Abstract
Short interval intracortical facilitation (SICF) can be elicited by transcranial magnetic stimulation (TMS) of the motor cortex (M1) with a suprathreshold first stimulus (S1) followed by a subthreshold second stimulus (S2). SICF occurs at three distinct phases and is likely to be related to the generation of indirect (I) waves. Short interval intracortical inhibition (SICI) is an inhibitory phenomenon and intracortical facilitation (ICF) is an excitatory phenomenon occurring in the M1 that can be studied with TMS. We studied the interactions between SICI/ICF and SICF in 17 healthy subjects. Six experiments were conducted. The first experiment examined the effects of different S1 intensities on SICI, ICF and SICF at three peaks. The effects of SICI on SICF were tested by a triple-pulse TMS protocol in the second experiment. We performed Experiments 3–5 to further test the interactions between SICI and SICF with various strengths of SICI, at SICF peaks and troughs, and with SICF generated by different current direction which preferentially generates late I waves. The effects of ICF on SICF were examined in Experiment 6. The results showed that ICF and SICF decreased whereas SICI increased with higher S1 intensities. SICI facilitated SICF mediated by late I waves both at the peaks and the troughs of SICF. The increase of SICF in the presence of SICI correlated to the strength of SICI. ICF decreased the third peak of SICF. We conclude that SICI facilitates SICF at neuronal circuits responsible for generating late I waves through disinhibition, while ICF may have the opposite effects.
Introduction
Cortical output depends on the balance between different inhibitory and facilitatory circuits (Ni & Chen, 2008; Rothwell et al. 2009). Transcranial magnetic stimulation (TMS) is a widely used technique to examine motor cortical physiology in humans. Depending on the stimulus parameters, TMS can be used to test different inhibitory and facilitatory circuits in the motor cortex (M1) (Hallett, 2000; Chen et al. 2008). With a subthreshold conditioning stimulus (CS) followed by a suprathreshold test stimulus (S1) at interstimulus interval (ISI) of 1–6 ms, the motor evoked potential (MEP) generated by the S1 is inhibited and this is known as short interval intracortical inhibition (SICI). On the other hand, the MEP generated by S1 is facilitated at ISI of 8–30 ms and this is termed intracortical facilitation (ICF) (Kujirai et al. 1993). If the S1 is followed by a second pulse (S2) at threshold intensity, another type of facilitation, known as short interval intracortical facilitation (SICF) or indirect (I) wave facilitation, can be elicited (Tokimura et al. 1996; Ziemann et al. 1998b; Rothwell, 1999). There are three SICF peaks, termed SICF-1, SICF-2 and SICF-3, at distinct ISIs of ∼1.5, 2.9 and 4.5 ms (Ziemann et al. 1998b; Chen & Garg, 2000). These SICF peaks are likely to be related to I wave generation. I waves were first described by Patton & Amassian (1954) in a classic study in non-human primates. They demonstrated two types of corticospinal waves following electrical stimulation of M1. The first wave was the direct (D) wave due to direct activation of the axon of corticospinal neurons and the subsequent I waves were due to trans-synaptic activation of these output neurons. I waves appeared at regular clocklike intervals of 1.5 ms. Since the three peaks of SICF also occur at about 1.5 ms intervals, it has been suggested that SICF is due to interaction of I waves generated by the two stimuli (S1 and S2) (Ziemann et al. 1998b). SICF originates in the cortical level because there was no facilitation if electrical stimulation was used to elicit S2 (Ziemann et al. 1998b) and it is associated with increased amplitudes of the I waves generated by the S1 (Di Lazzaro et al. 1999). SICF-1 is likely to be due to I2 waves from S1 interacting with I1 waves from S2; SICF-2 is likely to be due to I3 waves from S1 interacting with I1 waves from S2; and SICF-3 is likely to be related to I4 waves from S1 interacting with I1 waves from S2 (Hanajima et al. 2002). Additionally, anterior–posterior (AP) directed current in the M1 preferentially induces I3 waves whereas the usual posterior–anterior (PA) directed currents induce I1 waves (Sakai et al. 1997). SICF-1 elicited by S1 and S2 in the AP direction is likely to be due to I3 waves from S1 interacting with I2 waves from S2 (Hanajima et al. 2002).
