Abstract
Transcranial direct current stimulation (tDCS) when applied over the motor cortex, modulates excitability dependent on the current polarity. The impact of this cortical modulation on spinal cord network excitability has rarely been studied. In this series of experiments, performed in healthy subjects, we show that anodal tDCS increases disynaptic inhibition directed from extensor carpi radialis (ECR) to flexor carpi radialis (FCR) with no modification of presynaptic inhibition of FCR Ia terminals and FCR H-reflex recruitment curves. We also show that cathodal tDCS does not modify spinal network excitability. Our results suggest that the increase of disynaptic inhibition observed during anodal tDCS relies on an increase of disynaptic interneuron excitability and that tDCS over the motor cortex in human subjects induces effects on spinal network excitability. Our results highlight the fact that the effects of tDCS should be considered in regard to spinal motor circuits and not only to cortical circuits.
Introduction
It has been shown that transcranial direct current stimulation (tDCS) can modulate brain excitability in humans (Nistche & Paulus, 2000). This modification of brain excitability depends on current polarity, which is in accordance with previous results obtained in animals (Purpura & McMurtry, 1965). Indeed, when applied over the motor cortex, tDCS stimulation with the anode over the motor cortex contralateral to the target muscles and the cathode over the ipsilateral orbit (anodal tDCS) increases the motor cortex excitability as revealed by transcranial magnetic stimulation (TMS), whereas, with the cathode over the motor cortex and the anode over the orbit (cathodal tDCS), motor cortex excitability is decreased (Nitsche & Paulus, 2000; Lang et al. 2004; Nitsche et al. 2005; Power et al. 2007; Furubayashi et al. 2008). These effects of tDCS on cortical activity have also been revealed with BOLD MRI (Baudewig et al. 2001), functional MRI (Kwon et al. 2008) and PET scan (Lang et al. 2005).
tDCS in humans also seems to have an influence on motor abilities. Indeed, Vines et al. (2006) showed that tDCS over the left motor area influences both contralateral and ipsilateral finger sequence movements. These effects of tDCS are different for each hand: left primary motor cortex (M1) anodal stimulation improves right-hand performance significantly more than cathodal stimulation, whereas left M1 cathodal stimulation improves left-hand performance significantly more than anodal stimulation. Such an effect has also been observed in hemiplegic patients: anodal tDCS improves motor skills used in activities of daily living, reduces reaction times and improves pinch force in the paretic hand (Hummel et al. 2005).
These results obtained both in healthy subjects and hemiplegic patients suggest that modulation of motor cortex excitability induced by tDCS affects motor skill acquisition, raising the hypothesis that tDCS may have an impact on connected structures distant to the site of stimulation including the spinal cord networks. The hypothesis that modulation of brain excitability may modify spinal cord network excitability is supported by results obtained with repetitive transcranial magnetic stimulation (rTMS): Berardelli et al. (1998), Valero-Cabréet al. (2001) and Perez et al. (2005) showed that stimulation of motor cortex either below or above the motor threshold can modify excitability of monosynaptic and non-monosynaptic spinal reflex pathways. Indeed, although different, rTMS and tDCS can produce bidirectional effects on cortical excitability (Siebner et al. 2004). To our knowledge, only Nitsche et al. (2003a,c); have studied the effects of tDCS on spinal excitability using H-reflex or F-waves in just four subjects and did not test for changes in spinal network excitability.
The purpose of this series of experiments was, therefore, to determine if tDCS applied over the motor cortex hand area induces changes in spinal cord network excitability. To that end, the effects of anodal tDCS, cathodal tDCS and sham tDCS on disynaptic inhibition directed from ECR to FCR, on presynaptic inhibition of FCR Ia terminals and on FCR H-reflex excitability were studied in healthy subjects.
Methods
General experimental set-up
Thirteen healthy subjects (aged from 24 to 59 years) were included in the study. All subjects gave their written informed consent before participation. This study was performed in accordance with the ethical codes of the World Medical Association (Declaration of Helsinki) and was approved by the local ethical committee (CPP Ile de France VI – Pitié-Salpêtrière).
During all the experiments, the subjects were comfortably seated in an armchair; the shoulder was slightly abducted (60 deg) and elbow semi-flexed (100 deg) with the forearm pronated and supported by the arm of the chair.
All subjects took part in the three types of tDCS experiments which consisted of (i) an anodal polarisation condition (anode placed over the contralateral motor cortical hand area and cathode over the ipsilateral orbit), (ii) a cathodal polarisation condition (cathode applied over contralateral motor cortical hand area and anode placed over the ipsilateral orbit) and (iii) a sham condition (electrode placed as described for anodal and cathodal conditions but the current was delivered for only 2 min in order to induce a similar cutaneous sensation to that in the two first conditions).
Subjects were blind to the type of stimulation and the order of the experiments was randomised across subjects. The time between each experiment was at least one week.
Transcranial direct current stimulation of motor cortex
Before each experiment, TMS elicited by a Magstim 200 (Magstim, Dyfed, UK) with a double 70 mm figure-of-eight coil, was used to determine the optimum position for stimulating the motor cortex corresponding to the contralateral FCR muscle. The optimum position for tDCS was defined as the site where TMS consistently resulted in the largest FCR motor-evoked potential (MEP).
When the hot spot was defined, the position was marked on the scalp to place the active electrode of the tDCS. The other electrode was placed above the contralateral orbit. The two rectangular electrodes used were identical. They were 7 cm long and 5 cm wide (35 cm2). Both of them were recovered by a sponge soaked in saline. Current was delivered by a constant-current electrical stimulator (Eldith DC-Stimulator, Germany).
For both anodal and cathodal conditions, the intensity was set to 1.75 mA and was applied for 20 min. We stimulated with a current density of 0.05 mA cm−2 and delivered a total charge density of 0.06 C cm−2. These criteria are well below the threshold for tissue damage (Nitsche et al. 2003b).
