 6) (Hashimoto et al. 2005). Again use of primary antibodies and fluorescently labelled secondary antibodies along with dual-wavelength confocal scanning microscopy showed colocalization of MCT1 and COX at the sarcolemmal surface as well as throughout oxidative fibres. Results for MCT2 were less robust (Fig. 6B2 and B3).
Figure 6. Cellular locations of MCT1 and MCT2 lactate transporter isoforms and the mitochondrial reticulum (cytochrome oxidase, COX) in adult rat plantaris muscle determined using confocal laser scanning microscopy (CLSM) and fluorescent probes for the respective proteins.
Comparisons for MCT1 are shown in the first row (A1–A3), and for MCT2 in the second row (B1–B3). The localization of COX was detected in rat plantaris muscle (A1 and B1). MCT1 was detected throughout the cells including subsarcolemmal (arrowheads) and interfibrillar (arrows) domains (A2). MCT1 abundance was greatest in oxidative fibres where COX is abundant and the signal strong. When these MCT1 (green) and COX (red) were merged, superposition of the two probes was clear (yellow), a finding prominent at interfibrillar (arrows) as well as sarcolemmal (arrowheads) cell domains (A3). In contrast, the signal for MCT2 (B2) was weak, relatively more noticeable in fibres denoted by strong staining for COX (B1 and B3, broken line is delineated around oxidative fibre to distinguish the faint signal for MCT2). Overlap of MCT2 and COX is insignificant, denoted by absence of yellow in B3. Scale bar = 50 μm. Sections are from the same animal. Reprinted from Hashimoto et al. (2005).
Generality and applicability
Before summing up it is important to acknowledge the limited coverage provided in this review of the diverse aspects of lactate metabolism and cell signalling. In particular, it needs to be acknowledged that the above described the effects of lactate on gene expression have yet to be demonstrated in vivo. Still, the literature contains ample reports showing a role of lactate in metabolic regulation at diverse levels of physiological organization. Cell–cell and intracellular lactate shuttles are well described in exercise physiology and neurophysiology. Elucidation of the role of lactate in gluconeogenesis (i.e. the Cori cycle) demonstrated that lactate is the major 3-C gluconeogenic precursor. The Cori cycle was the first recognized example of a cell–cell lactate shuttle. First seen in muscle, mitochondrial lactate transporters and LDH have been demonstrated to exist in astrocytes and neurons (Hashimoto et al. 2008) and diverse other tissues including sperm (Hochachka, 1980). Recently, Chari et al. (2008) found that intra-cerebroventricular (ICV) lactate administration lowered glucose Ra and insulin action in rats with streptozotocin- and dietary induced diabetes. And, during human exercise arterial lactate is taken up and oxidized by the brain (Van Hall et al. 2009). These and other findings can be interpreted to mean that lactate is involved in the response to alterations in nutrient sensing. It is likely that other roles of lactate shuttles will be discovered.
The good, the bad (yin–yang, Darth Vader vs. Jedi Knight) of lactate is perhaps no more elegantly illustrated than in the recent work of Sonveaux et al. (2008). Tumour cell growth and metabolism involves lactate shuttling between glycolytic and rapidly respiring cells. Therefore, an opportunity exists to target and block MCT-mediated lactate exchange, thereby killing cancer cells.
Summary
This is a rapidly changing field and contemporary understanding of the role of lactate metabolism has changed dramatically from classic views. Once thought to be the consequence of oxygen lack in contracting skeletal muscle, we know now that lactate is formed and utilized continuously under fully aerobic conditions. Lactate is actively oxidized at all times, especially during exercise when oxidation accounts for 70–75% of removal and gluconeogenesis for most of the remainder. Working skeletal muscle both produces and uses lactate as a fuel, with much of the lactate formed in glycolytic fibres being taken up and oxidized in adjacent oxidative fibres. Because it is more reduced that its keto-acid analogue, sequestration and oxidation of lactate to pyruvate affects cell redox state, both promoting energy flux and signalling cellular events. Lactate diffusion and carrier-mediated lactate exchange occur down proton and concentration gradients. These gradients are established by mitochondrial respiration that is responsible for oxidation and gluconeogenesis. Thus, training that gives rise to mitochondrial biogenesis (Holloszy, 1967) establishes the diffusion gradients for increased lactate clearance during exercise. Facilitated lactate transport is accomplished by a family of monocarboxylate transport proteins (MCTs) that are differentially expressed in cells and tissues. The mitochondrial lactate/pyruvate transporter appears to work in conjunction with mitochondrial LDH that permits lactate to be oxidized within actively respiring cells, thereby establishing the gradients driving lactate flux. Glycolysis accompanied by lactate oxidation within cells permits high flux rates and maintenance of redox balance in cytosol and mitochondrial compartments. Because of its powerful effects on cell redox, lactate influences metabolic regulation at diverse levels in multiple cells. The presence of cell–cell and intracellular lactate shuttles gives rise to the notion that glycolytic and oxidative pathways be viewed as linked, as opposed to alternative, processes, because lactate, the product of one pathway, is the substrate for the other.
