Abstract
During maximal exercise, supraspinal fatigue contributes significantly to the decline in muscle performance but little is known about intracortical inhibition during such contractions. Long-interval inhibition is produced by a conditioning motor cortical stimulus delivered via transcranial magnetic stimulation (TMS) 50–200 ms prior to a second test stimulus. We aimed to delineate changes in this inhibition during a sustained maximal voluntary contraction (MVC). Eight subjects performed a 2 min MVC of elbow flexors. Single test and paired (conditioning–test interval of 100 ms) stimuli were delivered via TMS over the motor cortex every 7–8 s throughout the effort and during intermittent MVCs in the recovery period. To determine the role of spinal mechanisms, the protocol was repeated but the TMS test stimulus was replaced by cervicomedullary stimulation which activates the corticospinal tract. TMS motor evoked potentials (MEPs) and cervicomedullary motor evoked potentials (CMEPs) were recorded from biceps brachii. Unconditioned MEPs increased progressively with fatigue, whereas CMEPs increased initially but returned to the control value in the final 40 s of contraction. In contrast, both conditioned MEPs and CMEPs decreased rapidly with fatigue and were virtually abolished within 30 s. In recovery, unconditioned responses required <30 s but conditioned MEPs and CMEPs required ∼90 s to return to control levels. Thus, long-interval inhibition increased markedly as fatigue progressed. Contrary to expectations, subcortically evoked CMEPs were inhibited as much as MEPs. This new phenomenon was also observed in the first dorsal interosseous muscle. Tested with a high intensity conditioning stimulus during a fatiguing maximal effort, long-interval inhibition of MEPs was increased primarily by spinal rather than motor cortical mechanisms. The spinal mechanisms exposed here may contribute to the development of central fatigue in human muscles.
Introduction
During a fatiguing maximal effort, the level of voluntary activation of the muscle declines progressively and can contribute to more than one-quarter of the force loss (Taylor & Gandevia, 2008; see Gandevia 2001 for review). However, the extent to which this central fatigue develops in the motor cortex versus the spinal cord is unclear. Transcranial magnetic stimulation (TMS) of the motor cortex produces a short-latency motor evoked potential (MEP) and an interruption of ongoing electromyographic activity known as the silent period. As fatigue develops, the MEP increases and the silent period lengthens (Gandevia et al. 1996; McKay et al. 1996; Taylor et al. 1996, 2000; Benwell et al. 2007). The changes in both measures recover rapidly and are believed to be cortical in origin.
Although changes in the MEP fail to identify if the alteration in excitability occurs at a motor cortical or spinal level, stimulation of the corticospinal tract at the level of the mastoids produces a cervicomedullary motor evoked potential (CMEP) which assesses the motor pathway below the motor cortex (e.g. Ugawa et al. 1991; Gandevia et al. 1999). Hence, comparison of MEPs and CMEPs enables one to localise changes in excitability to either cortical or spinal sites. The CMEP decreases during the final third of a 2 min sustained MVC (Butler et al. 2003; Martin et al. 2006b). Moreover, if tested in a relaxed muscle, CMEP size is reduced for ∼2 min after a sustained MVC regardless of its duration (Gandevia et al. 1999). Thus, the increase in the MEP during a sustained MVC reflects an increase in cortical excitability. In contrast, the lengthening of the silent period suggests an increase in cortical inhibition as the latter part of the silent period is thought to be due to intracortical inhibition of voluntary motor output. These apparently opposite changes make the role of the motor cortex during fatiguing contractions unclear.
Paired TMS pulses can explore intracortical inhibitory and facilitatory mechanisms (e.g. Reis et al. 2008). At intensities above motor threshold, a test stimulus delivered in the silent period which was produced by a preceding conditioning stimulus (i.e. at an interstimulus interval of 50–200 ms) results in a smaller MEP than when the test stimulus is delivered alone (Valls-Sole et al. 1992). This effect is frequently termed ‘long-interval intracortical inhibition.’ Corticospinal volleys recorded in conscious humans suggest that, like the silent period, suppression of the conditioned MEP is mediated by spinal mechanisms at ∼50 ms but cortical mechanisms at longer interstimulus intervals (100–200 ms) (Nakamura et al. 1997; Chen et al. 1999; Di Lazzaro et al. 2002). In contrast to the myriad conditions studied with single-stimulus TMS, the effect of fatigue on long-interval intracortical inhibition has been tested only once. Inhibition of the test MEP decreased with fatigue. However, because measurements were made during relaxation after brief MVCs (Benwell et al. 2007), the implications for central fatigue are unclear.
The primary purpose of the current study was to use pairs of motor cortical stimuli to measure long-interval inhibition during and after a sustained maximal effort. A secondary purpose was to delineate cortical versus spinal mechanisms for any inhibition by replacement of the motor cortical test stimulus with cervicomedullary stimulation which activates the corticospinal tract at a subcortical site. We hypothesised that the level of inhibition tested during voluntary contraction would increase with the development of fatigue and that this inhibition would occur principally at the motor cortex. While we observed a complete inhibition of the response to the second of two cortical stimuli, this was the result of primarily spinal rather than motor cortical changes produced by fatigue.
Methods
Subjects
Eight healthy subjects (28 ± 12 years, mean ±s.d.; 1 female) participated in the main experiment, which comprised four protocols which were performed on separate days in a pseudo-random order. Four of these subjects and an additional four subjects (34 ± 5 years; 1 female) participated in the first additional experiment. One subject from the main experiment as well as one additional subject (49 year old female) participated in the second additional experiment, which comprised two protocols performed on separate days. Unless otherwise stated, all descriptions which follow in the Methods, Results and Discussion sections refer to the main experiment in the elbow flexors. All studies were approved by the institutional ethics committee and conformed to the Declaration of Helsinki. Written consent was obtained from each of the participants.
