Abstract
Differential A-fibre block of human peripheral nerves changes the sensation evoked by innocuous cooling (∼24°C) of the skin from ‘cold’ to ‘hot’ or ‘burning’, and this has been attributed to activity in unidentified unmyelinated fibres that is normally masked or inhibited by activity in Aδ cold fibres. Application of the TRPM8 agonist menthol to the skin evokes ‘burning/stinging’ as well as ‘cold’, and the unpleasant sensations are also enhanced by A-fibre block. In this study we used microneurography to search for C fibres in human skin activated by cooling and menthol, which could be responsible for these phenomena. Afferent C fibres were classified by activity-dependent slowing as Type 1A (polymodal nociceptor), Type 1B (mechanically insensitive nociceptor) or Type 2 (cold sensitive), and their responses to heating and cooling ramps were measured before and after topical application of menthol preparations (2–50%). The only C fibres activated by menthol were the Type 2 fibres, which discharged vigorously with innocuous cooling and were strongly activated and sensitized to cooling by menthol. Unlike an Aδ cold fibre, they continued to discharge at skin temperatures down to 0°C, and most (13/15) were also activated by heating. We propose that the Type 2 C fibres, although resembling Aδ cold fibres in their responses to innocuous cooling and menthol, have a more complex sensory function, colouring with a ‘hot-burning’ quality the perceptions of low and high temperatures. Their bimodal thermoreceptive properties may help account for several puzzling psychophysical phenomena, such as ‘innocuous cold nociception’, ‘paradoxical heat’ and the thermal grill illusion, and also for some neuropathic pains.
Introduction
The first molecular studies on cold thermoreception implicated the menthol receptor TRPM8 in innocuous cold sensation and the mustard oil and cinnamaldehyde-sensitive receptor TRPA1 in cold-induced pain (McKemy et al. 2002; Reid, 2005; Foulkes & Wood, 2007) but it is now clear that menthol can also evoke unpleasant sensations. In human studies, Wasner et al. (2004) showed that 40% menthol induces spontaneous burning pain and cold hyperalgesia, which was enhanced during an A-fibre block that abolished cold sensation. They also reported a flare reaction to menthol, suggesting activation of the C nociceptors responsible for axon reflex vasodilatation. However, a subsequent study by Namer et al. (2005), while confirming that menthol induced cold hyperalgesia, found that it evoked only local vasodilatation and not an extended axon reflex flare, in contrast to the TRPA1 agonist cinnamaldehyde. These studies indicate that there is a class of C afferent that evokes unpleasant sensations that is activated by menthol, but which class is unclear.
In our previous microneurographic studies of human cutaneous sensation, we have described (a) a class of polymodal C nociceptor that is activated by cooling below about 19°C (Campero et al. 1996), i.e. CMHC units, and (b) a class of C fibre that is activated by innocuous cooling, and behaves as expected for specific cold receptors (Campero et al. 2001). The latter units are readily distinguished from all other C afferents by their ‘Type 2’ pattern of activity-dependent slowing, i.e. when the stimulation rate is increased from 0.25 to 2 Hz, they reach a latency plateau after 1 min, corresponding to a slowing of conduction velocity that is close to 5% (Serra et al. 1999; Campero et al. 2001; Serra et al. 2004). They also have distinctive velocity recovery cycles (Bostock et al. 2003), which are helpful in recognizing this class of C fibre in rats (George et al. 2007). For convenience, and to avoid any implication of sensory function, we will refer to these ‘Type 2’ C fibres in this text as ‘C2’ fibres. Whereas the sensory function of several types of myelinated and unmyelinated fibres identified by microneurography has been clarified by intra-neural microstimulation (Ochoa & Torebjörk, 1983; Vallbo et al. 1984; Ochoa & Torebjörk, 1989), this technique has never, so far as we are aware, been reported to evoke the sensation of cold, and the contribution to sensation of the C2 units has never been established. While the C2 receptor properties were similar to those of Aδ specific cold afferents described in mammals (Iggo, 1969; Darian-Smith et al. 1973) their average conduction velocity (CV) of 0.98 m s−1 (Campero et al. 2001) appeared incompatible with the evidence from differential nerve blocks that cold sensation is mediated only by Aδ fibres (Mackenzie et al. 1975). We previously considered two hypotheses: (1) that the C2 fibres recorded in the superficial peroneal nerve at the ankle were the unmyelinated distal portions of non-uniform, incompletely myelinated Aδ specific cold fibres, structurally similar to fibres described in primates by Iggo & Ogawa (1971) and in the cat by Duclaux et al. (1976); or (2) that the C2 fibres do not contribute to cold sensation, perhaps having a purely unconscious role in temperature regulation (Campero et al. 2001). In the light of experiments reported here and in the literature, we will consider in the discussion another hypothesis: that C2 fibres do contribute to sensation, but that this contribution is complex, and quite different from that of the Aδ-cold fibres.
The primary aim of this study was to determine which class or classes of C fibre are activated by cooling and menthol, and are therefore candidates to mediate the burning sensations reported by Wasner et al. (2004). We have found that the only plausible candidates are the C2 fibres, and that these fibres often fire more vigorously on heating than on cooling. These bimodal, thermoreceptive and quasi-nociceptive fibres may provide the key to understanding a number of puzzles in thermal psychophysics, such as the changing quality of sensations evoked by innocuous cooling during differential A-fibre block, the phenomenon described alternatively as ‘synthetic heat’ or the painful thermal grill illusion, the ‘paradoxical heat’ induced by cooling warm spots, and the sensations referred to by Green and Schoen (2005) as ‘low threshold thermal nociception’. A role of these units as symptom generators in neuropathy is predicted.
Methods
Subjects and ethical approval
The results are drawn from 37 recording sessions on 18 adult volunteers, spread over a period of 4 years. There were 11 males and 7 females, with ages ranging from 17 to 52 years (mean 27.3 years) (see Tables 1 and 2). The study had the approval of the Legacy Health System Institutional Review Board (ID no. 1280) and conformed to the Declaration of Helsinki. All subjects gave their informed, written consent.
Table 1.
Summary of results from Type 2 units recorded, including 2 Aδ units
| Unit ID | Subject age/sex | Unit type | CV (m s−1) | 2 Hz (%) | RF | MT (mN) | CT (°C) | HT (°C) | Lat/spike | Menth sens (c/h) | Menth conc (%) | Delay activ (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L2a | 46M | 2 | 0.96 | 6.9 | ss | X | — | — | L/S | — | 50 | 5.6 |
| L2b | 46M | 2 | 0.91 | 4.5 | ss | X | — | — | L | — | 50 | 5.8 |
| L1b | 46M | 2 | 1.31 | 5.1 | ss | X | — | — | L/S | — | 50 | 25.0 |
| C1b | 22M | 2 | 1.50 | 5.7 | ss | X | 30.0 | 36.0 | L | — | 30 | 12.0 |
| N1 | 37F | 2 | 1.50 | — | ss | X | 31.8 | 37.0 | S | y/y | 30 | 3.0 |
| J3 | 22M | 2 | 1.11 | 3.9 | ss | X | 28.5 | 43.0 | S | y/y | 30 | 9.6 |
| D3 | 28M | 2 | 1.1 | 4.2 | ss | X | 29.8 | X | S | y/y | 40 | 1.0 |
| O1 | 24M | 2 | 0.86 | 5.6 | ss | X | 31.0 | 40.6 | S | — | 40 | 9.5 |
| O3 | 24M | 2 | 0.83 | 4.9 | ss | X | 30.1 | 34.4 | S | y/y | 40 | 6.8 |
| I2 | 25F | 2 | 1.01 | 5.8 | ss | X | 35.7 | 41.8 | S | y/y | 40 | 4.1 |
| K2 | 33F | 2 | 0.94 | 3.5 | ss | X | 29.3 | 35.0 | S | — | 40 | X |
| L3 | 48M | 2 | 0.71 | 6.6 | ss | X | 30.0 | 34.0 | L/S | — | 2 | 2.8 |
| J4 | 24M | 2 | 0.92 | 4.8 | ss | X | 29.8 | X | S | y/y | 2 | 6.9 |
| B2 | 27M | 2 | 0.99 | 3.2 | ss | X | — | >43.0 | L/S | — | 2 | 6.8 |
| R1 | 24F | 2 | 0.96 | — | ss | X | 38.1 | 39.5 | S | — | — | — |
| O2 | 24M | 2 | 0.97 | 3.4 | ss | X | 29.9 | 37.5 | S | — | — | — |
| F2b | 18M | 2 | — | — | ss | X | 33.0 | 33.0 | S | — | — | — |
| P1 | 20F | 2 | 1.07 | 3.7 | ss | X | 29.9 | 37.6 | S | — | — | — |
| Q1 | 52M | 2 (Aδ) | 3.80 | 8.4 | ss | X | 31.9 | X | L/S | y/n | 40 | 2.8 |
| J1b | 22M | 2 (Aδ) | 4.40 | 5.2 | ss | X | — | — | L | — | 40 | 17.0 |
Unit ID: letters refer to subjects, numbers designate recording session and small letters represent different fibres. 2 Hz (%), percentage slowing during a 3 min 2 Hz stimulation; RF, receptive field; ss, single spot; MT, mechanical threshold; HT, heat threshold; CT, cold threshold; Lat/spike indicates whether menthol-induced activity was monitored by recording latency to electrical stimulus (L) and/or by counting spontaneous spikes (S); Menth sens, sensitized by menthol; c, to cold; h, to heat; Menthol conc, menthol concentration; Delay activ, delay to discharge following application of menthol; X, no response; ‘–’, not tested or not testable.
Table 2.