The current was ramped up to 1.75 mA over an 8 s period and a similar but descending current ramp was used at the end of the stimulation period. Nearly all subjects felt the current flow as an itching sensation during the first 2 min following the start of current delivery. After this period, the scalp sensation disappeared such that subjects were unable to determine if the electrical stimulator was on or off.
For the sham condition, the intensity was set to 1.75 mA as for anodal and cathodal conditions but the current was applied for only 2 min, since Nitsche & Paulus (2000) had previously reported that at least 3 min of tDCS were necessary to induce after-effects. At the end of the sham condition, the tDCS stimulator was switched on for 30 s in order to mimic the sensation of the ramp-down current perceived at the end of anodal or cathodal conditions.
Method for assessment of spinal cord network excitability
Surface electrodes were used for both stimulation and recording. EMG activity was recorded with bipolar surface electrodes (0.8 cm2 silver plates, 1.5 cm apart) contacting the skin over the corresponding muscle belly. EMG signals were filtered (0.1 Hz to 1 kHz) and digitised at 1–2 kHz.
Test reflex: the FCR H-reflex
Percutaneous electrical stimulation of the median nerve (rectangular pulse of 1 ms duration, 0.33 Hz) was used to evoke an H-reflex in the wrist flexors. A marked increase in the H-reflex during wrist flexion but not during pure pronation or finger flexion was used as a criterion indicating that the reflex originated mainly from FCR. The FCR H-reflex was evoked by stimulating the median nerve through a bipolar electrode placed 2 cm below the elbow in the medial side of the arm. Before each experiment, the maximal H-reflex response (Hmax) and the maximal M response (Mmax) were recorded. As the sensitivity of the H-reflex to facilitatory or inhibitory conditioning effects is known to depend on its size (Crone et al. 1990), the size of the FCR control H-reflexes was systematically adjusted to between 10% and 15% of Mmax.
Conditioning stimulations
Disynaptic inhibition. The method described by Day et al. (1984) was used to assess disynaptic group I inhibition of the FCR H-reflex. The conditioning stimulus (rectangular pulse of 1 ms duration) was applied to the radial nerve. The radial nerve was stimulated through bipolar electrodes placed 2 cm above the elbow on the external part of the arm. The intensity of the conditioning stimulus was adjusted below ECR motor threshold (MT) in order to prevent recurrent inhibition (Aymard et al. 1995) and was between 0.7 and 0.9MT depending on the subject. The inter-stimulus interval (ISI) at which the early radial-induced inhibition of the FCR H-reflex was maximum (see Fig. 1A baseline, ▴) was determined using 0.5 ms steps and kept constant throughout the experiments. For all subjects except one, the ISI used was 0 ms.
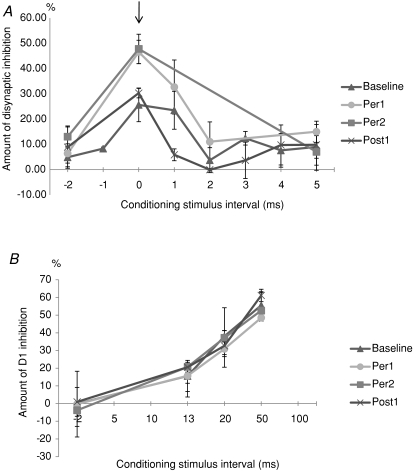
Figure 1. Time course of radial-induced inhibition of the FCR H-reflex.
A, time course of disynaptic inhibition of FCR H-reflex before, during and after 20 min of anodal tDCS applied over the hand motor cortex in one representative subject. Ordinate: amount of disynaptic inhibition. Amount of disynaptic inhibition is calculated using the following formula: ((mean control H value − mean conditioned H value)/mean control H value) × 100. Mean control H value and mean conditioned H value were obtained from 20 H-reflexes each. Vertical bars represent the standard error of the mean (±1 s.e.m.). ▴, amount of disynaptic inhibition during baseline time period. •, amount of disynaptic inhibition during Per1 time period. ▪, amount of disynaptic inhibition recorded during Per2 time period. ×, amount of disynaptic inhibition recorded in the Post1 time period. Arrow indicates the peak of disynaptic inhibition. Abscissa: conditioning test interval in milliseconds. B, time course of the presynaptic inhibition of FCR Ia afferents assessed by D1 method before, during and after 20 min of anodal tDCS applied over the hand motor cortex in one representative subject. Ordinate: amount of D1 inhibition. Amount of D1 inhibition is calculated using the following formula: ((mean control H value − mean conditioned H value)/mean control H value) × 100. Mean control H value and mean conditioned H value were obtained from 20 H-reflexes each. Vertical bars represent ±1 s.e.m.). ▴, amount of D1 inhibition during baseline time period. •, amount of D1 inhibition during Per1 time period. ▪ amount of D1 inhibition recorded during Per2 time period. ×, amount of D1 inhibition recorded in the Post1 time period. Abscissa: conditioning test interval in milliseconds.
Presynaptic inhibition. The method used to assess presynaptic inhibition of group I afferents was the D1 method, originally described in the lower limb by Mizuno et al. (1971). The conditioning radial nerve stimulation was set at 0.9MT. Radial nerve stimulation elicits a long-lasting depression of the FCR H-reflex called ‘D1 inhibition’ (see Fig. 1B baseline, ▴) which at an ISI of 13 ms is in all likelihood caused by presynaptic inhibition of FCR Ia afferents (see Discussion and Berardelli et al. 1987).
Organisation of the experiments
The experimental protocol comprised three different conditions: anodal tDCS, cathodal tDCS and sham. Each condition included alternate recordings of presynaptic inhibition and disynaptic group I inhibition.