Experimental set-up
Subjects were seated with their right arm positioned in an isometric myograph and an angle of ∼90 deg flexion at both the shoulder and elbow joints. The forearm was supinated and a strap at the wrist tightly secured the arm to the myograph. Elbow flexor torque was measured with a linear strain gauge (Xtran, Melbourne, Australia). EMG of the biceps brachii was recorded via adhesive Ag–AgCl electrodes (10 mm diameter) arranged in a monopolar configuration. The recording electrode was positioned on the belly of the muscle and the reference electrode over the distal tendon.
In the additional experiment involving contractions of the first dorsal interosseous (FDI), the right forearm was pronated and firmly secured to a table with two straps. The thumb was extended and held in place by an adjustable post. Middle, ring and little fingers were separated from the index finger by a bar and clamped. The index finger was positioned in an adjustable ring and abduction force was measured with a linear strain gauge (Xtran, Melbourne, Australia). The recording EMG electrode was positioned on the belly of the FDI and the reference electrode over the metacarpophalangeal joint.
In all experiments, torque and EMG data were recorded to computer using a 12-bit A/D converter (CED 1401 Plus; Cambridge Electronic Design Ltd., Cambridge, UK) in conjunction with Spike2 software (version 6.06; Cambridge Electronic Design). The torque and EMG data were sampled at 1000 and 2000 Hz, respectively. EMG data were amplified (×100) and bandpass filtered (16–1000 Hz) using CED 1902 amplifiers (Cambridge Electronic Design).
Transcranial magnetic stimulation
Stimulation to the motor cortex was delivered over the vertex using a circular coil (13.5 cm outside diameter) attached via a BiStim unit to two Magstim 200 stimulators (Magstim, Dyfed, UK). One stimulator delivered the conditioning stimulus and the other delivered the test stimulus. Three different configurations of stimuli were used: a single conditioning stimulus, a single test stimulus (producing what is henceforth referred to as the unconditioned response), and paired-stimuli in which the conditioning stimulus preceded the test stimulus by 100 ms (producing what is henceforth referred to as the conditioned response). The intensity of the conditioning cortical stimulus was set to produce a silent period of greater than 150 ms during brief (∼2 s) MVCs; mean of 181.1 ± 30.4 ms across the four protocols. For each subject, the same conditioning stimulus intensity (90–100% stimulator output) was used in all experiments. In the additional experiments involving FDI, conditioning stimulus intensities of 80 and 100% stimulator output were used in the two subjects and induced a mean silent period duration of 211.1 ± 25.9 ms. The test stimulus intensity (47–66% stimulator output) was set to match the size of the conditioned MEP during brief unfatigued MVCs to that of the conditioned CMEP (see Cervicomedullary stimulation section for details). Because the range in the size of the conditioned CMEP was narrower than that for the MEP, the MEP could always be matched to the largest CMEP, whereas the reverse was not necessarily true. For protocols with paired TMS, brief MVCs separated by 90 s of rest were performed with various intensities of the test stimulus until the conditioned MEP was matched to the conditioned CMEP. On average, this intensity was near to resting motor threshold as the mean amplitude of the MEP in relaxation was small (0.5 ± 0.6 mV) and potentials were absent in three subjects.
Cervicomedullary stimulation
In two of the four protocols of the main experiment and one of the additional FDI protocols, the TMS test stimulus was replaced by stimulation of the corticospinal tract at the cervicomedullary level with a high-voltage electrical current (100 μs duration, 200–320 mA, model DS7AH, Digitimer Ltd, Welwyn Garden City, UK) passed between adhesive Ag–AgCl electrodes fixed to the skin over the mastoid processes (Ugawa et al. 1991; Gandevia et al. 1999). To produce the largest possible CMEP, stimulator output was increased until the amplitude of the CMEP evoked in the relaxed muscle reached a plateau or there was a decrease in the latency indicative of activation of the ventral roots of the spinal nerves rather than the corticospinal tract (Taylor & Gandevia, 2004). In the case of a shift in latency, the stimulus intensity was decreased by the minimal amount required to restore the appropriate latency.
Experimental procedures
Protocol A
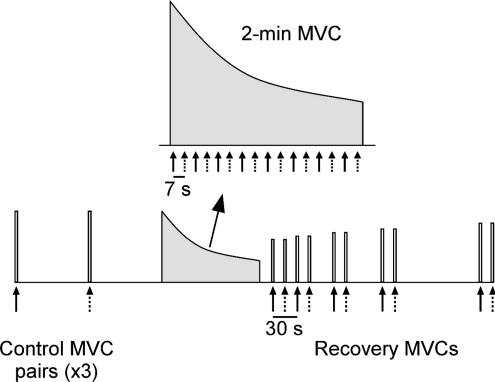
MEPs were evoked by paired stimuli or a single test stimulus during and following a sustained 2 min MVC. Six brief control MVCs were performed to establish unfatigued values for conditioned and unconditioned MEPs as well as long-interval inhibition. Paired stimuli and single test stimuli were delivered on alternate MVCs and 90 s of rest separated each effort (Fig. 1). Following the sixth control MVC, 90 s of rest were provided before the fatigue protocol, a sustained 2 min MVC, was initiated. Strong verbal encouragement and visual feedback of elbow flexion torque were provided throughout the contraction to motivate subjects to maintain maximal effort. Paired stimuli and single test stimuli were alternated at intervals of 7 s for the first six stimuli and at intervals of 8 s thereafter. Thus, the paired stimuli were delivered at 2, 16, 30, 45, 61, 77, 93 and 109 s, whereas the single test stimuli were delivered at 9, 23, 37, 53, 69, 85, 101 and 117 s (Fig. 1). Pairs of brief MVCs, separated by 15 s, were performed to assess recovery of the conditioned and unconditioned MEPs. Paired stimuli were delivered during the first, whereas the single test stimulus was delivered during the second, MVC of each pair. Thus, MVCs with paired stimuli were performed at 15, 45, 90, 150 and 270 s following the end of the sustained contraction and MVCs with a single test stimulus were performed at 30, 60, 105, 165 and 285 s (Fig. 1). Raw traces showing the conditioned MEPs before, during and after the 2 min MVC are displayed for a single subject in Fig. 2 (left panel).