Acknowledgments
This work was supported by National Institute of Health grants DK19577, AR42906 and AR050459.
References
- Baba N, Sharma HM. Histochemistry of lactic dehydrogenase in heart and pectoralis muscles of rat. J Cell Biol. 1971;51:621–635. doi: 10.1083/jcb.51.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE, Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab. 2000;278:E244–251. doi: 10.1152/ajpendo.2000.278.2.E244. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk G, Casazza GA, Horning MA, Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol. 1999;87:1684–1696. doi: 10.1152/jappl.1999.87.5.1684. [DOI] [PubMed] [Google Scholar]
- Bertocci LA, Lujan BF. Oxidative incorporation and utilization of [3-13C]lactate and [1,2-13C]acetate by rat skeletal muscle. J Appl Physiol. 1999;86:2077–2089. doi: 10.1152/jappl.1999.86.6.2077. [DOI] [PubMed] [Google Scholar]
- Brandt RB, Laux JE, Spainhour SE, Kline ES. Lactate dehydrogenase in mitochondria. Arch Biochem Biophys. 1987;259:412–422. doi: 10.1016/0003-9861(87)90507-8. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Lactate: Glycolytic end product and oxidative substrate during sustained exercise in mammals – the ‘lactate shuttle’. In: Gilles R, editor. Circulation, Respiration, and Metabolism: Current Comparative Approaches. Berlin: Springer-Verlag; 1985. pp. 208–218. [Google Scholar]
- Brooks GA. Mammalian fuel utilization during sustained exercise. Comp Biochem Physiol. 1998;120:89–107. doi: 10.1016/s0305-0491(98)00025-x. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002;30:258–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Brown MA, Butz CE, Sicurello JP, Dubouchaud H. MCT1 in cardiac and skeletal muscle mitochondria. J Appl Physiol. 1999a;87:1713–1718. doi: 10.1152/jappl.1999.87.5.1713. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE, Reeves JT. Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J Appl Physiol. 1991;71:333–341. doi: 10.1152/jappl.1991.71.1.333. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Donovan CM. Effect of training on glucose kinetics during exercise. Am J Physiol Endocrinol Metab. 1983;244:E505–E512. doi: 10.1152/ajpendo.1983.244.5.E505. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactic dehydrogenase and lactate oxidation in the ‘intra-cellular lactate shuttle’. Proc Natl Acad Sci U S A. 1999b;96:1129–1134. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari M, Lam CK, Wang PY, Lam TK. Activation of central lactate metabolism lowers glucose production in uncontrolled diabetes and diet-induced insulin resistance. Diabetes. 2008;57:836–840. doi: 10.2337/db07-1464. [DOI] [PubMed] [Google Scholar]
- Chatham JC, Des Rosiers C, Forder JR. Evidence of separate pathways for lactate uptake and release by the perfused rat heart. Am J Physiol Endocrinol Metab. 2001;281:E794–E802. doi: 10.1152/ajpendo.2001.281.4.E794. [DOI] [PubMed] [Google Scholar]
- Donovan CM, Brooks GA. Endurance training affects lactate clearance, not lactate production. Am J Physiol Endocrinol Metab. 1983;244:E83–E92. doi: 10.1152/ajpendo.1983.244.1.E83. [DOI] [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression and physiology of LDH, MCT1 and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E571–579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, Casazza GA, Horning MA, Huie MJ, Piacentini M-F, Trimmer JK, Brooks GA. Training-induced alterations of carbohydrate metabolism in young women: women respond differently from men. J Appl Physiol. 1998;85:1175–1186. doi: 10.1152/jappl.1998.85.3.1175. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labelled carbohydrate isotope experiments. J Clin Invest. 1988;82:2017–2025. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Brooks GA. Colocalization of MCT1, CD147 and LDH in mitochondrial inner membrane of L6 cells: Evidence of a mitochondrial lactate oxidation complex. Am J Physiol Endocrinol Metab. 2006;290:E1237–E1244. doi: 10.1152/ajpendo.00594.2005. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Cho H-S, Kaufer D, Brooks GA. Evidence for a mitochondrial lactate oxidation complex in rat neurons: a crucial component for a brain lactate shuttle. PloS One. 2008;3:e2915. doi: 10.1371/journal.pone.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 myocytes: activation of MCT1 expression and mitochondrial biogenesis. FASEB J. 2007;21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Masuda S, Taguchi S, Brooks GA. Immunohistochemical analysis of MCT1, MCT2 and MCT4 expression in rat plantaris muscle. J Physiol. 2005;567:121–129. doi: 10.1113/jphysiol.2005.087411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GC, Horning MA, Lehman SL, Wolfel EE, Bergman, Brooks GA. Pyruvate shuttling during rest and exercise in men before and after endurance training. J Appl Physiol. 2004;97:317–325. doi: 10.1152/japplphysiol.01367.2003. [DOI] [PubMed] [Google Scholar]