Summary of results from all C nociceptor units analysed
| Unit ID | Subject age/sex | Unit type | CV (m s−1) | 2 Hz (%) | Pause (%) | MT (mN) | HT (°C) | CT (°C) | Lat/spike | Menth resp | Menth (%) | Expos (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1a | 25M | 1A | 0.74 | 23.7 | 0.3 | 52.3 | — | X | L | X | 30 | 46 |
| A1b | 25M | 1A | 0.74 | 24.3 | -0.1 | 52.3 | — | X | L | X | 30 | 46 |
| A1c | 25M | 1A | 0.62 | — | 0.7 | 52.3 | — | X | L/S | X | 30 | 46 |
| B1 | 27M | 1A | 1.12 | 12.3 | — | 6.1 | >42.0 | — | L | X | 30 | 20 |
| C1a | 22M | 1A | 1.31 | 22.8 | 1.0 | — | 46.0 | X | L | X | 30 | 26 |
| D1a | 26M | 1A | 0.55 | 33.2 | 0.8 | 52.3 | — | X | L/S | X | 30 | 15 |
| D1b | 26M | 1A | 0.53 | 28.6 | 0.5 | 107.8 | 49.0 | X | L/S | X | 30 | 15 |
| C2a | 22M | 1A | 0.71 | 30.3 | 0.9 | 52.3 | 40.0 | X | L/S | X | 30 | 12 |
| E1 | 25M | 1A | 0.68 | 42.6 | 0.3 | 52.3 | 40.0 | X | L/S | X | 30 | 14 |
| C3 | 22M | 1A | 0.84 | 22.4 | 0.7 | 52.3 | — | X | L/S | X | 30 | 15 |
| A2 | 25M | 1A | 0.65 | 24.7 | 0.1 | 7.89 | 40.0 | X | S | X | 30 | 15 |
| C4a | 22M | 1A | 0.91 | 27.8 | 0.1 | 52.3 | 41.0 | X | L | X | 30 | 16 |
| C4b | 22M | 1A | 0.76 | 31.5 | 1.4 | 52.3 | 39.0 | X | L | X | 30 | 16 |
| C5 | 22M | 1A | 0.42 | — | — | 7.89 | 38.0 | X | L/S | X | 30 | 15 |
| A3a | 25M | 1A | 0.69 | 29.7 | 0.7 | 52.3 | 40.0 | X | L/S | X | 30 | 15 |
| A3b | 25M | 1A | 0.71 | 31.7 | 0.3 | 7.89 | — | X | L | X | 30 | 15 |
| F1 | 17M | 1A | 0.72 | 19.7 | — | 52.3 | 40.0 | 15.0 | S | X | 30 | 15 |
| F2a | 17M | 1A | 1.02 | — | — | 107.8 | 39.0 | 5.0 | S | X | 30 | 15 |
| G1 | 24F | 1A | 0.88 | 23.4 | — | 107.8 | 41.6 | -4.3 | S | X | 30 | 15 |
| H1 | 31M | 1A | 0.39 | — | — | — | 39.7 | 14.2 | S | X | 30 | 15 |
| I1a | 23F | 1A | 0.67 | 26.4 | 0.4 | 67.62 | — | — | L | X | 30 | 20 |
| J1a | 22M | 1A | 1.00 | 17.0 | 1.2 | — | — | — | L/S | X | 40 | 23 |
| D2 | 26M | 1A | 0.89 | 36.4 | — | 52.3 | 39.5 | X | S | X | 40 | 16 |
| K1a | 28F | 1A | 0.42 | 65.0 | 0.9 | 52.3 | — | X | L | X | 40 | 15 |
| D1c | 26M | 1B | 0.26 | 22.4 | 4.9 | X | 46.0 | X | L | X | 30 | 15 |
| C2b | 22M | 1B | 0.20 | 67.7 | 4.6 | X | X | X | L | X | 30 | 12 |
| I1b | 23F | 1B | 0.37 | 24.7 | 4.0 | X | X | X | L | X | 40 | 20 |
| I1c | 23F | 1B | 0.38 | Blk | 3.3 | X | X | X | L | X | 40 | 20 |
| J2 | 22M | 1B | 0.47 | — | 4.7 | X | X | X | L | X | 40 | 20 |
| L1a | 45M | 1B | 0.61 | 35.6 | 3.2 | X | X | X | L | X | 30 | 30 |
| M1 | 28F | 1B | 0.32 | 41.0 | 8.3 | — | 48.0 | X | L | X | 40 | 15 |
| K1b | 28F | 1B | 0.85 | 60.0 | 6.1 | X | X | X | L | X | 40 | 15 |
| K1c | 28F | 1B | 0.30 | 37.9 | 6.8 | X | 48.0 | X | L | X | 40 | 15 |
Unit ID: letters refer to subjects, numbers designate recording session and small letters represent different fibres. 2 Hz (%), percentage slowing during a 3 min 2 Hz stimulation; Pause (%), percentage change in latency during 0.25 Hz stimulation after the 3 min pause; MT, mechanical threshold; HT, heat threshold; CT, cold threshold; Lat/spike indicates whether menthol-induced activity was monitored by recording latency to electrical stimulus (L) and/or by counting spontaneous spikes (S); Menth resp, whether activated or sensitized by menthol; Menth (%), menthol concentration; Expos (min), menthol exposure time; X, no response; ‘—’, not tested; Blk, blocking.
Microneurographic recordings
Microneurography was used to record action potentials of human C fibres from cutaneous nerve fascicles of the superficial peroneal nerve at the ankle. The subjects sat relaxed with the leg firmly supported in a padded platform. Intraneural recordings were obtained using a 0.2 mm diameter lacquer-insulated tungsten microelectrode (MNG active/1 MΩ) FHC Inc., Bowdoinham, ME, USA), which was inserted percutaneously into the nerve. A subcutaneous reference electrode was inserted 1–2 cm away from the nerve trunk. The neural signals were amplified and filtered (band-pass 100–5000 Hz) with an isolated microelectrode amplifier (FHC Inc., Xcell 3+). Line interference was removed with an on-line noise eliminator (HumBug, Quest Scientific, North Vancouver, Canada). Signals were digitised at 20 kHz (PCI-6221 data acquisition board, National Instruments Corporation, Austin, TX, USA) and displayed and recorded by Qtrac software (written by H. Bostock, © University College London), which also controlled electrical and thermal stimulation.
Search for the electrical receptive fields (RF) of C fibres was conducted in areas of skin where intraneural electric microstimulation evoked painful sensations at near threshold levels (Torebjörk and Ochoa, 1990). This area of the skin was stimulated electrically with a pair of needle electrodes resting on the surface of the skin, using rectangular pulses of 0.25–0.3 ms duration (Grass S48, stimulus isolation unit SIU 5) at a rate of 0.25 Hz. Multiple units recorded at the same site could be recognized by the formation of lines on a raster plot of latency against time.
Temperature of the skin was measured with a thermocouple placed on the skin adjacent to the RFs of the units under study. Skin temperature was maintained above 30°C with an infrared lamp. This temperature control was maintained, even when the temperature of the RF was separately controlled by a Peltier device (see below).
Responses to thermal and menthol stimulation were recorded either (1) by stopping electrical stimulation and recording the spontaneous activity in a single, large amplitude unit, or (2) by continuing the stimulation at 0.25 Hz and detecting deviations from the steady-state latency due to the activity-dependent slowing caused by the spontaneous (i.e. not stimulus-evoked) activity, or (3) by stimulating at 0.25 Hz and recording both the stimulus-evoked and spontaneous discharges. On average only 85% of spontaneous action potentials were recorded, since Qtrac only allowed 850 ms sweeps to be recorded every second. Since conduction slowing can be caused either by activity or by cooling the axon, in many cases the responses to cooling could only be determined reliably by counting the spikes in a single, large amplitude unit.
Categorisation of C fibres
Only fibres with latencies compatible with conduction velocities in the C fibre range (<2 m s−1) were studied. When time-locked responses with such latencies were recorded at 0.25 Hz baseline stimulation, a sequence of 3 min pause followed by 6 min baseline and 3 min 2 Hz train was given to enable the types of fibre present to be categorised (Serra et al. 1999). Units which slowed progressively at 2 Hz were classified as Type 1 (nociceptors) and further subdivided according to the effects of the 3 min pause into Type 1A units (mechano-responsive), which were unaffected by the pause, and Type 1B units (mechano-insensitive), which slowed appreciably (by more than 1.5%) at 0.25 Hz following the pause. This ‘pause protocol’ has been found to unambiguously separate mechano-responsive and mechano-insensitive Type 1 units (Serra et al. 2004) in accordance with the findings of Weidner et al. (1999). Units which reached a plateau of slowing at ∼5% within 1 min of stimulation at 2 Hz were activated by cooling and classified as Type 2 units (Campero et al. 2001) or C2 units. The C2 units are similar in some respects to sympathetic efferents (Type 4), which also slow by about 5% at 2 Hz, but these two types of fibre can be distinguished unambiguously by their initial rate of slowing at 2 Hz, as well as by their responses to cooling and sympathetic manoeuvres (Campero et al. 2004). For all sensory units with adequate signal-to-noise to allow spike counting (see below), the typing by repetitive stimulation was then confirmed by natural stimulation of their receptors. In all cases, a mechanical RF was found for Type 1A fibres only, and a cold-sensitive RF found for C2 fibres only. Since the 1A fibres, when tested, were invariably activated by heat as well as mechanical stimuli, we will refer to them by the more common label of CMH units, and the Type 1B C fibres will be referred to as mechanically insensitive afferents (MIAs).
Mapping of receptive fields
The presence of a C2 unit was usually recognized by its spontaneous low firing rate at room temperature. The search for its RF was conducted by first identifying the area where cooling (by an air puff or by shielding from the infrared lamp) resulted in an increase in firing rate. Within this area, a search was made for RFs with a cold (∼2°C) metal rod with a rounded tip (radius ∼2 mm). An RF was recognised by a sudden increase in the firing rate and the spot marked with ink.
The RFs of CMH units were mapped by the ‘marking’ technique, by following changes in latency on the raster plot due to activity-dependent slowing, during electrical stimulation of the skin at 0.25 Hz as described above. The skin around the stimulation site was probed with a calibrated monofilament (176 mN), and every time a unit was excited a burst of action potentials was heard and there was an immediate increase in its latency. The mechanical RFs of CMH units were thus delineated and marked in ink, with a different colour for each unit.
Unlike the C2 and CMH units, which were initially detected by natural stimulation, the MIA units were only revealed by the presence of a unit with the Type 1B pattern of activity-dependent slowing on a raster plot during electrical stimulation. Having confirmed that the unit was mechanically insensitive, by failing to activate it with a strong monofilament (307 mN), a search was made for its electrical RF using additional electrical impulses (0.2 ms width, 20–50 mA) from a second stimulator (Teca TD20) via needle electrodes that were touched to the skin. The electrical RF was mapped as the sites where the extra impulses produced a marked slowing of conduction, similar to the mechanical marking of CMH units.