Figure 1. Schematic diagram of the 2 min MVC protocol.
Six brief control MVCs were performed during which paired conditioning–test stimuli (continuous arrows) or a single test stimulus (dotted arrows) were delivered. Paired and single stimuli were delivered alternately at regular intervals during the sustained 2 min MVC. Brief recovery MVCs were performed after the 2 min MVC.
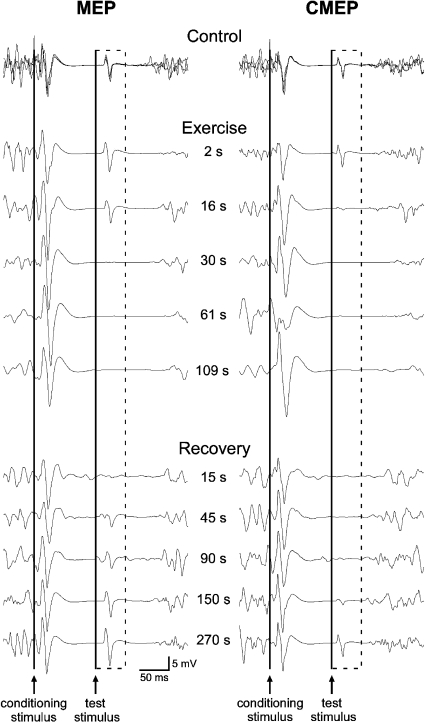
Figure 2. Individual traces of biceps EMG recorded from a single subject in brief control MVCs, a sustained 2 min MVC, and brief recovery MVCs.
Responses obtained during the three brief control MVCs with paired conditioning–test stimulation are overlaid. The time course of stimulation during the 2 min MVC and the recovery period is indicated between the two sets of traces. The dashed box surrounds the conditioned test MEPs (left) and CMEPs (right) evoked in the silent period following the conditioning TMS stimulus. The continuous vertical lines indicate the timing of the conditioning and test stimuli. For this subject, conditioned MEPs and CMEPs are completely abolished 30 s into the sustained contraction but reappear and progressively return to control size during the brief recovery MVCs.
Protocol B
To differentiate cortical and spinal contributions to the changes in MEPs observed during pilot testing for protocol A, in protocol B electrical cervicomedullary stimulation replaced TMS as the test stimulus. Subjects completed the control, fatigue and recovery procedures as outlined in protocol A. The right panel of Fig. 2 shows data from a single subject for the conditioned CMEPs before, during and after the 2 min MVC.
Protocols C and D
These experiments were conducted to assess if the changes to the MEP and CMEP observed during protocols A and B were affected by the duration of the fatiguing contraction. Procedures were identical to protocols A and B except that the sustained MVC lasted only 10 s rather than 2 min. A single test stimulus and paired stimuli were delivered at 2 and 9 s of contraction, respectively.
Additional experiments
The first additional experiment was designed to record changes to the silent period during and following a sustained 2 min MVC. The design was the same as protocols A and B in all respects except that single conditioning stimuli were delivered in place of the paired and single test stimuli.
The second additional experiment assessed if the findings of protocols A and B were reproducible in FDI, the muscle most commonly investigated in TMS studies. Subjects completed protocols A and B but performed a sustained 2 min MVC of index finger abduction rather than elbow flexion.
Data analysis, statistics and terminology
During off-line analysis, Signal software (version 3.05; Cambridge Electronic Design, Cambridge, UK) was used to determine all measures. Mean torque was calculated over 100 ms (in the interval 250 to 150 ms prior to the test stimulus). Non-fatigued MVC torque was calculated as the mean value of the six control contractions. Duration of the silent period was determined as the time from conditioning stimulus to the return of voluntary EMG. The areas of MEPs and CMEPs were measured between cursors marking the initial deflection from the baseline to the second crossing of the horizontal axis (Martin et al. 2006a). Long-interval inhibition was calculated as the ratio (expressed as a percentage) between the area of a conditioned response and the area of the next unconditioned response [conditioned/unconditioned × 100]. The control level of inhibition was obtained using the mean values of the three conditioned and unconditioned responses.
Univariate ANOVAs were used to compare non-fatigued control MVC and silent period data across protocols A–D (SPSS version 15.0; SPSS Inc., Chicago, IL, USA). Student's t test for paired samples was used to compare the area and amplitude of conditioned versus resting MEPs and conditioned versus resting CMEPs (data pooled across protocols A and C for MEPs and protocols B and D for CMEPs). Univariate ANOVAs were also used to compare torque at the end of exercise and the end of the recovery period across protocols of the same duration. Two-way repeated measures ANOVAs, with time as one factor and protocol as the other, were used to compare unconditioned and conditioned responses and the inhibition ratio (each normalised to the control value) across protocols of the same duration. For each protocol, a one-way repeated measures ANOVA was used to assess a main effect for time, and the t-statistics of paired-samples t tests were compared to a two-tailed Dunnett's table to determine which time points were different from the control value. Exercise and recovery data were assessed separately. The recovery of conditioned CMEPs (normalised to the control value) was compared across protocols B and D with a two-way repeated measures ANOVA, and Tukey's post hoc test was performed to indicate at which time points significant differences occurred. Given the sample size of only two subjects, statistical tests were not performed on data collected from FDI during the second additional experiment. All data are reported in the text as means ±s.d. The significance level was P < 0.05.