A recent study suggested that SICI may be contaminated by facilitatory components at SICF peaks (Peurala et al. 2008). However, how SICI and ICF interact with SICF has not been determined. The objective of the present study is to further understand the physiology of I waves by studying the interactions between SICF and SICI/ICF. Since SICI is likely to be mediated by γ-aminobutyric acid type A (GABAA) receptors (Ziemann, 2004) and SICF is diminished by drugs that enhance GABAA activity (Ziemann et al. 1998c), our first hypothesis is that SICI suppresses SICF. Since SICI decreases the late I waves while the early I wave was unaffected (Nakamura et al. 1997; Hanajima et al. 1998; Di Lazzaro et al. 1998b), our second hypothesis is that SICI will have greater influence on late I wave mediated SICF peaks (SICF-2 and SICF-3) than the early I wave mediated SICF peak (SICF-1). Since AP current direction preferentially induces the I3 wave and PA current direction preferentially induces the I1 wave (Sakai et al. 1997), our third hypothesis is that SICI will have greater influence on SICF-1 elicited by the AP current direction than SICF-1 elicited by the PA current direction. These hypotheses were tested by examining the effects of SICI/ICF on three phases of SICF and by examining the effects of different current directions that preferentially induced I1 and I3 waves.
Methods
Subjects
We studied 17 right-handed healthy volunteers (9 men, 8 women, mean age 30.4 ± 7.1 years, range 18–50 years). Eleven subjects participated in Experiment 1. Eight of the 11 subjects also participated in Experiment 2 and seven of them participated in Experiments 3 and 6. Six subjects participated in Experiments 4 and 5. All subjects gave their written informed consent. The experimental protocol was approved by the University Health Network Research Ethics Board in accordance with the Declaration of Helsinki on the use of human subjects.
EMG recording
Surface electromyogram (EMG) was recorded from the right first dorsal interosseous (FDI) muscle with disposable surface Ag–AgCl electrodes in a tendon-belly arrangement. Subjects maintained relaxation throughout the experiment. The EMG was monitored on a computer screen and via speakers at high gain. The signal was amplified 1000× (Intronix Technologies Corp., Model 2024F, Bolton, Ontario, Canada), filtered (bandpass 2 Hz to 2.5 kHz), digitized at 5 kHz (Micro 1401, Cambridge Electronic Design, Cambridge, UK) and stored in a laboratory computer for off-line analysis.
Transcranial magnetic stimulation
TMS was performed with a 7 cm figure-of-eight coil and four Magstim 200 stimulators (Magstim, Whitland, Dyfed, UK) connected via three Bistim modules in a ‘pyramid’ set-up (Sanger et al. 2001). The output of each of the two pairs of stimulators was connected to one Bistim module. The output from the two Bistim modules was directed to a third Bistim module which in turn was connected to the TMS coil. This set-up allowed us to deliver up to four pulses with different stimulus intensities through the same coil at very short ISIs. The coil was placed at the optimal position for eliciting MEPs from the FDI muscle where slightly suprathreshold stimulation produces the largest MEP in the target muscle. The optimal position was marked on the scalp to ensure identical placement of the coil throughout the experiment. The handle of the coil pointed backwards and rotated about 45 deg to the mid-sagittal line. The induced current was perpendicular to the central sulcus in the PA direction and was optimal to activate the corticospinal neurons trans-synaptically (Werhahn et al. 1994; Kaneko et al. 1996).
To simplify the discussion of the results, we will define a consistent terminology for the various experiments. Each experiment consisted of several different conditions. Each condition included a test stimulus (S1). S1 was preceded by CS and/or followed by S2. CS for SICI appeared 3 ms before S1 and was labelled CS3. We selected this ISI to allow the use of a current reversal device in Experiment 3, which needs at least 2.5 ms to reverse the current direction. The CS for ICF preceded S1 by 10 ms and was labelled CS10. CS10 was chosen because it consistently produces ICF (Kujirai et al. 1993; Ridding et al. 1995). The S2 for SICF was 1.5, 2.9 or 4.5 ms after S1. Since the intensities of S1 and S2 were adjusted to generate specific MEP amplitudes for each subject, we labelled the stimulus intensity according to the target MEP size. The stimulus intensity of ‘1 mV’ indicates the minimum stimulator output (determined to the nearest 1%) to produce MEPs of ∼1 mV in amplitude in at least 5 out of 10 trials. ‘0.2 mV’, ‘0.5 mV’ and ‘4 mV’ are defined similarly. A previous study showed that SICI, ICF and SICF can be observed with S1 intensity of ‘0.5 mV’ (Chen & Garg, 2000). This intensity is named S10.5mV for the PA direction and S10.5mV(AP) for the AP direction. S10.5mV(AP) is higher than S10.5mV although they generate a similar MEP size. The CS intensities were expressed as a percentage of the rest motor threshold (RMT). RMT was determined at rest and was the minimum stimulator output that produced MEPs of >50 μV in at least 5 out of 10 trials. The CS intensity used for SICI (CS3) and ICF (CS10) was 80% RMT and was labelled as CS30.8RMT and CS100.8RMT. It should be noted that CS30.8RMT and CS100.8RMT represented the same CS intensity but were delivered at different ISIs before S1. S2 intensity was set at RMT and was named S2RMT.