For each type of unit, menthol was applied to the relevant (thermal, mechanical or electrical) RF, and when more than one unit was being tracked by raster plot during menthol application, a unit was only included in the analysis if the menthol application had covered its RF.
Thermal stimulation
Thermal stimuli were applied to the RFs of selected units by a feedback-controlled Peltier device (Yale Electronic and Machine Shop, Yale Medical School) with an area of 1 cm2. This device provided a measurement of temperature of the skin touching the device, and also allowed the temperature to be controlled by Qtrac from an analog output of the PCI-6221 data acquisition board. The holding temperature of the Peltier device was set to 32°C, and three types of temperature control were built into the Qtrac recording protocol (850RCF.QRP) used in these experiments: (1) square wave temperature pulses, programmed as a series of 5 s warming pulses or 10 s cooling pulses at intervals of 2 min; (2) warming or cooling ramps at a rate of 0.2°C s−1; or (3) warming or cooling staircase ramps, with 2°C steps every 10 s.
Menthol application
An alcohol-soaked swab was cut to size (approx. 10 by 10 mm), dried, soaked with menthol solution (40 or 30% l-menthol in ethanol) and placed on the skin. In some experiments, instead of using the a swab a thin layer of menthol ointment (50%l-menthol in white petrolatum) or gel (2%dl-menthol) (Deep Freeze Cold Gel, Mentholatum Co. Ltd., East Kilbride, Scotland, UK) was spread onto the RF with a cotton-tipped applicator. In each case the menthol preparation was warmed close to skin temperature before application, to minimize thermal stimulation. The ethanol swab had the advantage that pre- and post-menthol temperature response profiles could be compared without the complication of the presence or absence of ointment on the Peltier device. On the other hand, the ethanol swabs caused a temporary cooling while the ethanol evaporated.
Spike identification, counting and waveform analysis
Although a large unit might appear to be easily identified from all others on the basis of amplitude alone, there was always a possibility that an apparent spike occurring during thermal or menthol stimulation might be caused by an electronic artefact, or that it might represent an impulse in a previously silent sympathetic or other fibre. In the case of the responses to heating and cooling temperature ramps, it was particularly important to establish whether the fibre responding to cooling was the same as the fibre responding to warming, and whether it was the same unit as previously typed in a raster plot by repetitive and natural stimulation. To aid spike separation and artefact removal, the analysis program QtracP provides for the use of two separate measurements on each spike (e.g. peak-to-peak height and width at half-height) to determine the position of a point on a peak vs. width or other ‘shape index’ plot. The points for a single unit normally fall within an ellipse on such a plot, and a manually fitted ellipse can be used for excluding artefacts, and to a limited extent other units, from further analysis. An additional check on the identity of a unit in two sections of recording can be made by comparing averaged waveforms of the selected spikes. Thus electrically evoked spikes corresponding to a specific line on a raster plot can be used to define an ellipse on the shape index plot, which can then be used to discriminate spikes evoked by thermal stimulation or by menthol.
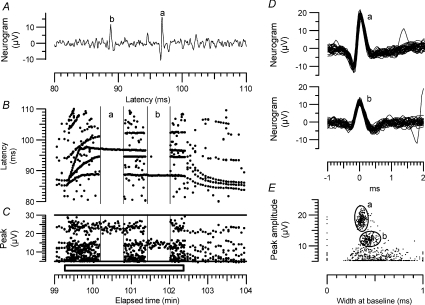
This method is illustrated in Fig. 1. A multi-unit recording is shown in which several units were excited between 80 and 110 ms, with the largest amplitude spikes labelled ‘a’ and ‘b’ (Fig. 1A). During repetitive stimulation at 2 Hz the units showed different patterns of activity-dependent slowing (Fig. 2B), with spike ‘b’ showing the typical Type 2 pattern of C2 units. To help separate this unit from the other, larger and smaller units, its latency was tracked throughout the 5 min period, and the spikes centred on those latencies measured and displayed on a plot of peak amplitude vs. width at baseline. (Width at half height was more normally used, but in this case was less effective than width at baseline for separating unit ‘b’ from other units.) This single-unit plot is not illustrated, but an ellipse fitted to just enclose nearly all the points is shown as ellipse ‘b’ (Fig. 1E), which also shows points corresponding to all the other peaks in the raster plot, and an ellipse fitted to the larger unit ‘a’. To demonstrate the use of the ‘shape ellipses’ to separate the units, the portion of the record between 100.2 and 100.8 min was filtered to exclude all points outside ellipse ‘a’, and the portion between 101.4 and 102 min was filtered to exclude all points outside ellipse ‘b’. The waveforms of all the spikes fitting these two filters are plotted in Fig. 1D, and Fig. 1B and C shows the effects of the filtering on the latency rasters and peak amplitudes. In general, we found that this method of filtering the recordings, by abstracting only spikes of a defined amplitude and shape, was very effective at separating an identified unit from electronic and muscle artefacts, and was more effective at separating action potentials than peak amplitude alone. However, most C units have rather similar shapes, so it is never possible to identify all spontaneous spikes with certainty.
Figure 1. Spike discrimination in multiunit recordings.
A, part of a single sweep in a multi-unit recording following electrical stimulation. The larger spike is labelled ‘a’ and the next largest ‘b’. Sweep recorded at 101 min. B, raster plot showing all peaks above 5 μV in amplitude during 5 min of recording, including a 3 min period in which stimulation rate was increased from 0.25 Hz to 2 Hz (indicated by bar at the bottom). Individual units firing consistently in response to the stimulus give rise to continuous lines in the raster plot. Recording during 36 s periods marked ‘a’ and ‘b’ indicate periods when recording was filtered to select spikes ‘a’ and ‘be respectively. C, peak amplitudes of all points in the raster plot, showing overlap of amplitudes. D, superimposed waveforms of all spikes in the periods marked ‘a’ and ‘b’ in parts B and C. E, plot of peak amplitude vs. width at baseline for all points in the recording, before filtering, showing clustering of points corresponding to the largest fibres. The ellipses ‘a’ and ‘b’ were drawn to fit the distribution of points (not shown) for the lines in the raster plot corresponding to each of the two largest peaks. These ellipses were subsequently used to filter the recording during the two 36 s periods indicated in parts B and C. (N.B. a 4 kHz low-pass filter frequency was used in the original recording, but a 2 kHz cut-off in the re-analysis, which is why the peak amplitudes are larger in C than in D and E).
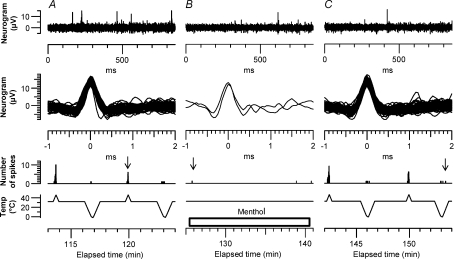
Figure 2. Response of C2 fibre to application of menthol on the skin.
The unit (L2a in Table 1) was stimulated electrically at 0.25 Hz throughout. Top, neurogram, recorded at time indicated by vertical dashed line in the lower plots. S indicates stimulus artefact and R the response to the electrical stimulus. Four other similar spikes are also visible. Second plot, latency of spikes to electrical stimulus. Third plot, number of spikes in sweeps without stimulation (counted as the number with amplitudes greater than the horizontal dashed line in the top plot). Bottom, skin temperature near the receptive field. About 5 min after menthol application, the rate of spontaneous firing increased. The activity-dependent slowing increased the latency to the electrical stimuli, but also disrupted the responses by collision. Warming the skin reduced the menthol-induced firing. Menthol shifts the temperature threshold for cold activation of the C2 fibres to a higher temperature, so that the effect of menthol can be counteracted by warming the skin. (NB Sweep duration was 0.85 s, so that 5 spikes per sweep = 5.88 Hz).
Results
Effects of menthol on C2 units
Table 1 summarizes the results of the recordings from 18 C2 fibres. Thirteen units were activated by menthol, with delays between 1 and 25 min (median 6.8 min) and with no clear relationship between the delay and menthol concentration. In five cases we were able to stimulate the unit at 0.25 Hz throughout this period, while also counting spontaneous spikes. One fibre (K2) was not activated during 16 min of menthol exposure, although it was still functioning at the end of the recording. Four other C2 fibres were treated with menthol but not recordable afterwards. No conclusion about their sensitivity to menthol was possible, but they are included because their baseline sensitivity to cooling and heating ramps was measured and relevant to an understanding of C2 function. In six cases it was possible to record responses to heating ramps both before and after menthol, and in all of these cases there was evidence of sensitization to warming as well as to cooling.
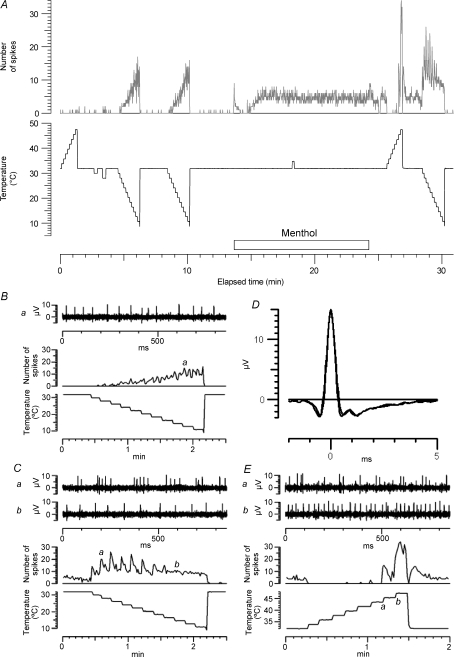
Figure 2 illustrates an experiment in which a single Type 2 unit was much the largest excited by electrical stimulation, and its punctate RF for stimulation with a cold rod was several millimetres away from the stimulating electrodes. We were therefore able to record both the spontaneous and the electrically evoked responses during application of menthol (50% ointment) to its RF. This fibre conducted at 0.96 m s−1, and on stimulation at 2 Hz the latency increased by 6.9%, consistent with previously described Type 2 fibres (Campero et al. 2001, 2004). The fibre was initially firing spontaneously at a low rate (∼1 Hz), but 5.6 min after application of menthol to its RF this rate increased progressively to about 6 Hz. As the spontaneous activity increased, so did the latency, but the latency recording became patchy as the spontaneous action potentials interfered with the electrically excited ones. The spontaneous activity was reduced when the skin was warmed further with the radiant heat lamp. A second, much smaller Type 2 unit (velocity 0.91 m s−1, slowing at 2 Hz: 4.5%) was visible in the recordings (not shown), and exhibited a similar degree of activity-dependent slowing, starting 5.8 min after menthol application. However the signal-to-noise ratio was too low to count the spontaneous spikes from this second unit.