Note, in the text we have often used the term ‘long-interval inhibition’ when the conditioned response is smaller than the unconditioned response. Others have termed this reduction ‘long-interval intracortical inhibition’ (e.g. Di Lazzaro et al. 2002; Chen, 2004; Benwell et al. 2007; Reis et al. 2008), but our results suggest that a critical site for the ‘inhibition’ is at a spinal level. In the Discussion we consider whether any ‘inhibition’ represents, in part, a disfacilitation of motoneurones.
Results
Control measures prior to fatigue
Maximal voluntary torque (MVC) was similar across the four protocols of the main experiment. The group mean across all sessions was 62.6 ± 10.4 N m (range of 61.6 ± 10.7 to 63.5 ± 10.0 N m for individual sessions; mean ±s.d.). In the first additional experiment, duration of the silent period was 187.1 ± 27.5 ms (Fig. 3). The areas of MEPs and CMEPs during control MVCs were similar across protocols. Conditioned and unconditioned MEPs were 4.4 ± 1.4 and 17.0 ± 5.2 mV, respectively whereas conditioned and unconditioned CMEPs were 3.8 ± 1.7 and 13.4 ± 4.3 mV, respectively. Thus, the control level of long-interval inhibition was similar across visits, with the conditioned MEPs and CMEPs being ∼25% of the area of the unconditioned responses (Fig. 4C and F). Compared to conditioned responses in control MVCs, MEPs recorded during relaxation were markedly smaller in area (0.003 ± 0.003 mV s; P < 0.001) and amplitude (0.5 ± 0.6 mV; P < 0.001) whereas CMEPs had an equivalent area (0.014 ± 0.010 mV s; P= 0.836) but a smaller amplitude (2.4 ± 1.5 mV; P= 0.034).
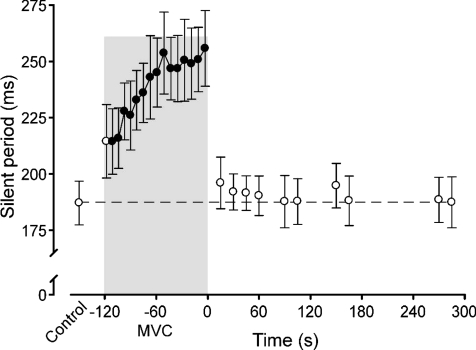
Figure 3. Duration of the silent period produced by a single motor cortical stimulus in brief control MVCs, a sustained 2 min MVC, and brief recovery MVCs.
Data are mean durations (±s.e.m.) and filled circles indicate data points that are significantly different from the control value (P < 0.05). The shaded box indicates the sustained MVC and time ‘zero’ corresponds to the beginning of the recovery period.
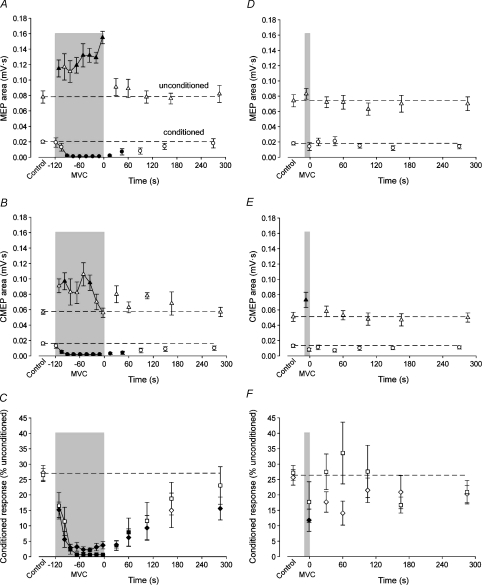
Figure 4. Test responses and inhibition ratios obtained in brief control MVCs, a sustained 2 min or 10 s MVC, and brief recovery MVCs.
Data are mean values (±s.e.m.) for unconditioned (▵) and conditioned (ô) test responses as well as the MEP (□) and CMEP (◊) inhibition ratios. Filled symbols indicate data points that are significantly different from the control value (P < 0.05). Data from the sustained 2 min and 10 s MVCs are in the left and right panels, respectively. In each panel, the shaded box indicates the sustained MVC and time ‘zero’ corresponds to the beginning of the recovery period. A, area of the unconditioned and conditioned MEPs of the 2 min MVC protocol. B, area of the unconditioned and conditioned CMEPs of the 2 min MVC protocol. C, comparison of the MEP and CMEP inhibition ratios of the 2 min MVC protocols. Ratios (expressed as a percentage) are calculated as the conditioned response divided by the subsequent unconditioned response. D, area of the unconditioned and conditioned MEPs of the 10 s MVC protocol. E, area of the unconditioned and conditioned CMEPs of the 10 s MVC protocol. F, comparison of the MEP and CMEP inhibition ratios of the 10 s MVC protocols.
Fatigue and recovery measures
Fatigue and recovery of torque, expressed as the percentage reduction from the control MVC, were similar across the MEP and CMEP protocols for both contraction durations. At the end of exercise, the mean reductions for the 2 min and 10 s protocols were 68.9 ± 7.8% and 14.3 ± 6.5%, respectively. At the end of the recovery period, mean torque was 20.6 ± 8.1% and 9.2 ± 6.4% less than the control MVC for the 2 min and 10 s protocols, respectively. In the first additional experiment, when only the conditioning cortical stimulus was delivered, the silent period increased linearly from the control value of 181.1 ± 30.4 ms to 255.8 ± 47.5 ms just prior to the end of the 2 min MVC (Fig. 3). The silent period recovered rapidly to the control level (within 15 s; 196.0 ± 32.6 ms).