Experiment 1: Effects of S1 intensity on SICI, ICF and SICF
We examined whether S1 intensities had different effects on SICI, ICF and SICF. CS intensities were CS30.8RMT for SICI and CS100.8RMT for ICF. S2 intensity was S2RMT. Six conditions were tested and are listed in Table 1: S1 alone (1A), CS3–S1 (1B), CS10–S1 (1C) and S1–S2 at three different ISIs for SICF (1D for 1.5 ms, 1E for 2.9 ms and 1F for 4.5 ms). Each run consisted of 10 trials for each of the six conditions delivered in a random order (60 trials in total). Three different S1 intensities of ‘0.2 mV’ (S10.2mV), ‘1 mV’ (S11mV) and ‘4 mV’ (S14mV) were studied in separate runs. These S1 intensities were chosen to investigate a wide range of test MEP amplitudes.
Table 1.
Stimulus conditions used in Experiment 1
| Stimulus |
|||
|---|---|---|---|
| Condition | CS | S1 | S2 |
| A | — | S10.2mV, S11mV, S14mV | — |
| B | CS30.8RMT | S10.2mV, S11mV, S14mV | — |
| C | CS100.8RMT | S10.2mV, S11mV, S14mV | — |
| D | — | S10.2mV, S11mV, S14mV | S2RMT (ISI 1.5 ms) |
| E | — | S10.2mV, S11mV, S14mV | S2RMT (ISI 2.9 ms) |
| F | — | S10.2mV, S11mV, S14mV | S2RMT (ISI 4.5 ms) |
S10.2mV, S11mV, S14mV represent different S1 intensities. These intensities were tested in separate runs.
Experiment 2: Effects of SICI on SICF
Experiment 2 tested the effects of SICI on SICF in eight subjects. CS preceded S1 at an ISI of 3 ms at the intensity of CS30.8MT. S1 intensity was set at S10.5mV (able to generate 0.5 mV MEP in the PA current direction). This intensity was chosen to avoid floor or ceiling effects. In addition, the AP current direction was used in Experiment 3, which required higher stimulus intensity than the PA direction (Hanajima et al. 2002) for the same MEP amplitude. S10.5mV allowed us to match the test MEP amplitudes produced by the AP and PA current directions. Since the response to S1 was likely to be inhibited by the preceding CS3, in some experimental conditions we adjusted the S1 intensity to generate ∼0.5 mV MEP in the presence of CS3. We named this intensity S10.5mV(CS3). S2 was delivered following S1 at an intensity of S2RMT. Seven conditions were included in this experiment and are listed in Table 2: S1 alone (2A), CS3–S1 (2B), S1–S2 (2C), S1 alone with adjusted intensity (2D), CS3–S1 with adjusted intensity (2E), S1 with adjusted intensity–S2 (2F) and triple-pulse CS3–S1 with adjusted intensity–S2 (2G). Each run consisted of 10 trials for each of the seven conditions delivered 6 s apart in random order (70 trials in total). Three different S1–S2 ISIs for SICF-1 (1.5 ms), SICF-2 (2.9 ms) and SICF-3 (4.5 ms) were tested in separate runs using the same stimulus configuration. The averaged MEP amplitudes for conditions 2A and 2E were calculated immediately after each experimental run. This was done to ensure that they were within 30% of their own target MEP amplitudes. Additionally, the average MEP amplitudes of 2A and 2E were also required to be within 30% of each other. The experimental run was repeated if the above conditions were not met.
Table 2.