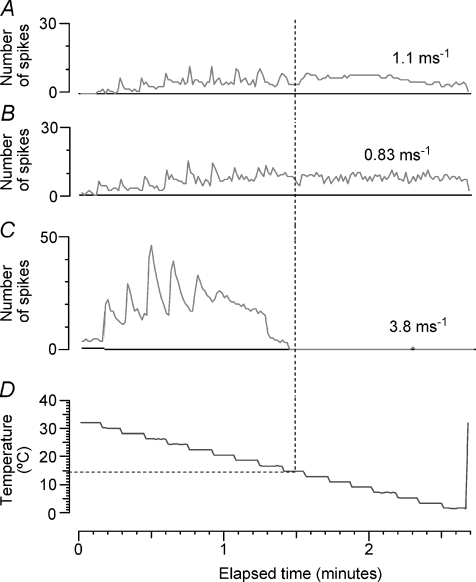
To investigate in more detail the effects of menthol on thermal sensitivity, we also recorded the responses to temperature ramps applied by the Peltier device, before and after menthol application. Figure 3 illustrates such an experiment, carried out on the unit that responded most rapidly to menthol. This C2 unit had a resting CV of 1.1 m s−1, and slowed by 4.2% after 3 min at 2 Hz. Figure 3A shows the time course of the recording, with the number of spikes per 850 ms sweep compared with the temperature of the Peltier device, which was ramped up from the holding temperature of 32 to 48°C, and then down to 10°C, in 2°C steps. Initially the unit responded only to cooling. Application of menthol (40% in ethanol) caused a brief initial discharge, presumably because of direct cooling. Spontaneous activity built up again just over 1 min after menthol application. When the Peltier device was reapplied, the unit was found to be not only more sensitive to cooling, with a vigorous phasic response at each temperature step, but also to be activated by heating, which actually produced a higher firing rate than the cooling. The other parts of the figure illustrate these temperature responses in more detail. Before menthol (Fig. 3B), the firing rate was fairly uniform and increased slowly with lowering temperature. After menthol, the unit fired more phasically, and in rapid bursts, to the first cooling steps (Fig. 3Ca), but more uniformly when the temperature was lowered further (Fig. 3Cb). The unit also fired in bursts when heated to 46°C (Fig. 3Ea), and reached a higher firing rate on warming than cooling (Fig. 3Eb and Fig. 4A(b)). To check that it was the same unit firing on warming as on cooling, averaged waveforms of 400 action potentials evoked by cooling (Fig. 3C) and heating (Fig. 3E) post menthol are superimposed in Fig. 3D.
Figure 3. Response of C2 fibre to temperature ramps and menthol.
A top, time course of changes in firing rate (in spikes per 850 ms sweep); bottom, temperature of Peltier device. B, detail of response to second pre-menthol cooling ramp; top, neurogram at time marked a in middle trace; middle, spikes per sweep; bottom, temperature. C, detail of response to post-menthol cooling ramp; traces as in B, except that two sweeps are illustrated, marked a and b to show changing firing pattern of unit. D, superimposed mean action potential wave forms from post-menthol responses to cooling (C) and heating (E) to confirm identity of unit. E, detail of post-menthol response to heating, traces as in C. This unit was D3 in Table 1.
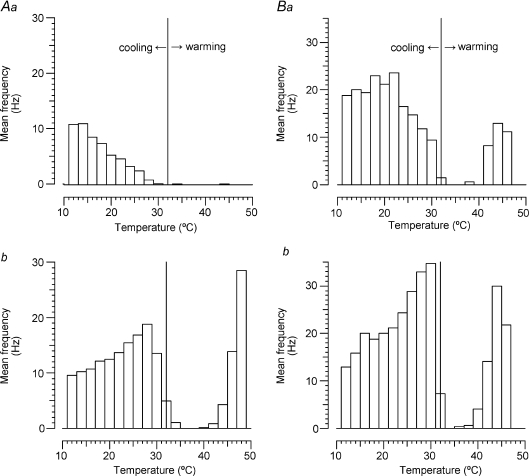
Figure 4. Effects of menthol on temperature responses of two C2 units.
A, mean firing rates of unit D3 illustrated in Fig. 3: a, pre-menthol; b, post-menthol. Vertical lines indicate holding temperature of 32°C. B, similar recordings from a second C2 unit (N1 in Table 1).
Figure 4 summarizes the results of applying temperature ramps to the C2 unit of Fig. 3 (Fig. 4A) and another well-resolved C2 unit in Fig. 4B. Following menthol treatment, each became more spontaneously active at the holding temperature of 32°C and much more sensitive to small cooling steps. Unexpectedly, both units also became more sensitive to heating, although we don't have adequate controls (e.g. by monitoring heat sensitivity at regular intervals) to be sure that this increased sensitivity was due to the menthol.
Additional C2 responses to temperature ramps
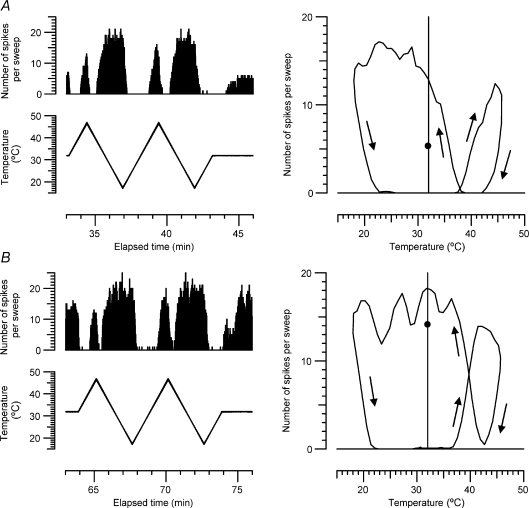
In eight recordings we were able to record responses of a C2 unit to temperature ramps before applying menthol, but the unit was lost or inactivated during or after the menthol application. Three of these pre-menthol responses are illustrated in Fig. 5, because they provide further evidence of the sensitivity of some of these units to warming. The unit in Fig. 5C is of particular interest, in being as sensitive to warming as to cooling. We were so surprised by this unit that the ramps were repeated 3 times with very similar results. We also checked for each of these recordings that it was the same unit responding to warming as to cooling, by averaging the spike waveform separately for each direction of temperature change. In each case the waveforms were indistinguishable. Of 14 C2 units tested with cooling and heating ramps before menthol application, all responded to cooling and 12 responded to warming. The median threshold of all 14 to warming was 37.55°C and to cooling was 30.0°C.
Figure 5.
Aa–Ca, responses to temperature ramps of 3 other C2 units, plotted as in Fig. 3. Ab–Cb, for each unit, the mean action potential waveforms during cooling and warming are compared, to show that the unit responsible is the same. (Units in A–C are O2, O1 and F2b respectively in Table 1).
Hysteresis and overlap of C2 responses to cooling and warming
In Fig. 3E it can be seen that on return to 32°C after the end of the heating ramp, the unit transiently stopped firing, but then picked up to a rate higher than before the heating, but over then next 15 s or so the unit settled back to its original rate. To explore further the after-effects of heating or cooling C2 units, which may be relevant to the phenomenon of paradoxical warmth (see Discussion), in one unit that responded to both cooling and warming we explored the effects of continuously ramping the temperature up and down. For these slow, continuous ramps we used the same average rate of change of temperature as for the stepped ramps in Fig. 3, i.e. 0.2°C s−1, but the temperature was altered every second, rather than every 10 s. Even at this rate, which is much slower than the 1°C s−1 usually used in the Marstock method of thermal threshold estimation, there was very considerable hysteresis. Because of the high dynamic sensitivity of the unit, low temperature responses occurred at a much higher temperature when cooling, and high temperature responses at a much lower temperature when warming (see Fig. 6). After applying menthol, responses to cooling started at a temperature 4.5°C higher, and responses to warming at a temperature ∼1°C lower, so that there was a substantial overlap of about 5.5°C between the cooling (after warming) and warming (after cooling) responses.
Figure 6. Hysteresis and overlap of responses of C2 unit to cooling and warming before (A) and after (B) application of 40% menthol.
Left, number of spikes per 850 ms sweep as skin temperature was ramped up and down at 0.2°C s−1. Right, mean number of spikes per sweep, averaged over 12 s, plotted as a function of skin temperature. Arrows indicate direction of change. After menthol, the unit fired at 40°C both when warming after cooling and when cooling after warming. (The unit is I2 in Table 1.) Small circles at 32°C indicate mean rate of firing at baseline temperature, before and after the temperature ramps.
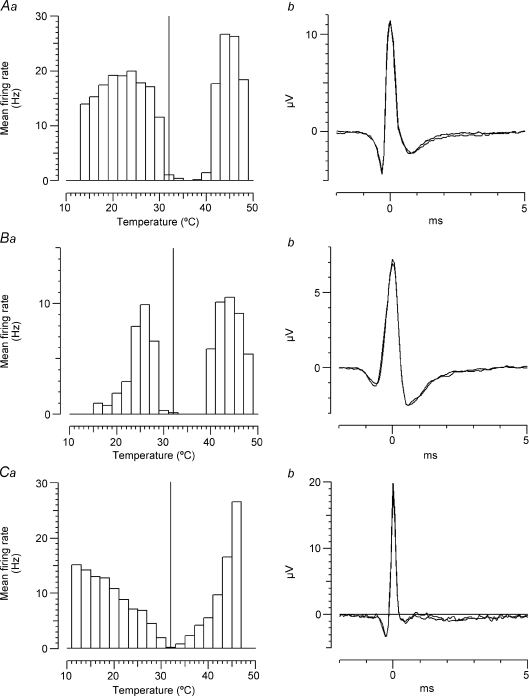
Comparison between C2 units and an Aδ-cold unit
It was previously noted that C2 fibres fire maximally at an appreciably lower temperature than has been described for the Aδ-cold units in monkey glabrous skin, but no human Aδ-cold units were recorded with adequate signal-to-noise to compare thermal response profiles directly (Campero et al. 2001). In this study we were fortunate enough to come across one such Aδ fibre and the response of this unit to a standard cooling staircase is compared with that of two C2 units in Fig. 7. It can be seen that not only do the C2 units fire maximally at a lower temperature than the Aδ-cold unit, but they also continue firing down to a much lower temperature. All of the 13 C2 fibres tested have continued to fire down to 0°C, but the Aδ-cold unit did not fire more than a few spikes below 14°C. The Aδ-cold unit in Fig. 7 was later activated by 40% menthol in ethanol after a delay of 5 min, in a similar way to the C2 units, and it was also sensitized to cooling by menthol, but it did not respond to heating up to 48°C, either before or after the menthol exposure (not illustrated).