MEP versus CMEP responses
MEPs and CMEPs behaved in the same way in the matching protocols. In a comparison across the 2 min protocols, normalised unconditioned and conditioned responses and the inhibition ratio were all similar during exercise (P= 0.282, P= 0.929, P= 0.980, respectively) and recovery (P= 0.417, P= 0.677, P= 0.439, respectively; Fig. 4A–C). Similarly, in a comparison across the 10 s protocols, there were no differences in normalised unconditioned or conditioned responses or the inhibition ratio during exercise (P= 0.064, P= 0.815, P= 0.592, respectively) or recovery (P= 0.375, P= 0.432, P= 0.233, respectively; Fig. 4D–F).
MEP changes during and following a 2 min MVC
The area of the unconditioned MEP increased progressively during the fatiguing contraction (F8,56= 5.96; P < 0.001; Fig. 4A). In contrast, as shown for a single subject, the conditioned MEP decreased rapidly and was virtually abolished within 30 s after the start of the maximal effort (Fig. 2, left panel). Data for the group are shown in Fig. 4A (F8,56= 11.01; P < 0.001). As a consequence of the divergent behaviour of conditioned and unconditioned responses during fatigue, the ratio of the conditioned to unconditioned MEP (given as a percentage) quickly decreased, such that it was only 2.4 ± 4.5% within the first 40 s of sustained contraction and less than 1% thereafter (F8,56= 18.91; P < 0.001; Fig. 4C). After the 2 min MVC, the unconditioned MEP was statistically similar to control within 30 s, whereas the conditioned MEP required 90 s to recover (Fig. 4A). In line with the recovery of the conditioned response, the MEP ratio was not different from control when assessed ∼105 s after the end of the 2 min MVC (Fig. 4C).
CMEP changes during and following a 2 min MVC
To determine if spinal mechanisms contributed to the profound reduction in the conditioned MEP, a test stimulus was delivered at the level of the mastoids to activate the corticospinal tract and enable production of a subcortical conditioned response. Data for a single subject are shown in Fig. 2 (right panel). The unconditioned CMEP had a rapid increase at the onset of exercise but rather than continuing to increase throughout the fatiguing contraction as the unconditioned MEP, the CMEP returned to the control level in the final 30 s (F8,56= 3.88; P= 0.001; Fig. 4B). Similar to the conditioned MEP, the conditioned CMEP area decreased rapidly and was virtually abolished within 30 s of the start of the sustained MVC (F8,56= 24.92; P < 0.001; Fig. 4B). Again, the ratio of conditioned to unconditioned CMEP (expressed as a percentage) quickly decreased to ∼3% within the first 40 s of contraction (F8,56= 42.82; P < 0.001; Fig. 4C). After the 2 min MVC, the unconditioned CMEP did not deviate significantly from the control value, but the conditioned CMEP remained smaller than control values until ∼90 s (Fig. 4B). The CMEP ratio was not different from the control value at 150 s (Fig. 4C).
MEP changes during and following a 10 s MVC
Neither the conditioned nor unconditioned MEPs deviated from the control values at any point during this protocol (Fig. 4D). Although the ratio of the conditioned to unconditioned MEP dropped slightly during the contraction and increased in the initial stages of recovery, these changes were not statistically different from the control value due to large variability among subjects (Fig. 4F).
CMEP changes during and following a 10 s MVC
The unconditioned CMEP increased during exercise, whereas the conditioned CMEP was unchanged from the control value (Fig. 4E). Despite the maintenance of the conditioned CMEP, the ratio between the two responses was reduced during the brief contraction (Fig. 4F). Both conditioned and unconditioned responses (Fig. 4E) as well as the ratio between the two (Fig. 4F) were similar to control values throughout recovery.
Additional experiment in FDI
In two subjects, prior to fatigue, conditioned and unconditioned MEPs were 6.5 ± 0.2 and 6.9 ± 1.4 mV, respectively, whereas conditioned and unconditioned CMEPs were 5.3 ± 1.4 and 13.3 ± 4.5 mV, respectively. Thus, the control level of long-interval inhibition was dissimilar for MEPs and CMEPs as the conditioned responses were 97% and 41% of the amplitude of the unconditioned responses, respectively. Despite less baseline inhibition, the same pattern of a rapid and equivalent decrease of the conditioned MEPs and CMEPs that was observed in biceps during the main experiment also occurred in FDI. Figure 5 plots the conditioned MEP and CMEP responses recorded from biceps (Fig. 5A) and FDI (Fig. 5B) in the one subject to perform the experiment with each muscle. In both subjects, the ratio of conditioned to unconditioned MEPs and CMEPs decreased to less than 2% within 30 s and had minimal recovery.
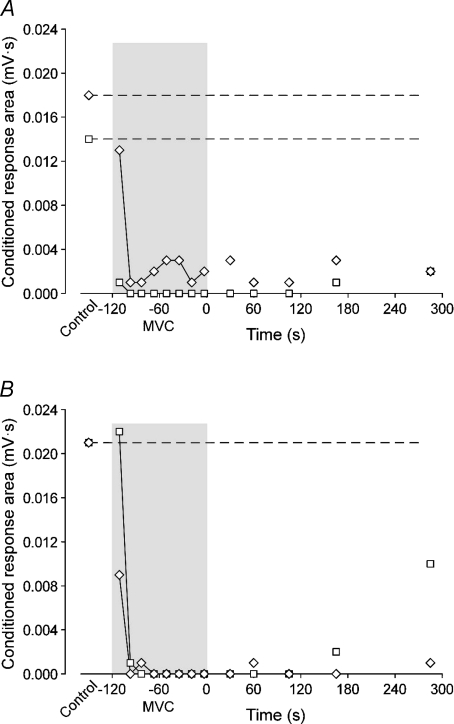
Figure 5. Comparison of conditioned responses from biceps brachii and first dorsal interosseous during fatigue in a single subject.