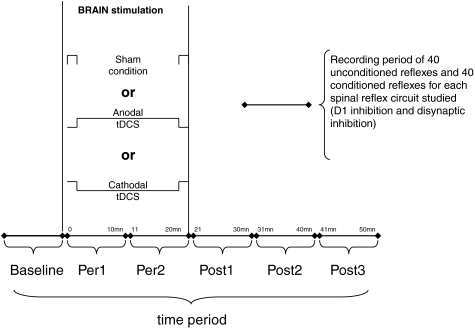
Each experimental condition was composed of six time periods (see Scheme 1). The first time period (baseline) corresponded to the period during which disynaptic inhibition and presynaptic inhibition were recorded before applying tDCS. The second time period (Per1) corresponded to the first 10 min of recording which followed the beginning of tDCS (0–10 min after the onset of tDCS). The third time period (Per2) corresponded to the next 10 min of recording during tDCS (11–20 min after the onset of tDCS). After the end of tDCS, three time periods were distinguished: Post1 (21–30 min after the onset of tDCS), Post2 (31–40 min after the onset of tDCS) and Post3 (41–50 min after the onset of tDCS)) corresponding to the first, second and third 10 min intervals of recordings, respectively, following the termination of tDCS.
Scheme 1.
Schematic diagram of the experimental procedure
For both disynaptic group I inhibition and presynaptic inhibition recordings, 40 conditioned and 40 control H-reflexes, elicited every 3 s, were recorded during each time period. Control and conditioned reflexes were randomly alternated.
In order to relieve the experimental procedure, the same sham condition was used as reference for anodal and cathodal conditions. In sham condition only the reflexes (control and conditioned) recorded after the end of the sham stimulation (2 min) were taken into account for analysis of the result.
FCR H-reflex recruitment curves
FCR H-reflex recruitment curves were also performed in 9 of the 13 subjects, before (baseline), during (Per1 and Per2 time periods) and after (Post1 and Post2 time periods) anodal tDCS. Ten FCR H-reflexes were averaged at each stimulus intensity. H-reflex threshold was defined as the minimum intensity necessary to elicit an H-reflex equal to 2% of Mmax. The intensity was progressively increased from below H-reflex threshold to Hmax response (Hmax). The Mmax response was also recorded.
Statistical analysis
The reflex response was measured as peak to peak amplitude of the non-rectified reflex response. For D1 inhibition and disynaptic inhibitions, 40 conditioned and 40 control H-reflexes were averaged for each condition. The mean value of unconditioned and conditioned test reflexes was determined with its standard error of the mean (s.e.m.). The amount of inhibition was defined as: ((mean control H value − mean conditioned H value)/mean control H value) × 100.
The amounts of D1 inhibition and disynaptic group I inhibition were each averaged among subjects for each time period (baseline, Per1, Per2, Post1, Post2, Post3).
A two-way repeated measures ANOVA, with ‘time period’ as first factor (baseline, Per1, Per2, Post1, Post2, Post3), and ‘condition’ as second factor (anodal, cathodal and sham) was used to determine the effects of tDCS on disynaptic and D1 inhibition. A Scheffépost hoc test was performed on significant comparisons.
A repeated measures single-factor ANOVA on time period (baseline, Per1, Per2, Post1, Post2, Post3) was also used for each condition to determine its impact on disynaptic and D1 inhibition among the six time periods. A Scheffépost hoc test was performed on significant comparisons.
For the analysis of the FCR H-reflex recruitment curves, the H-reflex threshold (intensity evoking an H-reflex of 2% of Mmax), the Hmax/Mmax ratio and the slope of the ascending limb of the recruitment curve were calculated for each subject during each time period: before the onset of tDCS (baseline period), during tDCS (Per1 and Per2 time periods) and after its end (Post1 and Post2 periods). The slope was obtained by fitting a linear regression function to the steepest part of the ascending limb of each recruitment curve. These three parameters were averaged across subjects for each period (baseline, Per1, Per2, Post1, Post2). A repeated measures ANOVA was used to determine the effects of anodal tDCS (with ‘time period’ as factor: baseline, Per1, Per2, Post1, Post2) on Hmax/Mmax ratio, the slope of the ascending part of the recruitment curve, and H-reflex threshold.
Results
Disynaptic group I inhibition of FCR H-reflex
Anodal tDCS
The main finding of these series of experiments is that anodal tDCS induced changes in the extent and the time course of disynaptic inhibition, as illustrated in Fig. 1A (results obtained in one representative subject). In Per1 and Per2 time periods (•, ▪), the amount of disynaptic inhibition was enhanced for −1, 0 and 1 ms ISI, while no changes were observed in Post1 (×) time period.
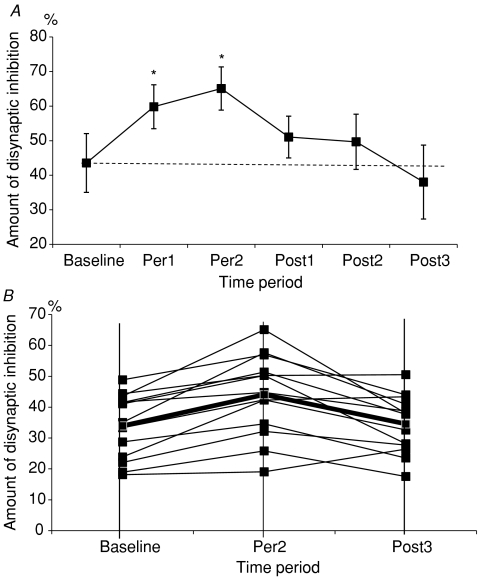
In Fig. 2A (obtained in one subject), the amount of disynaptic inhibition at the peak of inhibition (ISI 0 ms in Fig. 1A) is plotted against the tDCS time period. The amount of disynaptic inhibition directed from ECR to FCR increases progressively after the onset of anodal tDCS from 44.6% in the baseline period to 61.9% in the Per1 time period. It reaches its maximum in Per2 time period (65.8%). Then the amount of disynaptic inhibition decreases progressively after the end of the tDCS to 49.0% in Post1 time period, 46.0% during Post2 and returned to its baseline 38.0% over the Post3 time period.
Figure 2. Changes in disynaptic inhibition in the anodal tDCS condition.