Figure 7. Comparison of C2 and Aδ cold units to standard cooling staircase.
Both types of cold-sensitive unit initially showed a high dynamic sensitivity to the 2°C cooling steps, but lost this at lower temperatures. The two C2 units in A and B (P1 and O3 in Table 1) continued to discharge all the way down to 0°C, but the Aδ unit in C (Q1) stopped firing altogether at about 14°C.
Effects of menthol on Type 1A (CMH) and Type 1B (MIA) units
We tested the effects of menthol on 33 nociceptors, and the results are summarized in Table 2. These nociceptors were divided into CMH (n= 24) and MIAs (n= 9) according to their sensitivity to mechanical stimulation and their activity-dependent slowing profile. The CVs of the CMHs averaged 0.74 ± 0.22 m s−1 (mean ±s.d., n= 24), whereas MIAs mostly conducted much more slowly (0.42 ± 0.20 m s−1, n= 9); after a 3 min pause, CMHs slowed by 0.49 ± 0.62% (n= 17)) and MIAs by 4.86 ± 1.52% (n= 9), consistent with previous results (Serra et al. 2004).
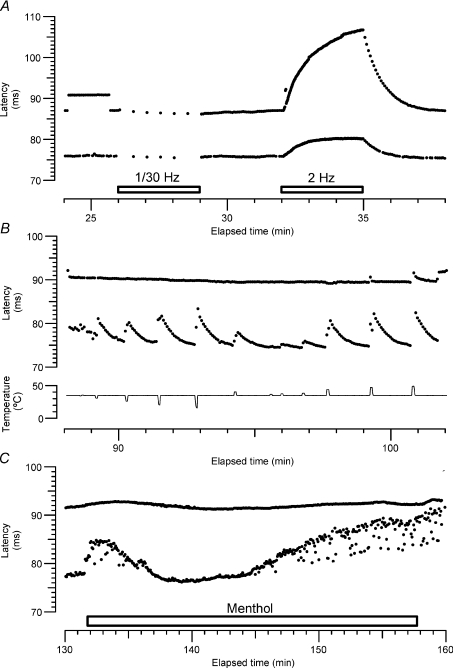
Figure 8 illustrates an experiment in which a C2 unit and a CMH nociceptor were recorded simultaneously. The RFs of both units were very close, covered completely by the menthol-soaked pad. The CV of the C2 was 1.5 m s−1, and it slowed by 5.7% during the 2 Hz tetanus. The CMH had a CV of 1.31 m s−1 and slowed by 22.8% during the tetanus. While the C2 responded readily to small thermal stimuli, both cooling and warming, the CMH was only activated by heating between 44 and 47°C. The CMH was insensitive to a cold ramp to 0°C (not shown). Forty per cent menthol caused a smooth cooling of the skin inducing a gradual and transient slowing of the CMH and activity-dependent slowing of the C2. After about 5 min the cooling due to evaporation of ethanol had finished and the C2 latency returned to baseline. After 13.2 min the C2 fibre started firing again, causing latency shifts and a general activity-dependent slowing (cf. Fig. 2). The latency of the nociceptor remained unchanged, suggesting that menthol caused no activation of this unit. Further negative responses to menthol treatment, for another CMH and 3 MIAs, are illustrated in Fig. 9. In each case the responses of the units to two extra stimuli (above the baseline stimulation rate of 0.25 Hz) are shown, to provide a calibration for any activity evoked by menthol. There was no evidence for even a single spike in any of the other nociceptors (18 CMHs and 9 MIAs in all) tested similarly by latency tracking.
Figure 8. Simultaneous recording of a C2 and a CMH nociceptor.
A, two fibres with CV of 1.5 m s−1 and 1.3 m s−1 (units C1b in Table 1 and unit C1a in Table 2). According to slowing profile during the changes to baseline stimulation frequency (0.25 Hz), the fast fibre corresponds to a type 2 (Serra et al. 1999) and the slow fibre to a mechano-heat sensitive (CMH) nociceptor. B, these two fibres responded differently to cold and heat ramps: whereas the type 2 responded readily to non-noxious cold and heat, the nociceptor responded only to noxious heat (46°C). C, when menthol was applied there was an immediate and transient response of the C2 caused by the low temperature of menthol. The nociceptor displayed only a smooth change in latency due to a general cooling effect. After 13 min of 40% menthol application, the C2 fibre started firing, while the nociceptor remained with a stable latency.
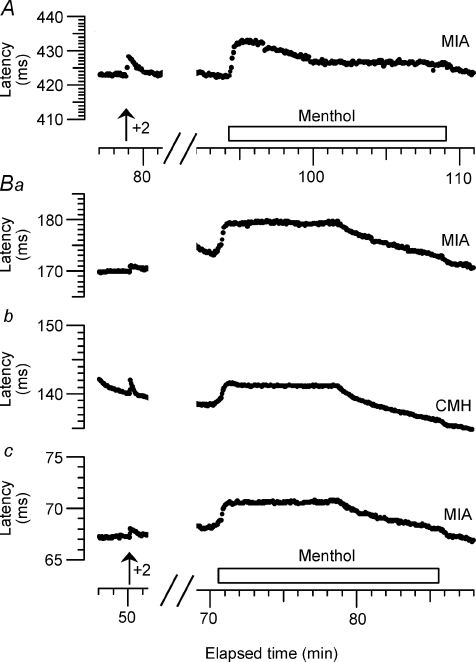
Figure 9. Recordings of 3 MIAs and 1 CMH treated with menthol.
In each trace, the left hand section of the recording shows the activity-dependent slowing caused by 2 extra stimuli (in addition to the baseline stimulation at 0.25 Hz), while the right hand portions show the responses to 40% menthol in ethanol. Unit in A from one subject and those in Ba–c from another. Each unit shows a smooth increase in latency due to cooling when the menthol is applied, but no latency changes corresponding to extra action potentials. (The slow acceleration of the B units was caused by an accidental displacement of the infra-red lamp.) The units A, Ba, Bb, Bc correspond to M1, K1b, K1a and K1c respectively in Table 2.
Although we could find no evidence that menthol evoked activity in any nociceptors at resting skin temperature, there remained the possibility that menthol would sensitize the units to cooling pulses. We therefore also checked for increased discharges to defined cooling pulses or temperature ramps, with a particular interest in CMHs which responded to cooling before menthol (i.e. CMHC units). In Fig. 8, the brief cooling pulses sufficient to activate the C2 unit did not cause too much conduction slowing to obscure the activity-dependent slowing of either unit. However, the much longer and colder pulses required to activate CMH units usually caused so much conduction slowing that they prevented detection of any activity-dependent slowing, since both phenomena cause a shift in latency in the same direction. This problem was made much worse by the very low firing rates (less than 1 spike per second) of CMHs to cooling. To measure these small responses we therefore restricted attention to units with sufficient amplitude to be able to count spikes, using a shape index to limit contamination by muscle artifacts and other units (see Methods). With this approach we recorded from 15 CMHs before and while menthol was applied to the RF for 12 to 46 min (median 15 min). Among these units, four responded to cold prior to menthol with very few spikes. Figure 10 illustrates the typical response of a CMH (CV 1.0 m s−1). Prior to menthol it gave a vigorous response to heating, with first spikes occurring at 37 and 41°C in two trials, but only two and four spikes were recorded during two cooling ramps to −1.5°C, with the first spikes at 2 and 8°C. During 15 min of menthol exposure there were only two spikes registered of similar size and shape. One, at the onset, may have been due to mechanical activation. The second, near the end, may have been from the same unit, but may not. After removing the menthol swab, two more heating and cooling ramps were given and the responses were similar to before. Heating responses started at 36 and 39°C, and four spikes were recorded to each cooling ramp, starting at 2 and 1°C. In recordings from three additional CMH fibres, a few spikes were counted during menthol application, but the signal-to-noise ratio did not allow unequivocal identification of these spikes by waveform as being from the same unit (rather than, for example, from a sympathetic unit of similar amplitude). We applied temperature ramps down to 0°C or below, before and after menthol, to the mechanical RFs of 14 other CMHs with similarly negative or equivocal results.
Figure 10. Responses of a CMH unit to heating, cooling and menthol.
A–C, 3 portions of the recording from a polymodal nociceptor (F2a in Table 2) subjected to heating and cooling ramps and exposure to 30% menthol in ethanol for 15 min. In each portion, the fourth (bottom) part shows the temperature of the Peltier element, the third the number of spikes per sweep, with an arrow indicating the single sweep illustrated at the top. The second part shows a superimposition of all the spikes recorded in the particular portion of the record. Clear responses were only seen to heating and close to 0°C, which were not appreciably changed by exposure to menthol (see text).
In summary, in recordings from 24 CMHs and nine MIAs we found no convincing evidence of either activation or sensitization to cooling or heating by menthol treatments which consistently activated and sensitized C2 fibres.