Data are areas for the conditioned MEPs (□) and CMEPs (◊) of the 2 min MVC protocol performed with the elbow flexors (A) or first dorsal interosseous (B). For this subject, conditioned MEPs and CMEPs are abolished 16 s into the sustained maximal contraction irrespective of the muscle involved. Amplitudes of the control responses in the biceps brachii were 3.8 and 5.1 mV for the MEP and CMEP, respectively, whereas control responses in the FDI were 6.7 and 6.3 mV for the MEP and CMEP, respectively.
Discussion
In accordance with the hypothesis that cortical inhibition increases during a fatiguing maximal contraction, the long-interval inhibition of the test MEP in biceps increased during a 2 min MVC of the elbow flexors. This resulted in abolition of the response to the second of a pair of motor cortical stimuli. The phenomenon was reproduced in an intrinsic hand muscle in two subjects. Contrary to the hypothesis, the same level of inhibition was obtained when the test stimulus was delivered to the corticospinal tract rather than to the motor cortex. Hence, in contrast to the label ‘long-interval intracortical inhibition’, the effects of the conditioning TMS on a test stimulus delivered during the silent period during maximal effort occur predominantly at the spinal motoneurones in both non-fatigued and fatigued muscle states. The abolition of the conditioned CMEP during fatigue implies that spinal mechanisms profoundly decrease the responsiveness of motoneurones to descending input and are likely to contribute to the development of central fatigue. Moreover, although GABAB receptors are implicated in the silent period and long-interval inhibition (Werhahn et al. 1999), two of our findings support the proposal (Berardelli et al. 1996; Benwell et al. 2007) that different mechanisms underlie the silent period and long-interval inhibition. First, MEP inhibition during a maximal effort is mediated primarily at a spinal level and second, after fatiguing exercise, the silent period recovers within 15 s whereas the conditioned MEP requires ∼90 s to return to its control value.
Paired-stimulus inhibition during a non-fatigued MVC
The silent period induced by the high-intensity conditioning TMS during a MVC demonstrates an interruption of the descending voluntary drive from the cortex (Day et al. 1989; Fuhr et al. 1991; Inghilleri et al. 1993) and thus the silent period could be considered akin to artificial ‘relaxation’. If so, the conditioned CMEP and MEP responses may be comparable to unconditioned responses in a relaxed muscle. For both the CMEP and MEP recorded from the biceps, the control conditioned response in the silent period is ∼75% smaller than the control unconditioned response during a MVC. However, the CMEP and MEP vary markedly when the conditioned responses in the silent period are expressed relative to values obtained in the relaxed muscle. The control conditioned CMEP area is equivalent to that in relaxation, whereas the area of the control conditioned MEP is several-fold greater than the MEP recorded from a relaxed muscle. Thus, 100 ms into the silent period, the biceps motoneurones are near to a resting state, but the motor cortex is strongly facilitated compared to rest. This facilitation of the cortex occurs despite the inability of volition to generate motor cortical output as signified by the EMG silence. The disparate behaviour of the evoked and voluntary activity suggests that the TMS input to the corticospinal neurones may be separate from the voluntary input to them (cf. Butler et al. 2007).
With muscles at rest or during weak contraction, TMS-evoked corticospinal volleys recorded with epidural electrodes are suppressed by a stimulus that precedes them by 100–200 ms (Chen et al. 1999; Di Lazzaro et al. 2002). Comparison of the descending volleys and MEPs suggested a spinal suppression of the conditioned MEP at an interstimulus interval of 50 ms but cortical suppression at longer intervals. These authors concluded that a spinal contribution to suppression of the MEP at an interstimulus interval of 100–200 ms was unlikely because H-reflexes in the silent period were not reduced in size relative to those measured in relaxed muscle (Fuhr et al. 1991; Roick et al. 1993; Uncini et al. 1993; Ziemann et al. 1993). Studies of long-interval intracortical inhibition during moderate voluntary contractions did not assess the possibility of a spinal contribution to the suppression of the MEP (Wassermann et al. 1996; Wu et al. 2000; Hammond & Vallence, 2007). However, in the present study, the equivalent attenuation of the biceps conditioned MEP and CMEP demonstrates that, during maximal efforts, altered excitability at a spinal level is largely responsible for the decreased test MEP at an interstimulus interval of 100 ms. As the biceps conditioned CMEP is only modestly greater than that in a relaxed muscle, it is likely that the similar reduction in both the conditioned CMEP and MEP in the silent period represents primarily the disfacilitation of the motoneurones by the withdrawal of descending drive. It remains possible that there is additional cortical suppression of the TMS-evoked output, but this is not evident in the comparison of the CMEP and MEP. A cortical component to long-interval inhibition tested with a high intensity conditioning stimulus during maximal efforts is even less likely in FDI as there was minimal attenuation of the conditioned MEP but the conditioned CMEP was only 40% the size of the unconditioned CMEP. In fact, the preservation of the conditioned MEP suggests a net excitation of the motor cortex which largely overcomes a reduction in spinal excitability.
Paired-stimulus inhibition during fatigue and recovery
Both the conditioned MEP and CMEP decreased rapidly within 16–30 s of exercise and were virtually abolished for the remainder of the contraction. During the fatiguing MVC, the duration of the silent period increased and the unconditioned MEP increased (Gandevia et al. 1996; Taylor et al. 1996, 2000, see also McKay et al. 1996; Benwell et al. 2007). As expected, the unconditioned CMEP increased initially then returned to control size (Butler et al. 2003; Martin et al. 2006b). The continued increase in the unconditioned MEP despite the return of the unconditioned CMEP to the control value suggests that the increase in the MEP represents an increase in excitatory output evoked from the cortex and that the multiple volleys evoked by TMS were sufficient to overcome the spinal mechanisms responsible for the late decrease in the CMEP (see the following section for details). The ratios of the conditioned to unconditioned responses mirrored the profound suppression of the conditioned response. Given that the conditioned CMEP was abolished during the fatiguing contraction, it was difficult to observe whether fatigue also increased intracortical inhibition. The slightly greater reduction of the inhibition ratio for the MEP versus CMEP (Fig. 4C) suggests that cortical inhibition may increase during a sustained 2 min MVC, but this remains uncertain.