A, changes in the amount of disynaptic inhibition between ECR and FCR induced by 20 min of anodal tDCS applied over the hand motor cortex in one subject. Ordinate: amount of disynaptic inhibition. Amount of disynaptic inhibition is calculated as in Fig. 1A and is represented by ▪. Mean control H value and mean conditioned H value were obtained from 40 H-reflexes each. Vertical bars represent ±1 s.e.m.). *P < 0.05. Abscissa: the 6 recording time periods, baseline corresponds to the period of recording before the onset of tDCS, Per1 to the first 10 min of recording during tDCS, Per2 to the second 10 min of recording during tDCS, Post1 to the first 10 min of recording after the end of tDCS, Post2 to the second 10 min of recording after the end of tDCS, Post3 to the last 10 min of recording after the end of tDCS. B, effect of anodal tDCS on disynaptic inhibition in each of the 13 subjects. Ordinate: as in Fig. 1A. Each full line represents one subject, the bold line represents the grand mean of the amount of disynaptic inhibition. Abscissa: time periods, baseline corresponds to the period of recording before the onset of tDCS, Per2 to the second 10 min of recording during tDCS, Post3 to the 10 min of recording after the end of tDCS.
As illustrated in Fig. 2B, similar results were observed for 11 of the 13 subjects. In all subjects, the amount of disynaptic inhibition increased in Per2 time period compared to its value during the baseline time period. The mean value of disynaptic inhibition of the 13 subjects, illustrated by the bold line in Fig. 2B, increases from 33.9 ± 2.9% before the start of stimulation (baseline time period) to 38.5 ± 3.5% during Per1 and to 44.0 ± 3.6% during Per2, returning to its baseline level after termination of tDCS (36.8 ± 3.1% in Post1, 37.6 ± 2.9% in Post2 and 34.5 ± 2.6% during the Post3 time period).
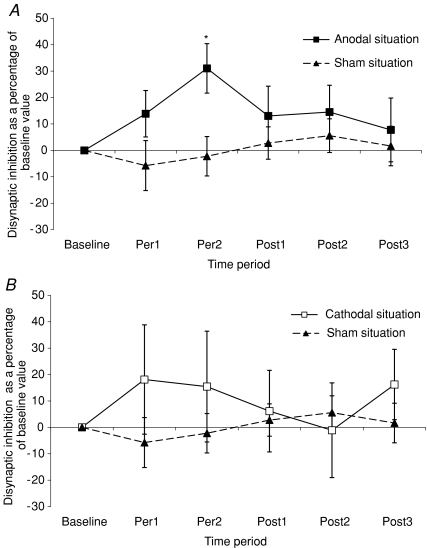
Figure 3A illustrates the comparison between variations of disynaptic inhibition (expressed as a percentage of its baseline value) in anodal and sham conditions. In the anodal condition, disynaptic inhibition increased during Per1 (+13.0% compared to its baseline value) and Per2 (+31.0% compared to its baseline value) time periods, but was not modified in the sham condition. The two-way repeated measures ANOVA gave a significant interaction between condition and time period (F= 2.062; P < 0.03). Anodal tDCS significantly increases disynaptic group I inhibition of the FCR H-reflex. Post hoc analysis showed that this increase reached statistical significance during the second part of the tDCS stimulation period (Per2) (P < 0.01). The one-way repeated measures ANOVA confirmed that anodal condition significantly increases disynaptic inhibition (F= 6.180, P < 0.001), post hoc analysis indicates that this increase of disynaptic inhibition reaches statistical significance in Per1 and Per2 time periods (P < 0.03 and P < 0.001, respectively) when compared to its baseline value.
Figure 3. Comparison between anodal (or cathodal) and sham condition.
A, changes in disynaptic inhibition in the anodal tDCS condition and in the sham condition. Ordinate: disynaptic inhibition as a percentage of baseline value. The percentage of variation of disynaptic inhibition is calculated using the following formula in each of the thirteen subjects studied: ((mean disynaptic inhibition during period of measurement − mean disynaptic inhibition in the baseline condition)/mean disynaptic inhibition in the baseline condition) × 100. Mean disynaptic inhibition is obtained in a subject and in a given period from 40 control H-reflexes and 40 conditioned H-reflexes. The grand mean of these 13 values is then plotted on the graph. ▪, grand mean of the amount of disynaptic inhibition recorded in anodal condition. ▴, grand average of the amount of disynaptic inhibition recorded in the sham condition. Vertical bars represent ±1 s.e.m.*P < 0.05. Same abscissa as in Fig. 2A. B, changes of disynaptic inhibition in the cathodal and sham tDCS conditions. Ordinate as in Fig. 2A. □, grand mean of the amount of disynaptic inhibition recorded in cathodal condition. ▴, grand mean of the amount of disynaptic inhibition recorded in sham condition. Vertical bars represent ±1 s.e.m. Abscissa as in Fig. 2A.
Cathodal tDCS
In the cathodal condition, the amount of disynaptic inhibition of the 13 subjects was 32.8 ± 2.9% during baseline time period. In Per1 and Per2 time periods, the amount of disynaptic inhibition was 35.0 ± 3.5% and 35.2 ± 4.4%, respectively. After the end of tDCS, the amount of inhibition was 32.6 ± 3.2% in Post1 time period, 31.1 ± 3.5% in Post2 and 34.9 ± 3.8% in Post3 time period.
Figure 3B illustrates the variations of disynaptic inhibition (expressed as a percentage of its baseline value) in cathodal condition and in sham condition. There is a weak and non-significant increase of disynaptic inhibition in Per1, Per2 and Post3 periods. ANOVAs confirmed the lack of modification of disynaptic inhibition in the cathodal condition compared to the sham condition (F= 1.543, P= 0.29) and the absence of significant change of disynaptic inhibition in the cathodal condition over the six recording time periods (F= 0.739, P= 0.59).