Discussion
This study has used microneurography to search for C fibres responsive to innocuous cooling and menthol that could be responsible for the reported sensations of burning evoked in healthy volunteers. Microneurography provides an unrivalled tool for detecting the impulses in primary sensory neurons that give rise to sensations, but some of our interpretations are necessarily speculative, because of the limited number of afferent fibres sampled, and the uncontrolled selectivity of the method. There is abundant evidence that cutaneous cold sensation is mediated primarily by Aδ fibres, but we have only ever recorded one with sufficiently good signal-to-noise ratio to determine its thermal response profile (see Fig. 7). It is therefore quite possible that there are other types of thermo-sensory fibre in human skin, that have so far eluded detection. (For example, we have found no fibres corresponding to the high-threshold cold fibres in the monkey described by LaMotte & Thalhammer (1982), which compared with C2 fibres had higher thresholds to cooling (26–10°C), larger receptive fields, and were insensitive to heating.) However, we consider that we have now observed sufficient responses to menthol and temperature changes to justify drawing some tentative conclusions about the role of the C2, or ‘Type 2’ C units, and to drastically revise our previous description of them as ‘specific cold fibres’ (Campero et al. 2001). (N.B. In the following discussion, temperature descriptors in inverted commas, such as ‘hot’ or ‘warm’ refer to sensations, and not stimuli.)
Our results show that the C fibres most strongly activated by both cold and menthol are the C2 fibres. Twenty-four polymodal (Type 1A, CMH) nociceptors were not significantly activated by menthol, whether they discharged slowly at very low temperatures or not (i.e. whether CMHC or CMHCi). Whereas all of the 14 CMH fibres, for which the Peltier device was positioned in their mechanical RFs, discharged vigorously on heating, the firing rates of the four units activated by cooling never exceeded 1 Hz, whether or not menthol had been applied. The remaining 13 were unresponsive to cold down to 0°C. A further nine CMH units were recorded while menthol was applied to their cutaneous RFs, and the latency profiles showed no evidence of spontaneous activity. ‘Silent’, i.e. mechano-insensitive (Type 1B, MIA), nociceptors were more difficult to test, since they had no mechanical RF. However, attempts to activate such units by applying menthol to their electrical RFs were all unsuccessful (in contrast to an earlier study where such units were found to respond to capsaicin applied even at considerable distances (Serra et al. 2004). Since the MIAs are thought to be the fibres responsible for axon reflex vasodilatation in humans (Schmelz et al. 2000), this negative result fits with the finding of Namer et al. (2005) that topical 40% menthol does not induce an axon-reflex flare, but not with the contradictory report by Wasner et al. (2004). Although we cannot exclude the possibility that there is a subpopulation of CMHs or MIAs that behave differently, we conclude on the basis of the present results that the C2 fibres are the most plausible candidates to mediate the compression block-resistant burning pain and cold allodynia/hyperalgesia induced by menthol (Wasner et al. 2004).
Temporal profile of menthol effect on C2 fibres
The latencies of the C2 responses to menthol application (median 6.8 min) were rather variable, but not dissimilar to psychophysical latencies for ‘cool’ and ‘burning’. Namer et al. (2005) found that 40%l-menthol in ethanol induced pain in 7/10 subjects with a latency of 4 min, rising to a maximum in 16 min, while it induced coldness with a latency of 5 min, rising to a maximum in 17 min, Wasner et al. (2004), also using 40%l-menthol in ethanol found a mean latency for pain in 8/10 subjects of ‘a few minutes’, reaching a plateau after 8 min, while a sensation of cold was felt within the first 2 min and reached a plateau after 9 min. Hatem et al. (2006) using 30%l-menthol in ethanol described that 90% of 39 volunteers reported cold at 10 min, whereas 10% reported warm. We used 30% and 40%l-menthol in ethanol to match these psychophysical studies, but we found that in three trials a 2%dl-menthol gel activated C2 units just as quickly.
C2 fibres and anomalous thermal sensations
Current clinical testing of thermoreception is based implicitly on the assumption that only four sensory channels are involved: innocuous ‘cold’ and ‘warm’ and noxious ‘cold pain’ and ‘heat pain’. As Green (2004) has pointed out, this simplistic scheme, which derived from a naive specificity theory, relegates many well-attested thermal sensations to the status of ‘paradoxical’ or ‘illusory’. Here we consider evidence that the C2 fibres may be responsible for some of these apparent anomalies.
Changes in cold sensation during differential A-fibre block
To test whether unmyelinated cold receptors contribute to cold sensations, Fruhstorfer (1984) used a pneumatic cuff to selectively block myelinated fibres. He found that in 25 out of 27 normal subjects the sensation induced by a cooling stimulus changed strikingly after about 13 min from cold to icy, stinging, burning or even hot, while warm sensation was little affected. The change in sensation was accompanied by a drop in threshold temperature from a mean of 28.4°C to 24.9°C, but as he noted, this change was likely to have been caused in part by his use of a 2°C s−1 temperature ramp to estimate threshold (Fruhstorfer, 1976), and the slower velocity of unmyelinated fibres. He concluded that the information from unmyelinated low-threshold cold receptors alone leads to an unpleasant sensation which is normally suppressed by the activity of myelinated cold afferents. Fruhstorfer's findings, that A-fibre block induces a form of cold allodynia, and his interpretation in terms of disinhibition of C fibre input, have been confirmed by Wahren et al. (1989) using radial nerve compression and by Yarnitsky & Ochoa (1990) who again used ischaemia. Of the types of C fibre recorded by microneurography, only the C2 fibres could be responsible for the ‘hot’ and ‘burning’ sensations evoked by cooling to temperatures above 20°C. Normal polymodal nociceptors are only activated at lower temperatures (Campero et al. 1996 and the present study).
Paradoxical warmth and heat
After the discovery of the punctate nature of skin sensibility by Blix (1882–3), Goldscheider (1884, 1912) reported that when warm spots in the skin are stimulated with a cool stimulus, they sometimes evoke a sensation of warmth, and this was confirmed by Pavlicek & Jenkins (1933), who found that most normal subjects sometimes described a 26°C stimulus applied to a warm spot as ‘warm’. A later study by Jenkins & Karr (1957), found that at 18 out of 74 warm spots on 16 subjects, temperatures 3°C below physiological zero were consistently misreported as ‘warm’ or ‘intensely warm’. They also found that paradoxical warmth was most frequently evoked just after a normal warm stimulus. This phenomenon was described by Susser et al. (1999) as ‘paradoxical heat’, and they concluded from CV estimates that the nerve fibres responsible are C fibres. Hamalainen et al. (1982) speculated that polymodal nociceptors were responsible, but they are never activated by the innocuous cooling that can effectively evoke paradoxical warmth and heat. The C2 fibres activated by warming as well as cooling are the only type of fibre identified in humans with appropriate properties to mediate similar thermal sensations on innocuous cooling as well as warming. Moreover, the unit illustrated in Fig. 5 was sensitized by prior warming, after which it discharged maximally at close to 26°C, conditions similar to those reported by Jenkins & Karr (1957) to be optimal for evoking paradoxical warmth.
‘Synthetic heat’ and the thermal grill illusion
Whereas paradoxical heat is best evoked by cooling soon after warming, the sensation of ‘synthetic heat’ is evoked by simultaneously cooling and warming neighbouring regions of skin. Thunberg (1896, as cited by Alrutz, 1898) originally reported that simultaneous application of non-noxious cold and warm stimuli to adjacent skin areas evokes the sensation of strong, but not painful, heat (preceded by transient cold). Craig & Bushnell (1994), however, reported that the ‘thermal grill illusion’ sensation was painful, and related it to cold-evoked burning pain. Other groups have variously stressed the thermal (e.g. Green, 2002; Fruhstorfer et al. 2003) or painful (e.g. Bouhassira et al. 2005; Defrin et al. 2008) aspects of the sensation evoked. Like paradoxical heat, synthetic heat appears to depend on transmission in C fibres, since Fruhstorfer et al. (2003) found that the sensation of synthetic heat was unchanged during A-fibre block but the cold component of sensation was lost. They concluded that the perception of synthetic heat most likely arises from the fusion of independent signals from unmyelinated low threshold cold and warm receptors. The C2 fibres are the only known C fibres with appropriate sensitivity to provide the cold-sensitive arm of this combination.
‘Innocuous cold nociception’
In his study of the thermal grill illusion, Green (2002) found that some normal subjects reported ‘stinging’ or ‘burning’ when the skin of the forearm was cooled only a few degrees below normal skin temperature, and Green & Pope (2003) coined the apparently self-contradictory term ‘innocuous cold nociception’ (ICN) to describe these sensations. The unpleasant sensations evoked by a mildly cool stimulus (27–31°C) are strongly inhibited when cooling is accompanied by touch (‘dynamic mechanical contact’) (Green & Pope, 2003; Green & Schoen, 2005). Further studies of the ICN phenomenon (Green & Schoen, 2007; Green et al. 2008) make the C2 units the most likely peripheral substrate for ICN. Thus Green & Schoen (2007) have found that menthol increases ICN during static contact, and that the menthol-induced or menthol-enhanced unpleasant sensations are also suppressed by dynamic contact. Although Green and colleagues have not reported the effects of A-fibre block, their experiments are consistent with the evidence from the ischaemia and compression-block experiments that the menthol-sensitive C2 fibres can evoke unpleasant sensations that are inhibited by A-fibres (which include low threshold mechanoreceptors or, as discussed above, Aδ cold fibres).
C2 fibres compared with ‘warm’ fibres
Of the 14 C2 fibres tested with the Peltier device, over half had thresholds to heating below 38°C, suggesting that they may act in part as ‘warm’ sensors. It is remarkable that, to the best of our knowledge, there have been no reports on human ‘warm’ fibres since a cluster of articles in the 1970s and early 1980s (Konietzny & Hensel, 1975; Hensel, 1976; Torebjörk & Hallin, 1976; Konietzny & Hensel, 1977; Hallin et al. 1982; Konietzny, 1984). In reviewing recordings from 44 of these units, Konietzny (1984) proposed that they fell into two groups: 21 were low threshold warm receptors (LWRs), active at 32°C and with firing rate falling off above a maximum rate at about 40°C, whereas 23 were high threshold warm receptors (HWRs), with thresholds at 35–38°C and firing rates increasing monotonically up to 45°C or higher. Our C2 fibres clearly differ from the LWRs, in that none exhibited spontaneous activity at 32°C that was inhibited by cooling, and on heating none had a maximum firing rate below 45°C. On the other hand the C2 behaviour on warming (median threshold 37.55°C) corresponds closely with the HWRs, which Konietzny (1984) thought primarily responsible for the sensation ‘hot’. There is no evidence that the HWRs were systematically tested with cooling, so that it is conceivable that HWR = C2. However, Konietzny does describe one fibre (CV unknown) that was activated by warming above 36°C, and also by cooling, so most likely a C2 fibre, which he refers to as a ‘cold’ fibre.