Stimulus conditions used in Experiments 2 to 6
| Stimulus |
||||
|---|---|---|---|---|
| CS |
||||
| Condition | Exp. 2–5 | Exp. 6 | S1 | S2 |
| A | — | — | S10.5mV | — |
| B | CS30.8RMT | CS100.8RMT | S10.5mV | — |
| C | — | — | S10.5mV | S2RMT |
| D | — | — | S10.5mV(CS) | — |
| E | CS30.8RMT | CS100.8RMT | S10.5mV(CS) | — |
| F | — | — | S10.5mV(CS) | S2RMT |
| G | CS30.8RMT | CS100.8RMT | S10.5mV(CS) | S2RMT |
| Acon | — | — | S10.5mV | — |
| Dcon | — | — | S10.5mV(CS) | — |
| Gcon | CS30.8RMT | CS100.8RMT | S10.5mV(CS) | S2RMT(CS) |
S1 intensity was adjusted according to the preceding CS (CS3 in Experiments 2 to 5; CS10 in Experiment 6). Various CS intensities were tested in Experiment 4.
Since a preceding CS3 may also inhibit the response to S2, we conducted an additional experiment with control conditions to address this issue. We determined the RMT in the presence of a preceding CS3 (actual ISI used was 3 ms + ISI between S1 and S2). It was named RMTCS3. S2 intensity used in the control conditions was named S2RMT(CS3). This intensity slightly varied with ISI between S1 and S2. Three control conditions were tested (Table 2): S1 alone (2Acon), S1 with adjusted intensity (S10.5mV(CS3)) (2Dcon) and triple-pulse with adjusted S1 and S2 intensities (2Gcon). Ten trials for each condition were delivered in random order (30 trials). Three different S1–S2 ISIs (1.5, 2.9 and 4.5 ms) were tested in separate runs.
SICI and SICF were derived from the ratios of the MEP amplitude induced in one condition compared to another. Conditions 2A to 2C were used to determine SICI (2B/2A) and SICF (2C/2A) for a test MEP of 0.5 mV. Conditions 2D to 2G used the adjusted S1 intensity to produce 0.5 mV MEPs in the presence of CS3 (S10.5mV(CS3)). E/D represents the SICI with the adjusted S1 intensity. The MEP amplitude in condition 2E was adjusted to 0.5 mV and this allowed us to match the MEP amplitude to produce a similar degree of corticospinal activation with a preceding CS3 (2E) and without the CS3 (2A). Therefore, SICF in the presence of SICI (2G/2E) was compared to SICF alone matched for MEP amplitude (0.5 mV) (2C/2A) and to SICF alone matched for S1 intensity (S10.5mV(CS3)) (2F/2D). In addition, 2Gcon/2E was compared to 2G/2E to address the question of whether the changed SICF in the presence of SICI was due to the preceding CS3 suppressing the response to S2 because condition 2Gcon used the adjusted S2 intensity (S2RMT(CS3)).
Experiment 3: Effects of SICI on SICF with AP current direction for S1 and S2
Experiment 3 was designed to test the effects of SICI on SICF elicited by the AP current direction. A custom-made current reversal device was connected to the final (third) Bistim module used in Experiment 2. This set-up allowed us to deliver up to four pulses with different intensities and current directions at very short ISIs. The induced currents were in the PA direction for CS, and the AP direction for S1 and S2. In each subject it was first confirmed that PA and AP current directions generated MEPs with different latencies under slight voluntary contraction (Hanajima et al. 2002). We then determined the RMT and S1 intensity for 0.5 mV MEP for the AP current direction. We named them RMTAP and S10.5mV(AP), respectively. These intensities were higher than those under the PA current direction (RMT and S10.5mV). The CS intensity was set at CS30.8RMT, the same as in Experiment 2. The S1 intensity was S10.5mV(AP) and the S2 intensity was S2RMT(AP). We also determined the S1 intensity for generating 0.5 mV MEP and RMT in the AP direction in the presence of CS3. These intensities were named S10.5mV(CS3AP) and RMTCS3AP, respectively. The same experimental conditions as Experiment 2 were used (Table 2) and we named them 3A to 3G. Ten trials for each condition (70 trials in total) were delivered in random order. A similar control experiment as described for Experiment 2 including conditions 3Acon, 3Dcon and 3Gcon was also conducted. Thirty trials for the three conditions were collected in random order. Only the S1–S2 ISI of 1.5 ms was tested. Similar to Experiment 2, ratios of 3C/3A, 3F/3D, 3G/3E, 3Gcon/3E were compared.