Sham condition
The amount of disynaptic inhibition of the 13 subjects was 33.1 ± 2.6% in baseline time period and 31.1 ± 3.2% in Per1, 31.8 ± 2.5% in Per2, 32.6 ± 2.3 in Post1, 33.2 ± 2.5% in Post2 and 31.5 ± 2.5% in Post3 time period. This absence of modification of disynaptic inhibition is confirmed by Fig. 3A (▴) and B (▴) illustrating changes in disynaptic inhibition as a percentage of its baseline value in sham condition. A one-way repeated measures ANOVA confirmed the absence of modification of disynaptic inhibition in the sham condition over the six recording time periods (F= 0.508, P= 0.79).
Presynaptic inhibition of FCR Ia afferents
Anodal tDCS
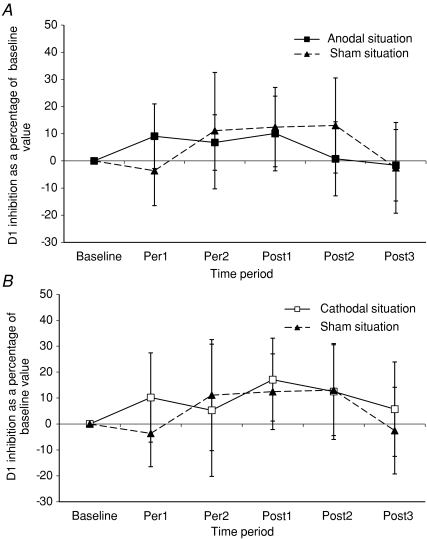
Figure 1B illustrates that the anodal tDCS did not induce changes in the time course of D1 inhibition in a representative subject. No change was observed whatever the time period (Per1, Per2, Post1). Results obtained in the whole population fully confirm this finding. Indeed, the amount of D1 inhibition at 13 ms ISI was 37.0 ± 2.9% in baseline time period. In Per1 and Per2 time periods, the amount of D1 inhibition was 39.5 ± 3.3 and 38.8 ± 2.8%, respectively. In the Post1 time period, the amount of D1 inhibition was 39.7 ± 3.3%, in the Post2 time period 37.2 ± 3.9% and in the Post3 time period 36.4 ± 3.8%. Figure 4A illustrates that the amount of D1 inhibition expressed as a percentage of its baseline value is similar in both anodal and sham conditions. The two-way repeated measures ANOVA indicates that there is no interaction between polarity and recording time period (F= 0.294, P= 0.98) and that the anodal condition is not statistically different from the sham condition (F= 0.53, P= 0.94). The one-way repeated measures ANOVA indicates also that the anodal condition has no significant effect on D1 inhibition over the six recording time periods (F= 0.506, P= 0.77).
Figure 4. Changes in D1 inhibition induced by tDCS.
A, anodal tDCS condition and sham condition. Ordinate: D1 inhibition as a percentage of baseline value. The percentage of variation in D1 inhibition is calculated using the following formula in each of the thirteen subjects studied: ((mean D1 inhibition during period of measurement − mean D1 inhibition in baseline condition)/mean D1 inhibition in baseline condition) × 100. Mean D1 inhibition is obtained in a subject and in a given period from 40 control H-reflexes and 40 conditioned H-reflexes. The grand mean of these 13 values is then plotted on the graph. ▪, grand mean of the amount of D1 inhibition recorded in anodal condition. ▴, grand mean of the amount of D1 inhibition recorded in sham condition. Vertical bars represent ±1 s.e.m. Abscissa as in Fig. 2A. B, cathodal tDCS condition and sham condition. Ordinate is the same as those used in Fig. 4A. □, grand mean of amount of D1 inhibition recorded in cathodal condition. ▴, grand mean of the amount of D1 inhibition recorded in sham condition. Vertical bars represent ±1 s.e.m. Abscissa as in Fig. 2A.
Cathodal tDCS
The amount of D1 inhibition in the 13 subjects was 31.9 ± 5.0% in the baseline time period, 30.8 ± 3.9% in the Per1 time period, 27.6 ± 5.5% in Per2, 33.8 ± 4.3% in Post1, 32.2 ± 4.7% in Post2 and 29.2 ± 4.0% in the Post3 time period. Figure 4B illustrates the variations of D1 inhibition (expressed as a percentage of its baseline value), in cathodal and sham conditions. ANOVAs show the absence of modification of D1 inhibition in the cathodal condition (F= 0.53, P= 0.80) compared to the sham condition and over the six recording time periods (F= 0.713, P= 0.61).
Sham condition
The amount of D1 inhibition in the 13 subjects (not illustrated) was 32.2 ± 3.7% in the baseline time period and 28.8 ± 3.3% in Per1, 31.5 ± 3.6% in Per2, 32.5 ± 2.5% in Post1, 31.9 ± 2.3% in Post2 and 29.3 ± 3.6% in the Post3 time period. The one-way repeated measures ANOVA test confirms the absence of modification of D1 inhibition (expressed as a percentage of its baseline value) in the sham condition over the six recording time periods (F= 0.763, P= 0.57).
Effect of anodal tDCS on FCR H-reflex recruitment curves
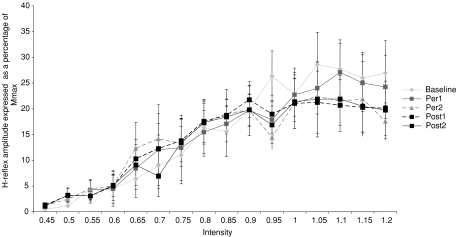
Figure 5 shows the FCR H-reflex recruitment curves obtained in nine subjects before, during and after anodal tDCS. No modification of the FCR H-reflex recruitment curves was seen either during or after tDCS. Moreover the FCR H-reflex threshold, the Hmax/Mmax ratio and the slope of the ascending part of the curves were not modified by anodal tDCS.
Figure 5. Mean FCR H-reflex recruitment curves before, during and after anodal tDCS.