- Hill AV, Lupton H. Muscular exercise, lactic acid and the supply and utilization of oxygen. Q J Med. 1923;16:135–171. [Google Scholar]
- Hochachka PW. Living Without Oxygen. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- Kline ES, Brandt RB, Laux JE, Spainhour SE, Higgins ES, Rogers KS, Tinsley SB, Waters MG. Localization of L-lactate dehydrogenase in mitochondria. Arch Biochem Biophys. 1986;246:673–680. doi: 10.1016/0003-9861(86)90323-1. [DOI] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The changes in respiration at the transition from work to rest. J Physiol. 1920;53:431–439. doi: 10.1113/jphysiol.1920.sp001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MR, Taylor J, Chesnick AS, DeGroot M, Balaban RS. Pyruvate and lactate metabolism in the in vivo dog heart. Am J Physiol Heart Circ Physiol. 1993;264:H2068–2079. doi: 10.1152/ajpheart.1993.264.6.H2068. [DOI] [PubMed] [Google Scholar]
- Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, Mazur C, Kamme F, Lovenberg TW. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- Margaria R, Edwards RHT, Dill DB. The possible mechanisms of contracting and paying the oxygen debt and the role of lactic acid in muscular contraction. Am J Physiol. 1933;106:689–715. [Google Scholar]
- Mazzeo RS, Brooks GA, Schoeller DA, Budinger TF. Disposal of [1-13C]-lactate during rest and exercise. J Appl Physiol. 1986;60:232–241. doi: 10.1152/jappl.1986.60.1.232. [DOI] [PubMed] [Google Scholar]
- McClelland GB, Khanna S, González G, Butz CE, Brooks GA. Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system? Biochem Biophys Res Commun. 2003;203:130–135. doi: 10.1016/s0006-291x(03)00550-3. [DOI] [PubMed] [Google Scholar]
- Meyerhof O. Die Energieumwandlungen im Muskel III. Kohlenhydrat und Milchsaureumsatz im Froschmuskel. Pflügers Arch ges Physiol. 1920;185:11–32. [Google Scholar]
- Miller BF, Fattor JA, Jacobs KA, Horning MA, Suh S-H, Navazio F, Brooks GA. Metabolic and cardiorespiratory responses to an exogenous lactate infusion during rest and exercise: ‘the lactate clamp’. Am J Physiol Endocrinol Metab. 2002a;283:E889–E898. doi: 10.1152/ajpendo.00266.2002. [DOI] [PubMed] [Google Scholar]
- Miller BF, Fattor J, Jacobs KA, Horning MA, Suh S-H, Navazio F, Brooks GA. Lactate–glucose interaction in men during rest and exercising using lactate clamp procedure. J Physiol. 2002b;544:963–975. doi: 10.1113/jphysiol.2002.027128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarella S, de Bari L, Valenti D, Pizzuto R, Paventi G, Atlante A. Mitochondria and L-lactate metabolism. FEBS Lett. 2008;582:3569–3576. doi: 10.1016/j.febslet.2008.09.042. [DOI] [PubMed] [Google Scholar]
- Pasteur L. Recherches sur putréfaction. Compt Rend. 1863;56:1189–1194. [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ. Evidence supporting the existence of an activity-dependent astrocyte–neuron lactate shuttle. Dev Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J. 1998;329:321–328. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DA, Brooks GA. Lactate and pyruvate transport is dominated by a pH gradient-sensitive carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys. 1990a;279:386–394. doi: 10.1016/0003-9861(90)90506-t. [DOI] [PubMed] [Google Scholar]
- Roth DA, Brooks GA. Lactate transport is mediated by a membrane-borne carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys. 1990b;279:377–385. doi: 10.1016/0003-9861(90)90505-s. [DOI] [PubMed] [Google Scholar]
- Saddik M, Gamble J, Witters, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem. 1993;268:25836–25845. [PubMed] [Google Scholar]
- Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WC, Gertz EW, Wisneski JA, Morris DL, Neese R, Brooks GA. Lactate metabolism in exercising human skeletal muscle: Evidence for lactate extraction during net lactate release. J Appl Physiol. 1986;60:1116–1120. doi: 10.1152/jappl.1986.60.4.1116. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Wisneski JA, Gertz EW, Neese RA, Brooks GA. Glucose and lactate interrelations during moderate intensity exercise in man. Metabolism. 1988;37:850–858. doi: 10.1016/0026-0495(88)90119-9. [DOI] [PubMed] [Google Scholar]
- Trimmer JK, Schwarz JM, Casazza GA, Horning MA, Rodriguez N, Brooks GA. Measurement of gluconeogenesis in exercising men by mass isotopomer distribution analysis. J Appl Physiol. 2002;93:233–241. doi: 10.1152/japplphysiol.01050.2001. [DOI] [PubMed] [Google Scholar]
- van Hall G, Strømstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]