Experiment 4: Effects of SICI on SICF elicited by different CS intensities
Experiment 4 with the CS intensity recruitment curve was performed to further explore the findings from Experiment 2. CS intensities are expressed as fractions of active motor threshold (AMT). AMT was defined as the minimum stimulator output that produced MEPs of more than 200 μV in at least 5 out of 10 trials while the subject performed voluntary muscle contraction of 20% maximum. Five CS intensities varying from 0.6 to 1.0 AMT with steps of 0.1 AMT were tested. AMT was used in order that the results are comparable to a study of SICI and SICF at different CS intensities (Peurala et al. 2008). The experimental conditions of 4A–4G were used (Table 2). Different CS intensities were tested in separate runs. Only S1–S2 at ISI of 4.5 ms was tested because Experiment 2 showed significant effects of SICI on SICF at the later SICF peaks (see Results).
Experiment 5: Effects of SICI on SICF at SICF peak and trough
Experiment 5 was designed to examine whether the interaction between SICI and SICF found in Experiment 2 also occurs at SICF troughs. Since we found significant facilitation of SICF in the presence of SICI at peaks 2 and 3 in Experiment 2 (see Results) and trough 2 is between these two peaks, SICF at trough 2 was tested. The second trough and the third peak of SICF with S1–S2 ISIs of 4.0 and 4.5 ms were compared. Ten experimental conditions were tested including conditions 5A–5G (Table 2) with conditions 5C, 5F and 5G administered twice (with S1–S2 ISIs of 4.0 and 4.5 ms).
Experiment 6: Effects of ICF on SICF
The experimental set-up was identical to Experiment 2 except that CS10 was used instead of CS3. The experimental conditions were named 6A–6G (Table 2). Before the experiment, we determined the S1 intensity which can generate 0.5 mV MEP in the presence of CS10. This intensity was named S10.5mV(CS10). RMT in the presence of CS10 (RMTCS10) was determined in a similar way. S10.5mV was used as S1 intensity in conditions 6A–6C. Adjusted S1 intensity (S10.5mV(CS10)) was used in conditions 6D–6G. S2RMT was used as S2 intensity. A control experiment including conditions 6Acon, 6Dcon and 6Gcon was also conducted. The effect of adjusted S2 intensity (S2RMT(CS10)) was tested. S1–S2 ISIs of 1.5, 2.9 and 4.5 ms were tested in separate runs. The ratios 6C/6A, 6F/6D, 6G/6E and 6Gcon/6E were compared.
Data analysis
The peak-to-peak MEP amplitude for each trial was measured off-line. Ratios for different conditions in each experiment were calculated. These ratios were B/A, C/A, D/A, E/A and F/A for Experiment 1 and C/A, F/D, G/E and Gcon/E for Experiments 2–6. Ratios of less than 1 indicate inhibition, and ratios greater than 1 indicate facilitation. Values were expressed as means ± standard deviation.
Statistical analysis
For Experiment 1, the effects of S1 intensities on SICI (B/A), ICF (C/A) and SICF at the three peaks (D/A for SICF-1, E/A for SICF-2, F/A for SICF-3) were evaluated by repeated measures analysis of variance (ANOVA). For Experiments 2, 3 and 6, ratios of G/E and Gcon/E were compared to C/A with repeated measures ANOVA because these three ratios represent the SICF in the presence of SICI/ICF with adjusted S2 intensity (Gcon/E), without adjusted S2 intensity (G/E) and SICF alone (C/A), and because they were matched for test MEP amplitude (∼0.5 mV). The Greenhouse–Geisser correction was used to correct for non-sphericity. If ANOVA showed significant main effects, post hoc Fisher's protected least significance difference tests were used for further analysis. In order to examine the effect of SICI/ICF on SICF matched for S1 intensity, a separate paired t test was used to compare G/E (SICF in the presence of CS with adjusted S1 intensity) with F/D (SICF alone with adjusted S1 intensity). For Experiment 4, a two-way repeated measures ANOVA was used to test the main effect of SICF in the presence of SICI (SICFSICI, G/E) vs. SICF alone (SICFAlone, C/A) and the main effect of CS intensity. In addition, SICFSICI and SICI for all subjects were averaged at each CS intensity, and the averaged values were used to test the relationship between SICF in the presence of SICI and the strength of SICI with Pearson's correlation coefficient. For Experiment 5, a two-way repeated measures ANOVA was used to examine whether SICF in the presence of SICI was different at SICF peaks and troughs. ISI (4.0 ms vs. 4.5 ms) and conditions with or without preceding CS (SICFSICIvs. SICFAlone) were the main factors. A post hoc Student's paired t test with Bonferroni's correction for multiple comparisons was used for Experiments 4 and 5 to examine whether SICFSICI and SICFAlone were different under different experimental conditions. The threshold for significance was set at P < 0.05. StatView 5.0.1 software was used for statistical analysis.