Regardless of the cortical contribution, the profound increase in long-interval inhibition contrasts with the only previous investigation of muscle fatigue in which inhibition was reduced when subjects performed brief (7 s), intermittent MVCs of the first dorsal interosseous for 10 min (Benwell et al. 2007). Methodological differences are likely to explain the discrepancy. Most importantly, the previous study assessed long-interval inhibition each minute with stimulation delivered during relaxation 7 s after a brief MVC. The marked inhibition at the motoneurones observed in the current study emphasises that the state of the muscle is critical to the assessment of long-interval inhibition. Indeed, the identical inhibition of the control conditioned MEP and CMEP suggests that, during a MVC of non-fatigued muscle, there is no cortical component to long-interval inhibition of the MEP. Thus, it is likely that any reduction in long-interval inhibition measured at rest (Benwell et al. 2007) is not relevant to the behaviour of the motor cortex during a fatiguing MVC. In contrast, the lengthening of the silent period during fatigue is a robust finding. Furthermore, its quick recovery after the sustained MVC when CMEPs continue to be depressed is consistent with a cortical rather than spinal origin for the increase in its duration (Taylor et al. 1996).
Motoneurone behaviour during fatigue
The behaviour of the unconditioned and the conditioned CMEP is consistent with increased ‘inhibition’ at a spinal level during a sustained maximal effort. Although the final unconditioned CMEP in the 2 min MVC was equivalent to control, the similarity of the data to those in our previous studies using identical contractions (Butler et al. 2003; Martin et al. 2006b) suggests that the CMEP is reduced compared to the maximal M wave, which increases during a 2 min MVC (Taylor et al. 1999; Butler et al. 2003; Martin et al. 2006b). Butler et al. (2003) concluded that this decrease in the CMEP during fatigue was not caused by reduced efficacy of the corticomotoneuronal synapse (Gandevia et al. 1999; Petersen et al. 2003) and a role of group III and IV muscle afferents was also excluded. However, changes to other reflex pathways, particularly disfacilitation via reduced muscle spindle discharge (Macefield et al. 1991), and changes to intrinsic motoneuronal properties occur (e.g. Kernell & Monster, 1982; Spielmann et al. 1993; Sawczuk et al. 1997). We propose that a combination of the two mechanisms causes the suppression of an unconditioned CMEP during fatigue, but only spindle-mediated disfacilitation contributes to the additional suppression of the conditioned CMEP during the silent period.
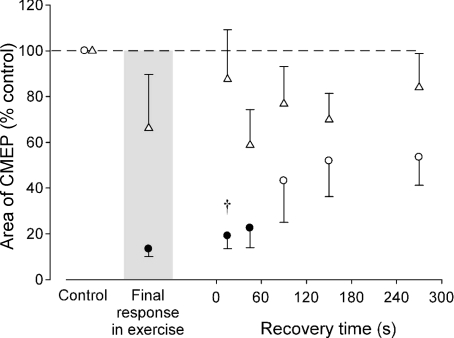
During fatiguing contractions, muscle spindle discharge decreases (Macefield et al. 1991) and muscle relaxation slows (e.g. Bigland-Ritchie et al. 1983). Moreover, muscle relaxation slows during the silent period in a 2 min MVC (Todd et al. 2005, 2007). This further decrease in spindle discharge will disfacilitate the motoneurones. Given the conditioning–test interval of 100 ms, motoneurone adaptation is unlikely to contribute to the greater fatigue-related impairment of the conditioned compared with unconditioned CMEP because 100 ms is much longer than the time between motoneurone firings. The interdischarge interval is ∼50 ms (biceps motor unit discharge of ∼20 Hz) at the end of a 20 s elbow flexor MVC (Bigland-Ritchie et al. 1986), a time when the conditioned CMEP was maximally suppressed in the present study. The interstimulus interval of 100 ms also excludes Renshaw cell inhibition, which is over within ∼30 ms (Bussel & Pierrot-Deseilligny, 1977). Finally, reduced efficacy of transmission at the corticomotoneuronal synapse can also be excluded because its recovery time is independent of contraction duration (Gandevia et al. 1999). In the present study, the conditioned CMEP required between 45 and 90 s to recover following the 2 min MVC but was minimally affected by the 10 s MVC (Fig. 6).
Figure 6. Comparison of conditioned CMEPs in the recovery period following sustained MVCs of different duration.
Data are mean areas (±s.e.m.) normalised to the values recorded during brief control MVCs prior to a sustained 2 min (ô) and 10 s (▵) MVC. Filled symbols indicate data points that are significantly different from the control value (P < 0.05). Normalised conditioned CMEP area was significantly smaller 15 s following the 2 min compared to 10 s MVC (†P < 0.05).
To summarise, we propose that the responsiveness of motoneurones to descending input decreases during a fatiguing maximal effort through altered motoneurone properties and through disfacilitation due to decreased muscle spindle discharge. In the silent period during a maximal fatiguing effort, the CMEP may be further depressed because of a further reduction in spindle input. Whatever the mechanisms, greater suppression of the conditioned compared to unconditioned CMEPs indicates that fatigue-related changes are ameliorated by continuous voluntary drive or enhanced by the synchronous conditioning stimulus.