Ordinate: H-reflex amplitude expressed as percentage Mmax value. Each point represents the grand mean of the amplitude of the H-reflex. Diamonds: H-reflex recruitment curve recorded during the baseline time period. Light squares: H-reflex recruitment curve recorded during the Per1 time period. Triangles: H-reflex recruitment curve recorded during Per2 time period. Black squares with dashed line: H-reflex recruitment curve recorded in the Post1 time period. Black squares with continuous line, H-reflex recruitment curve recorded in the Post2 time period. Vertical bars represent ±1 s.e.m. Abscissa: stimulus intensity expressed as a multiple of the intensity necessary to obtain a motor response equal to 25% of Mmax.
Indeed, the mean intensity necessary to elicit a FCR H-reflex equal to 2% of Hmax was not modified during or after anodal tDCS ranging from 0.61 ± 0.07 during the baseline period to 0.56 ± 0.06 in Per1 and Per2 time periods, respectively, and to 0.57 ± 0.07 during the Post1 and Post2 time periods (P= 0.21).
The Hmax value was also not modified, ranging from 28.9 ± 3.8% of Mmax during the baseline period to 28.8 ± 3.1% in the Per1 time period, 27.7 ± 2.8% in Per2, 27.3 ± 2.7% in Post1 and 26.7 ± 2.9% in Post2 time period (P= 0.83). The slope of the ascending part of the recruitment curves was 61.5 ± 10.1 in the baseline time period, 64.1 ± 12.3 in Per1, 64.1 ± 12.6 in Per2, 60.3 ± 10.5 in Post1 and 55.0 ± 8.4 in Post2 time periods. There was no statistically significant modification of the slope of the ascending part of the FCR H-reflex recruitment curves between the five time periods (P= 0.28).
Discussion
The main finding of this series of experiments is that disynaptic inhibition directed from ECR to FCR is enhanced during anodal tDCS. This enhancement is polarity-dependent since neither cathodal nor sham conditions modified disynaptic inhibition.
Our results also indicate that D1 inhibition is not modified by tDCS and that H-reflex recruitment curves are not modified in the anodal condition.
Methodological considerations
Before reaching the conclusion that modulation of the hand motor cortex excitability induced by tDCS results in changes in spinal pathway excitability, some methodological points need to be discussed.
Is D1 inhibition assessing presynaptic Ia inhibition? The D1 inhibition originally described in the lower limb by Mizuno et al. (1971) was demonstrated in the upper limb by Day et al. (1984). Indeed, in the upper limb, the conditioning volley to the radial nerve produces in the FCR H-reflex a threefold inhibition: (i) an early and short-lasting inhibition (the disynaptic inhibition) peaking at 0 ms; (ii) a second phase of inhibition between 5 and 50 ms (D1 inhibition); and (iii) a third phase of inhibition lasting up to 500 ms (Day et al. 1984). In 1987, Berardelli et al. demonstrated that, for 10–20 ms ISI, the radial conditioning volley inhibits the FCR H-reflex but not the FCR MEP elicited by cortical stimulation, whereas at 0 ms ISI, both FCR H-reflex and FCR MEP are inhibited. Since a postsynaptic effect at motoneuron level (as the disynaptic inhibition) will affect both H-reflex and MEP, it is thus likely that for 10–20 ms ISI, the conditioning radial volley acts at a premotoneuronal level. However, as stressed by Pierrot-Deseilligny & Burke (2005), even though the most parsimonious explanation for this differential behaviour between H-reflex and MEP is that the radial nerve-induced inhibition is presynaptic in origin, it has to be kept in mind that long-lasting cutaneous effects have been described following antagonistic nerve stimulation in soleus, ECR and FCR test reflexes (for references see Pierrot-Deseilligny & Burke, 2005). Interestingly, these long-lasting facilitatory effects are more potent at soleus level (Hultborn et al. 1987) and at ECR level (Burke et al. 1994) than at FCR level (Day et al. 1984; Burke et al. 1992). Furthermore, in 1990, Nakashima et al. found almost no effect in the FCR H-reflex after various cutaneous stimulations in the radial nerve area while they demonstrated that these radial cutaneous stimulations modify the FCR D1 inhibition for time intervals between radial cutaneous stimulations and FCR H-reflex stimulations longer than 33 ms. Put together these findings suggest that for 13 ms ISI, the radial-induced inhibition of the FCR H-reflex is in all likelihood presynaptic in origin.
The onset (and the offset) of tDCS is usually accompanied by a cutaneous sensation that might by itself result in changes in spinal network excitability. Indeed, it has been shown that cranial cutaneous stimulation could influence the soleus H-reflex (Delwaide & Crenna, 1983; Ghanim et al. 2009). Nevertheless it seems unlikely that the modification of disynaptic inhibition could be attributed to a cutaneous effect because none of the subjects was able to detect the presence or absence of current after 2 min of tDCS application.
Since each experiment lasted 1 h, it could be argued that the observed changes in spinal network excitability are linked to the duration of the experiment. Results obtained in the sham condition allow us to reject this hypothesis. If it were true, the same modification of disynaptic inhibition which we observed in the anodal condition should also have been observed in both cathodal and sham conditions.
Since it has been suggested that the order of experiments could interfere with the results (Boggio et al. 2006), the three conditions (anodal tDCS, cathodal tDCS or sham stimulation) performed during this study were randomised in order to avoid this bias.
We are therefore confident that the modification of spinal network excitability observed in this study is probably due to the tDCS applied over the hand motor cortex.
Anodal tDCS and spinal network excitability
The results of this series of experiments indicate that anodal tDCS increases disynaptic inhibition directed from ECR to FCR.
‘Unlike spinal cord motoneurons which are silent during skeletal muscle relaxation, most M1 (primary motor cortex area) neurons are spontaneously active at rest’ (Evarts, 1981), and it is likely that tDCS acts by modifying the rate of spontaneous discharge of M1 neurons. Indeed, in animals, Purpura & McMurtry (1965) showed that weak current polarisation modulates motor cortex activity by modifying the discharge rate of M1 neurons, and that anodal current increases the rate of spontaneous discharge. It has been suggested that such a mechanism is also acting in humans (Nitsche & Paulus, 2000). Thus, we favour the hypothesis that the increase in disynaptic inhibition observed in our series of experiments and induced by anodal tDCS is due to an increase in the efficiency of the descending volleys reaching spinal neurons. Obviously, our experimental design does not allow us to determine if this facilitatory effect is mediated through a direct corticospinal pathway or through a more indirect descending control.