C2 fibres and the spectrum of temperature sensations
Lowering skin temperature can evoke a variety of sensations that range from ‘cool’ through ‘cold’, ‘icy’ and ‘burning’ to ‘pain’. Sensory physiologists have wondered how we are able to discriminate static temperatures on either side of the bell-shaped curve relating the firing rate of Aδ cold fibres to temperature. One suggestion was the firing pattern, which might enable the CNS to distinguish between units firing in bursts or regularly at the same mean rate (Iggo, 1969). This hypothesis is not, however, supported by recordings of human cold fibres (Hensel, 1982), and the recordings in our Fig. 7 suggest a simpler solution: temperature may be determined by comparing the firing rate of Aδ cold fibres and C2 fibres. The ratio of C2 to Aδ activity increases as the skin gets colder, until the Aδ cold fibres stop firing altogether. A similar proposal was made by Fruhstorfer (1984) who deduced that cold-sensitive C fibres must exist, and suggested that strong cold stimuli would inactivate the Aδ fibres, leaving the C fibres to give rise to a ‘superficial stinging and burning pain which precedes the deep aching cold pain’.
Raising skin temperature can also evoke a variety of sensations that range from ‘warm’ through ‘hot’ and ‘burning’ to ‘pain’. While ‘warm’ may be mediated by selective activation of specific warm fibres (LWR), the C2 fibres activated by heating as well as cooling may contribute to the sensations of ‘hot’ and ‘burning hot’, at temperatures somewhat lower than those at which the CMH nociceptors add a definite pain component. On this hypothesis, ‘hot’ is normally signalled by the simultaneous activation of LWR and C2 fibres, so it is not surprising that simultaneous activation of some LWR fibres by innocuous warmth, and nearby C2 fibres by innocuous cold, should generate ‘synthetic heat’.
So the C2 fibres appear to play a similar function in high temperature thermoreception as they do in low temperature thermoreception, in colouring the sensation of the specific thermoreceptors to indicate that skin temperature is leaving the comfort zone (‘cool’, ‘warm’) and becoming uncomfortable (‘cold’, ‘hot’) or even very uncomfortable (‘burning cold’, ‘burning hot’) while not yet tissue-damaging (cold pain, heat pain) (see Table 3). It seems likely that when C2 fibres are active on their own, the predominant sensation is ‘burning’ or ‘hot/burning’, but more commonly they are active at the same time as other thermoreceptive fibres, when the sensation experienced may be a blend (like a mixture of different colours), not easily resolved into distinct components. The controversy over whether the thermal grill generates sensations of heat or pain may be due to the critical position of the LWR+C2 fibre combination in the spectrum of thermal sensations, filling the gap between innocuous warmth and noxious heat pain.
Table 3.
Proposed correlation between unit activation and sensation for thermal sensations
| Normal stimulus | Units activated | Sensation | Abnormal stimulation |
|---|---|---|---|
| Warm << C2, CMH | Heat pain | ||
 |
Warm < C2 | Burning hot | Thermal grill illusion |
| hot | Warm ∼ C2 | Hot | Synthetic heat |
| Warm | Warm | ||
| Neutral | Aδ-cold, C2, warm | ||
| Aδ-cold > C2 | Cool | ||
| Aδ-cold ∼ C2 | Cold | ||
 |
Aδ-cold < C2 | Icy | |
| cold | C2 | Hot, burning | Cool/cold during A-fibre block |
| C2, CMHC | Cold pain |
Returning to our finding that menthol sensitizes C2 fibres to cooling, Green's (1992) report that menthol treatment shifts sensations at 31°C and 29°C from ‘cool’ to ‘cold’, ‘hot’ and ‘burning’ fits well with this hypothesis for C2 function. However, our finding that menthol also apparently sensitizes C2 fibres to heating would lead to the expectation that menthol should also shift sensations of ‘warm’, on mild heating, to ‘hot’ and ‘burning’, but Green (1992) found the opposite. This discrepancy is unresolved.
What is the biological function of C2 fibres?
The biological function of the bimodal C2 fibres presents even more of a puzzle than their sensory function. Their high sensitivity to innocuous cooling of the skin is inappropriate and expensive (in terms of energy consumption) for a sensory channel evolved primarily to warn of excessive cold or heat. Furthermore, the powerful inhibition of their unpleasant sensations by touch and Aδ cold fibres indicates that their function is not to detect cold objects, but rather the temperature of skin that is not being touched. These considerations suggest that the original biological function of the C2 fibres may have been, as previously proposed (see hypothesis 2 in the Introduction), to provide information about skin temperature for the purposes of unconscious thermoregulation, for which the high sensitivity would be important. A rationale for thermoregulatory sensory activity acquiring an unpleasant conscious aspect was provided by Green & Schoen (2005), who suggested that ICN (which is most likely mediated by C2 fibres) may provide a warning of temperatures which, though not extreme enough to cause tissue damage, could represent a dangerous thermoregulatory challenge. The combination of sensitivity to cooling and warming in the same cell appears inappropriate for thermoregulatory control, but may provide a useful indication of departure from the ‘comfort zone’, in which thermoregulation can be maintained indefinitely.
What is the clinical role of C2 fibres?
Fruhstorfer (1984) was the first to note a clinical correlate of his evidence that A-fibre block releases unpleasant sensations from low-threshold cold-sensitive C fibres. He suggested that the abnormal perception of innocuous cold stimuli as ‘warm’ or ‘burning’ by patients with diabetic and uraemic polyneuropathy could be due to loss of Aδ cold afferents, and hence insufficient inhibition of the input from C fibres. However, he did not equate these abnormal sensations in the patients with cold hyperalgesia or cold allodynia. Abnormal thermal responses in uraemic neuropathy were later documented by Yosipovitch et al. (1995), who found a strong correlation between cold hypoaesthesia and paradoxical heat sensations, but they also did not equate these sensations with pain. On the other hand, Ochoa & Yarnitsky (1994) described a syndrome of cold hypoaesthesia and cold hyperalgesia in a variety of neuropathies. They linked the burning sensations reported by the patients in response to cold stimuli to the release of C-fibre mediated cold pain by A-fibre block (Yarnitsky & Ochoa, 1990). They supposed that polymodal nociceptors were responsible, but the cold pain was experienced at temperatures (>20°C) that do not excite normal polymodal nociceptors, so that either C2 fibres or sensitized nociceptors must have been involved. There is a striking parallel between the discrepant accounts of the thermal grill sensations as either ‘hot’ or ‘burning pain’ and these discrepant accounts of abnormal sensations linked to cold hypoaesthesia in neuropathies, both situations in which C2 activity is implicated. It seems unlikely that the ‘pain’ experienced by some subjects and patients due to disinhibited C2 activation can be equated with the pain due to activation of polymodal nociceptors, or that all types of cold hyperalgesia (e.g. toothache) can be explained by this mechanism. A patient was recently described with cold hyperalgesia due to sensitized nociceptors: Type 1A units, which were shown by microneurography to be activated by menthol, in clear contrast to the normal Type 1A units in the present study (Serra et al. 2009).
In conclusion, we have found that the only C-fibres appreciably activated by menthol and cold are the C fibres with a Type 2 profile of activity-dependent slowing, previously described as specific cold afferents (Campero et al. 2001). In the light of the psychophysical studies reviewed, it now seems unlikely that they ever evoke the sensations of ‘cool’ or innocuous ‘cold’. On the contrary, these fibres have been implicated in sensations of ‘hot’, ‘burning’ and ‘stinging’. The C2 fibres are the only known types of C-fibre that could be responsible for the ‘stinging’ and ‘hot/burning’ evoked by innocuous cold stimuli that are usually suppressed by A-fibre input (Aδ-cold and Aβ-touch fibres), but disinhibited when A-fibre input is blocked by ischaemia (Fruhstorfer, 1984) or avoided by cooling with a static thermode (Green & Pope, 2003; Green & Schoen, 2005) or by sliding a thermode over oiled skin (Green et al. 2008). The C2 fibres differ markedly in membrane properties from CMHs and MIAs (Bostock et al. 2003) and although the sensations they evoke can be unpleasant, they are seldom described as painful. In the case of the thermal grill illusion, the sensations that must be mediated in large part by C2 fibres are described by some groups as nociceptive and others as purely thermal. Green et al. (2008) recently used the term ‘low-threshold thermal nociception’ to allow for the bimodal nature of what was previously referred to as ‘innocuous cold nociception’ (Green & Pope, 2003), and C2 units are the best candidates for the ‘nociceptors’ involved. However, we are reluctant to classify the C2 fibres as nociceptors (Latin route: nocere= to harm), and prefer the neutral label ‘C2’, for these C fibres activated by cold, menthol and usually heat, which appear responsible for colouring thermal sensations on either side of the comfort zone.
Acknowledgments
This work was supported by NIH Grant no. R01-NS48932.
Glossary
Abbreviations
- C2
‘Type 2’ C fibre
- CMH
C nociceptor responding to mechanical and heat stimuli
- CMHC
as CMH but also responding to cold stimuli
- CV
conduction velocity
- HWR
high-threshold warm receptor
- ICN
innocuous cold nociception
- LWR
low-threshold warm receptor
- MIA
mechanically insensitive afferent
- RF
receptive field
- TRPA1
transient receptor potential cation channel, subfamily A, member 1
- TRPM8
transient receptor potential cation channel, subfamily M, member 8
Author contributions
All authors contributed to the experimental design, generation and interpretation of data, drafting the manuscript and critically reviewing the final version before its final approval.