In conclusion, during an unfatigued MVC, high-intensity conditioning stimulation of the motor cortex inhibits voluntary input to corticospinal neurones but not cortical output evoked by cortical stimulation. Similar reductions in conditioned MEPs and CMEPs compared to their unconditioned counterparts suggest that both responses are decreased due to the withdrawal of voluntary drive from the motoneurones. As fatigue develops, suppression of both conditioned MEPs and CMEPs increases due to spinal mechanisms. A fatigue-induced lengthening of the silent period suggests a concomitant increase in intracortical inhibition, but evidence of any long-interval intracortical inhibition is limited by the profound changes in excitability at a spinal level. These changes at a spinal level are likely to contribute to the decline in motor unit firing rates in fatigue and to the progressive reduction of voluntary drive (i.e. central fatigue) which develops during fatiguing maximal contractions.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and the Natural Sciences and Engineering Research Council of Canada.
Glossary
Abbreviations
- CMEP
cervicomedullary motor evoked potential
- FDI
first dorsal interosseous
- MEP
motor evoked potential
- MVC
maximal voluntary contraction
- TMS
transcranial magnetic stimulation
Author contributions
Each author contributed to all aspects of the study. All experiments were performed at the Prince of Wales Medical Research Institute in Sydney, Australia.
References
- Benwell NM, Mastaglia FL, Thickbroom GW. Differential changes in long-interval intracortical inhibition and silent period duration during fatiguing hand exercise. Exp Brain Res. 2007;179:255–262. doi: 10.1007/s00221-006-0790-2. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rona S, Inghilleri M, Manfredi M. Cortical inhibition in Parkinson's disease. A study with paired magnetic stimulation. Brain. 1996;119:71–77. doi: 10.1093/brain/119.1.71. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol. 1983;50:313–324. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussel B, Pierrot-Deseilligny E. Inhibition of human motoneurons, probably of Renshaw origin, elicited by an orthodromic motor discharge. J Physiol. 1977;269:319–339. doi: 10.1113/jphysiol.1977.sp011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Larsen TS, Gandevia SC, Petersen NT. The nature of corticospinal paths driving human motoneurones during voluntary contractions. J Physiol. 2007;584:651–659. doi: 10.1113/jphysiol.2007.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23:10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Maertens de Noordhout A, Nakashima K, Shannon K, Marsden CD. Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain. Brain. 1989;112:649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, Visocchi M, Colosimo C, Tonali PA, Rothwell JC. Direct demonstration of long latency cortico-cortical inhibition in normal subjects and in a patient with vascular parkinsonism. Clin Neurophysiol. 2002;113:1673–1679. doi: 10.1016/s1388-2457(02)00264-x. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol. 1999;521:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G, Vallence AM. Modulation of long-interval intracortical inhibition and the silent period by voluntary contraction. Brain Res. 2007;1158:63–70. doi: 10.1016/j.brainres.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Time course and properties of late adaptation in spinal motoneurones of the cat. Exp Brain Res. 1982;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Macefield G, Hagbarth KE, Gorman R, Gandevia SC, Burke D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Gandevia SC, Taylor JL. Output of human motoneuron pools to corticospinal inputs during voluntary contractions. J Neurophysiol. 2006a;95:3512–3518. doi: 10.1152/jn.01230.2005. [DOI] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci. 2006b;26:4796–4802. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay WB, Stokic DS, Sherwood AM, Vrbova G, Dimitrijevic MR. Effect of fatiguing maximal voluntary contraction on excitatory and inhibitory responses elicited by transcranial magnetic motor cortex stimulation. Muscle Nerve. 1996;19:1017–1024. doi: 10.1002/mus.880190803. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Butler JE, Gandevia SC. Depression of activity in the corticospinal pathway during human motor behaviour after strong voluntary contractions. J Neurosci. 2003;23:7974–7980. doi: 10.1523/JNEUROSCI.23-22-07974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roick H, von Giesen HJ, Benecke R. On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Exp Brain Res. 1993;94:489–498. doi: 10.1007/BF00230207. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Contribution of outward currents to spike-frequency adaptation in hypoglossal motoneurons of the rat. J Neurophysiol. 1997;78:2246–2253. doi: 10.1152/jn.1997.78.5.2246. [DOI] [PubMed] [Google Scholar]
- Spielmann JM, Laouris Y, Nordstrom MA, Robinson GA, Reinking RM, Stuart DG. Adaptation of cat motoneurons to sustained and intermittent extracellular activation. J Physiol. 1993;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol. 2000;89:305–313. doi: 10.1152/jappl.2000.89.1.305. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490:519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Gandevia SC. Altered responses of human elbow flexors to peripheral-nerve and cortical stimulation during a sustained maximal voluntary contraction. Exp Brain Res. 1999;127:108–115. doi: 10.1007/s002210050779. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol. 2004;96:1496–1503. doi: 10.1152/japplphysiol.01116.2003. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol. 2008;104:542–550. doi: 10.1152/japplphysiol.01053.2007. [DOI] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563:621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Butler JE, Martin PG, Gorman RB, Gandevia SC. Use of motor cortex stimulation to measure simultaneously the changes in dynamic muscle properties and voluntary activation in human muscles. J Appl Physiol. 2007;102:1756–1766. doi: 10.1152/japplphysiol.00962.2006. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Uncini A, Treviso M, Di Muzio A, Simone P, Pullman S. Physiological basis of voluntary activity inhibition induced by transcranial cortical stimulation. Electroencephalogr Clin Neurophysiol. 1993;89:211–220. doi: 10.1016/0168-5597(93)90098-a. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Sommer M, Tergau F, Paulus W. Modification of the silent period by double transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:1868–1872. doi: 10.1016/s1388-2457(00)00426-0. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Netz J, Szelenyi A, Homberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett. 1993;156:167–171. doi: 10.1016/0304-3940(93)90464-v. [DOI] [PubMed] [Google Scholar]