The fact that anodal tDCS does not induce significant changes in presynaptic inhibition directed to FCR Ia fibres favours the hypothesis that anodal tDCS increases disynaptic inhibitory interneuron excitability. Indeed, increase in disynaptic inhibition may theoretically be related either to an increase in disynaptic interneuron excitability or to a decrease in presynaptic inhibition of their afferents. Enríquez-Denton et al. (2000) showed in the cat lumbar spinal cord that presynaptic inhibition of Ia fibres directed to α-motoneurons and presynaptic inhibition of Ia fibres directed to Ia interneurons are similarly controlled. If their results could be transposed to disynaptic inhibition in the human cervical spinal cord, the absence of changes in presynaptic inhibition of FCR Ia terminals, assessed by the D1 method and observed in our series of experiments, suggests that the observed increase of disynaptic inhibition is mainly due to an increase of disynaptic interneuron excitability. Taking into account that presynaptic inhibition of FCR Ia terminals is not modified by anodal tDCS, the absence of modification of FCR H-reflex recruitment curves seems to reflect a lack of change in FCR α-motoneuron excitability. This may appear surprising, considering that Cowan et al. (1986) described an α-motoneuron facilitation following a transcranial anodal subthreshold single electrical stimulus. However, the anodal stimulation parameters were quite different in their experiments. In their study, Cowan et al. used a single subthreshold anodal stimulus and studied the time course of the changes in FCR H-reflex excitability induced by this single stimulus. In the present study, we applied current continuously for 20 min and we explored the ‘steady state’ induced by this continuous stimulation. Thus, if a continuous electrical stimulation results in cortical effects similar to those induced by a single subthreshold electrical stimulation, the absence of modifications of FCR H-reflex recruitment curves observed during anodal tDCS could be explained by the fact that α-motoneurons continuously receive both EPSPs and IPSPs with the net result of no change in FCR H-reflex excitability.
Cathodal tDCS and spinal network excitability
Nitsche et al. (2003c) have previously shown in four healthy subjects that cathodal tDCS applied over the hand motor cortex at an intensity of 1 mA decreased MEPs evoked by TMS without modifying FCR H-reflex recruitment curves. The results of our study support their results indicating that cathodal tDCS does not modify presynaptic Ia inhibition and disynaptic inhibition.
It could be argued that the absence of effects of cathodal tDCS on disynaptic and presynaptic inhibition could be due to insufficient stimulation intensity. In this respect, Nitsche & Paulus (2000) showed that the effects of tDCS on motor cortex excitability depend on tDCS intensity. However, these authors also showed that the minimum tDCS intensity required to induce a long-lasting change of motor cortex excitability is 0.6 mA (over 5 min) which is below the tDCS intensity used in this study. Moreover studies performed in healthy subjects and using cathodal tDCS with an intensity of 1 mA showed a significant decrease of hand motor cortex excitability (Nitsche et al. 2003a, 2005; Lang et al. 2004; Power et al. 2007; Furubayashi et al. 2008). Therefore it is likely that the intensity of tDCS used in the present study was sufficient to decrease motor cortex excitability but these modifications of motor cortex excitability have no impact on spinal neuronal network excitability. This finding seems at first sight surprising since (i) in the reverse condition (i.e. during the anodal condition) an increase in FCR MEP is accompanied by an increase in disynaptic interneuron excitability most likely due to an increase in descending volleys, and (ii) in the cathodal condition the decrease in FCR MEP is most likely due to a decrease in M1 spontaneous firing which probably results in a decrease in the descending volleys reaching the spinal neurons. However, it must be kept in mind that the reference disynaptic inhibition interneuron excitability used to detect changes in disynaptic inhibition is that measured during the baseline, i.e. at rest. In such conditions, to detect a decrease in the cathodal condition implies that at rest, there is a tonic descending volley sufficient to induce facilitation of the interneuron excitability that could decrease when this volley is reduced. If this is not the case, i.e. if at rest the descending volley is unable to induce a significant facilitation of the interneurons, no change will be seen following a decrease of cortical excitability. In other words, we favour the hypothesis, that at rest, disynaptic inhibitory interneurons behave as α-motoneurons (Evarts, 1981) i.e. that both are silent at rest. This hypothesis fits in with our results and those of Nitsche et al. (2003c) indicating that the cathodal condition modifies neither the disynaptic inhibition nor the H-reflex recruitment curves.
In conclusion, our study is the first to show that weak anodal transcranial direct current stimulation applied over hand motor cortex increases disynaptic inhibition between ECR and FCR muscle. This result indicates that modulation of motor cortex excitability induced by electrical cortical stimulation may have an impact on spinal network excitability. This is of importance taking into account the increasing usage of tDCS both in investigating fundamental mechanisms of motor control and in rehabilitation. For example, it has been shown that spastic stroke patients present a decrease of disynaptic inhibition at wrist level (Nakashima et al. 1989). Anodal tDCS is a powerful, simple and painless tool that could constitute a new therapeutic approach to increase and perhaps normalise the level of disynaptic inhibition in such patients.
Acknowledgments
The authors wish to express their gratitude to G. W. M. Westby (PhD) for having scrutinised the manuscript and to G. Bard for her technical support. This work was supported by grants from INSERM and MESR (Er 6 UPMC), APHP, ANR, IRME, FRM and Fondation Motrice.