References
- Alrutz S. On the temperature senses: II. The sensation ‘hot’. Mind. 1898;7:141–144. [Google Scholar]
- Blix M. Experimentela bidrag till lösning af frågan om hudnervnas specifika energi. Uppsala Läkareförenings förhandlingar. 1882:87–102. –3. [Google Scholar]
- Bostock H, Campero M, Serra J, Ochoa J. Velocity recovery cycles of C fibres innervating human skin. J Physiol. 2003;553:649–663. doi: 10.1113/jphysiol.2003.046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhassira D, Kern D, Rouaud J, Pelle-Lancien E, Morain F. Investigation of the paradoxical painful sensation (‘illusion of pain’) produced by a thermal grill. Pain. 2005;114:160–167. doi: 10.1016/j.pain.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol. 2001;535:855–865. doi: 10.1111/j.1469-7793.2001.t01-1-00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campero M, Serra J, Bostock H, Ochoa JL. Partial reversal of conduction slowing during repetitive stimulation of single sympathetic efferents in human skin. Acta Physiol Scand. 2004;182:305–311. doi: 10.1111/j.1365-201X.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- Campero M, Serra J, Ochoa JL. C-polymodal nociceptors activated by noxious low temperature in human skin. J Physiol. 1996;497:565–572. doi: 10.1113/jphysiol.1996.sp021789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science. 1994;265:252–255. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I, Johnson KO, Dykes R. ‘Cold’ fibre population innervating palmar and digital skin of the monkey: responses to cooling pulses. J Neurophysiol. 1973;36:325–346. doi: 10.1152/jn.1973.36.2.325. [DOI] [PubMed] [Google Scholar]
- Defrin R, Benstein-Sheraizin A, Bezalel A, Mantzur O, Arendt-Nielsen L. The spatial characteristics of the painful thermal grill illusion. Pain. 2008;138:577–586. doi: 10.1016/j.pain.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Duclaux R, Mei N, Ranieri F. Conduction velocity along the afferent vagal dendrites: a new type of fibre. J Physiol. 1976;260:487–495. doi: 10.1113/jphysiol.1976.sp011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes T, Wood JN. Mechanisms of cold pain. Channels. 2007;1:154–160. doi: 10.4161/chan.4692. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H. Conduction in the afferent thermal pathways of man. In: Zotterman Y, editor. Sensory Functions of the Skin of Primates with Special Reference to Man. Oxford: Pergamon Press; 1976. pp. 355–365. [Google Scholar]
- Fruhstorfer H. Thermal sensibility changes during ischemic nerve block. Pain. 1984;20:355–361. doi: 10.1016/0304-3959(84)90112-X. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H, Harju EL, Lindblom UF. The significance of A-δ and C fibres for the perception of synthetic heat. Eur J Pain. 2003;7:63–71. doi: 10.1016/s1090-3801(02)00056-3. [DOI] [PubMed] [Google Scholar]
- George A, Serra J, Navarro X, Bostock H. Velocity recovery cycles of single C fibres innervating rat skin. J Physiol. 2007;578:213–232. doi: 10.1113/jphysiol.2006.116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldscheider A. Die spezifische Energie der Temperaturnerven. Monat f Prakt Dermatol. 1884;3:198–208. [Google Scholar]
- Goldscheider A. Beiträge zur Lehre von der Hautsensibilität. 11. Über die Empfindung der Hitze. Z KIin Med. 1912;75:1–14. [Google Scholar]
- Green BG. The sensory effects of l-menthol on human skin. Somatosens Mot Res. 1992;9:235–244. doi: 10.3109/08990229209144774. [DOI] [PubMed] [Google Scholar]
- Green BG. Synthetic heat at mild temperatures. Somatosens Mot Res. 2002;19:130–138. doi: 10.1080/08990220220220131524. [DOI] [PubMed] [Google Scholar]
- Green BG. Temperature perception and nociception. J Neurobiol. 2004;61:13–29. doi: 10.1002/neu.20081. [DOI] [PubMed] [Google Scholar]
- Green BG, Pope JV. Innocuous cooling can produce nociceptive sensations that are inhibited during dynamic mechanical contact. Exp Brain Res. 2003;148:290–299. doi: 10.1007/s00221-002-1280-9. [DOI] [PubMed] [Google Scholar]
- Green BG, Roman C, Schoen K, Collins H. Nociceptive sensations evoked from ‘spots’ in the skin by mild cooling and heating. Pain. 2008;135:196–208. doi: 10.1016/j.pain.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Schoen KL. Evidence that tactile stimulation inhibits nociceptive sensations produced by innocuous contact cooling. Behav Brain Res. 2005;162:90–98. doi: 10.1016/j.bbr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Green BG, Schoen KL. Thermal and nociceptive sensations from menthol and their suppression by dynamic contact. Behav Brain Res. 2007;176:284–291. doi: 10.1016/j.bbr.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallin RG, Torebjörk HE, Wiesenfeld Z. Nociceptors and warm receptors innervated by C fibres in human skin. J Neurol Neurosurg Psychiatry. 1982;45:313–319. doi: 10.1136/jnnp.45.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamalainen H, Vartiainen M, Karvanen L, Jarvilehto T. Paradoxical heat sensations during moderate cooling of the skin. Brain Res. 1982;251:77–81. doi: 10.1016/0006-8993(82)91275-6. [DOI] [PubMed] [Google Scholar]
- Hatem S, Attal N, Willer J-C, Bouhassira D. Psychophysical study of the effects of topical application of menthol in healthy volunteers. Pain. 2006;122:190–196. doi: 10.1016/j.pain.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Hensel H. Correlation of neural activity and thermal sensation in man. In: Zotterman Y, editor. Sensory Functions of the Skin. Oxford: Pergamon Press; 1976. pp. 331–353. [Google Scholar]
- Hensel H. Thermoreception and Temperature Regulation. London, New York: Academic Press; 1982. [Google Scholar]
- Iggo A. Cutaneous thermoreceptors in primates and sub-primates. J Physiol. 1969;200:403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Ogawa H. Primate cutaneous thermal nociceptors. J Physiol. 1971;216:77P–78P. [PubMed] [Google Scholar]
- Jenkins WL, Karr AC. Paradoxical warmth: a sufficient condition for its arousal. Am J Psychol. 1957;70:640–641. [PubMed] [Google Scholar]
- Konietzny F. Peripheral neural correlates of temperature sensations in man. Hum Neurobiol. 1984;3:21–32. [PubMed] [Google Scholar]
- Konietzny F, Hensel H. Letters and notes: Warm fibre activity in human skin nerves. Pflugers Arch. 1975;359:265–267. doi: 10.1007/BF00587384. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JG. Response properties of high-threshold cutaneous cold receptors in the primate. Brain Res. 1982;244:279–287. doi: 10.1016/0006-8993(82)90086-5. [DOI] [PubMed] [Google Scholar]
- Konietzny F, Hensel H. The dynamic response of warm units in human skin nerves. Pflugers Arch. 1977;370:111–114. doi: 10.1007/BF00707956. [DOI] [PubMed] [Google Scholar]
- Mackenzie RA, Burke D, Skuse NF, Lethlean AK. Fibre function and perception during cutaneous nerve block. J Neurol Neurosurg Psychiatry. 1975;38:865–873. doi: 10.1136/jnnp.38.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Namer B, Seifert F, Handwerker HO, Maihofner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport. 2005;16:955–959. doi: 10.1097/00001756-200506210-00015. [DOI] [PubMed] [Google Scholar]
- Ochoa J, Torebjörk E. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J Physiol. 1983;342:633–654. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J, Torebjörk E. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J Physiol. 1989;415:583–599. doi: 10.1113/jphysiol.1989.sp017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa JL, Yarnitsky D. The triple cold syndrome. Cold hyperalgesia, cold hypoaesthesia and cold skin in peripheral nerve disease. Brain. 1994;117:185–197. doi: 10.1093/brain/117.1.185. [DOI] [PubMed] [Google Scholar]
- Pavlicek G, Jenkins JW. Paradoxical warmth. Am J Psychol. 1933;45:350–353. [Google Scholar]
- Reid G. ThermoTRP channels and cold sensing: what are they really up to? Pflugers Arch. 2005;451:250–263. doi: 10.1007/s00424-005-1437-z. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Michael K, Weidner C, Schmidt R, Torebjörk HE, Handwerker HO. Which nerve fibres mediate the axon reflex flare in human skin? Neuroreport. 2000;11:645–648. doi: 10.1097/00001756-200002280-00041. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Bostock H, Ochoa J. Two types of C nociceptors in human skin and their behaviour in areas of capsaicin-induced secondary hyperalgesia. J Neurophysiol. 2004;91:2770–2781. doi: 10.1152/jn.00565.2003. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol. 1999;515:799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Solà R, Quiles C, Casanova-Molla J, Pascual V, Bostock H, Valls-Solé J. C-nociceptors sensitized to cold in a patient with small-fibre neuropathy and cold allodynia. Pain. 2009 doi: 10.1016/j.pain.2009.07.028. (in press) [DOI] [PubMed] [Google Scholar]
- Susser E, Sprecher E, Yarnitsky D. Paradoxical heat sensation in healthy subjects: peripherally conducted by Aδ or C fibres? Brain. 1999;122:239–246. doi: 10.1093/brain/122.2.239. [DOI] [PubMed] [Google Scholar]
- Torebjörk E, Hallin RG. Skin receptors supplied by unmyelinated (C) fibres in man. In: Zotterman Y, editor. Sensory Functions of the Skin. Oxford: Pergamon Press; 1976. pp. 475–485. [Google Scholar]
- Torebjörk E, Ochoa JL. New method to identify nociceptor units innervating glabrous skin of the human hand. Exp Brain Res. 1990;81:509–512. doi: 10.1007/BF02423499. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olsson KA, Westberg KG, Clark FJ. Microstimulation of single tactile afferents from the human hand. Sensory attributes related to unit type and properties of receptive fields. Brain. 1984;107:727–749. doi: 10.1093/brain/107.3.727. [DOI] [PubMed] [Google Scholar]
- Wahren LK, Torebjörk E, Jørum E. Central suppression of cold-induced C fibre pain by myelinated fibre input. Pain. 1989;38:313–319. doi: 10.1016/0304-3959(89)90218-2. [DOI] [PubMed] [Google Scholar]
- Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol: a human model for cold pain by activation and sensitization of C nociceptors. Brain. 2004;127:1159–1171. doi: 10.1093/brain/awh134. [DOI] [PubMed] [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjörk HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci. 1999;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113:893–902. doi: 10.1093/brain/113.4.893. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Yarnitsky D, Mermelstein V, Sprecher E, Reiss J, Witenberg C, Hemli JA, Boner G. Paradoxical heat sensation in uremic polyneuropathy. Muscle Nerve. 1995;18:768–771. doi: 10.1002/mus.880180714. [DOI] [PubMed] [Google Scholar]