Glossary
Abbreviations
- ECR
extensor carpi radialis
- EPSP
excitatory postsynaptic potential
- FCR
flexor carpi radialis
- Hmax
maximal H-reflex response
- IPSP
inhibitory postsynaptic potential
- ISI
inter-stimulus interval
- MEP
motor-evoked potential
- Mmax
maximal M response
- MT
motor threshold
- PAD
primary afferent depolarisation
- tDCS
transcranial direct current stimulation
- TMS
transcranial magnetic stimulation
Author contributions
N.R., B.B. and R.K. contributed to the conception and design of experiments. N.R., A.L. and V.A. performed the experiments and analysed the data. N.R., B.B. and R.K. wrote the manuscript. All authors approved the final version of the paper for publication. The experiments were conducted in Er 6 Research Unit and in the rehabilitation and physical medicine department of Raymond Poincaré Hospital.
References
- Aymard C, Chia L, Katz R, Lafitte C, Pénicaud A. Reciprocal inhibition between wrist flexors and extensors in man: a new set of interneurones? J Physiol. 1995;487:221–235. doi: 10.1113/jphysiol.1995.sp020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudewig J, Nitsche MA, Paulus W, Frahm J. Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn Reson Med. 2001;45:196–201. doi: 10.1002/1522-2594(200102)45:2<196::aid-mrm1026>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Day BL, Marsden CD, Rothwell JC. Evidence favouring presynaptic inhibition between antagonist muscle afferents in the human forearm. J Physiol. 1987;391:71–83. doi: 10.1113/jphysiol.1987.sp016726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Currà A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79–84. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Castro LO, Savagim EA, Braite R, Cruz VC, Rocha RR, Rigonatti SP, Silva MT, Fregni F. Enhancement of non dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006;404:232–236. doi: 10.1016/j.neulet.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Non monosynaptic transmission of the cortical command for voluntary movement in man. J Physiol. 1994;480:191–207. doi: 10.1113/jphysiol.1994.sp020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of afferents to propriospinal-like neurones in man during voluntary contractions. J Physiol. 1992;449:673–687. doi: 10.1113/jphysiol.1992.sp019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan JMA, Day BL, Marsden C, Rothwell JC. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and the leg in intact man. J Physiol. 1986;377:333–347. doi: 10.1113/jphysiol.1986.sp016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazières L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Day BL, Marsden CD, Obeso JA, Rothwell JC. Reciprocal inhibition between the muscles of the human forearm. J Physiol. 1984;349:519–534. doi: 10.1113/jphysiol.1984.sp015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide P, Crenna P. Exteroceptive influences on lower limb motoneurons in man: spinal and supraspinal contributions. Adv Neurol. 1983;39:797–807. [PubMed] [Google Scholar]
- Enríquez-Denton M, Nielsen J, Perreault MC, Morita H, Petersen N, Hultborn H. Presynaptic control of transmission along the pathway mediating disynaptic reciprocal inhibition in the cat. J Physiol. 2000;526:623–637. doi: 10.1111/j.1469-7793.2000.t01-1-00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV. Handbook of Physiology section I, The Nervous System, Motor Control. II. Bethesda, MD, USA: American Physiological Society; 1981. Role of motor cortex in voluntary movements in primates; pp. 1083–1120. part 2, chapter 23 Role of motor control in voluntary movements in primates. [Google Scholar]
- Furubayashi T, Terao Y, Arai N, Okabe S, Mochizuki H, Hanajima R, Hamada M, Yugeta A, Inomata-Terada S, Ugawa Y. Short and long duration transcranial direct current stimulation (tDCS) over the human hand motor area. Exp Brain Res. 2008;185:279–286. doi: 10.1007/s00221-007-1149-z. [DOI] [PubMed] [Google Scholar]
- Ghanim Z, Lamy JC, Lackmy A, Achache V, Roche N, Penicaud A, Meunier S, Katz R. Effects of galvanic mastoid stimulation in seated human subjects. J Appl Physiol. 2009;106:893–903. doi: 10.1152/japplphysiol.90594.2008. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronicstroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibres: a study in man and the cat. J Physiol. 1987;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Ko MH, Ahn SH, Kim YH, Song JC, Lee CH, Chang MC, Jang SH. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci Lett. 2008;435:56–59. doi: 10.1016/j.neulet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res. 2004;156:439–443. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Wards NS, Lee L, Nistche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Tanaka R, Yanagisawa N. Reciprocal group I inhibition on triceps surae motoneurons in man. J Neurophysiol. 1971;34:1010–1017. doi: 10.1152/jn.1971.34.6.1010. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Rothwell JC, Day BL, Thompson PD, Marsden CD. Cutaneous effects on presynaptic inhibition of flexor Ia afferents in the human forearm. J Physiol. 1990;426:369–380. doi: 10.1113/jphysiol.1990.sp018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Rothwell JC, Day BL, Thompson PD, Shannon K, Marsden CD. Reciprocal inhibition between forearm muscles in patients with writer's cramp and another occupational cramps, symptomatic hemidystonia and hemiparesis due to stroke. Brain. 1989;112:681–697. doi: 10.1093/brain/112.3.681. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henshke U, Schlitterlau A, Liebantz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulations of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003a;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebantz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003b;114:2220–2222. doi: 10.1016/s1388-2457(03)00235-9. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003c;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nielsen JB. Short-term adaptations in spinal cord circuits evoked by repetitive transcranial magnetic stimulation: possible underlying mechanisms. Exp Brain Res. 2005;162:202–212. doi: 10.1007/s00221-004-2144-2. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord. Cambridge University Press; 2005. [Google Scholar]
- Power HA, Norton JA, Porter CL, Doyle Z, Hui I, Chan KM. Transcranial direct current stimulation of the primary motor cortex affects cortical drive to human musculature as assessed by intermuscular coherence. J Physiol. 2006;583:409. doi: 10.1113/jphysiol.2006.116939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Siebner RH, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: Evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cabré A, Oliveri M, Gangitano M, Pascual-Leone A. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport. 2001;12:3845–3848. doi: 10.1097/00001756-200112040-00048. [DOI] [PubMed] [Google Scholar]
- Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006;17:671–674. doi: 10.1097/00001756-200604240-00023. [DOI] [PubMed] [Google Scholar]