Results
Experiment 1: Effects of S1 intensity on SICI, ICF and SICF
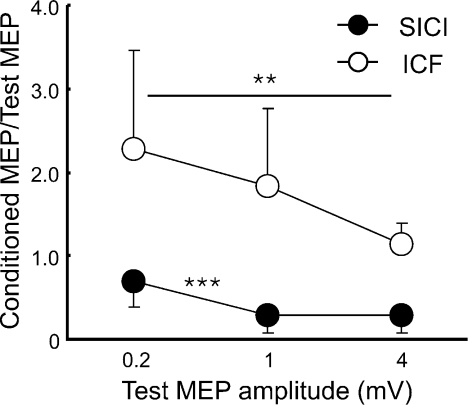
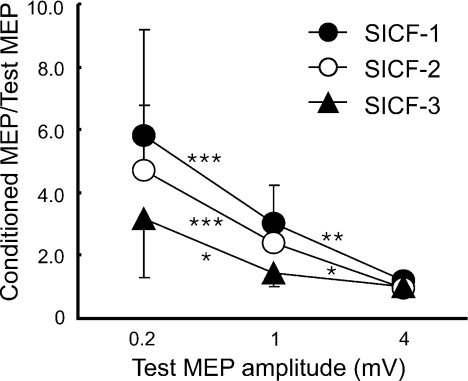
The MEP amplitudes (n= 11) were 0.29 ± 0.11 mV for S10.2mV, 0.89 ± 0.12 mV for S11mV and 3.33 ± 0.72 mV for S14mV. SICI, ICF (Fig. 1) and SICF at the three peaks (Fig. 2) varied with S1 intensities. For SICI, there was a significant effect of S1 intensity (ANOVA, F2,20= 24.15, P < 0.001). A post hoc test revealed that SICI increased when the test MEP amplitude increased from 0.2 to 1 mV (P < 0.001) with no significant difference between test MEP amplitudes of 1 and 4 mV. For ICF, facilitation decreased with higher S1 intensity (ANOVA, F2,20= 5.37, P= 0.014). A post hoc test confirmed stronger ICF at 0.2 mV compared to 4 mV MEP size (P= 0.005). ICF for test MEP amplitudes of 0.2 and 1 mV or for 1 and 4 mV showed no significant difference. Figure 2 shows that SICF-1 (1.5 ms), SICF-2 (2.9 ms) and SICF-3 (4.5 ms) all decreased with increasing S1 intensity (ANOVA; SICF-1, F2,20= 17.85, P < 0.001; SICF-2, F2,20= 36.19, P < 0.001; SICF-3, F2,20= 14.20, P < 0.001). Post hoc tests confirmed that SICF-1 decreased when MEP amplitudes increased from 0.2 to 1 mV (P < 0.001) and from 1 to 4 mV (P= 0.009). SICF-2 showed similar results (0.2 to 1 mV, P < 0.001; 1 to 4 mV, P= 0.011). SICF-3 decreased when MEP amplitude increased from 0.2 to 1 mV (P= 0.010) but a further increase of MEP amplitude from 1 to 4 mV did not change the degree of facilitation.
Figure 1. Effects of different test stimulus intensities on short interval intracortical inhibition and intracortical facilitation (Experiment 1).
Data from 11 subjects. The abscissa indicates the MEP size induced by test stimulus (S1) alone. The ordinate indicates the amplitude of conditioned MEP (CS3/CS10–S1 paired-pulse) expressed as a ratio of the MEP amplitude induced by S1 alone. Ratios less than 1 indicate inhibition and ratios greater than 1 indicate facilitation. Filled circles indicate short interval intracortical inhibition (SICI) and open circles indicate intracortical facilitation (ICF). **P < 0.01, ***P < 0.001, comparing different S1 intensities.
Figure 2. Effects of different test stimulus intensities on the three phases of short interval intracortical facilitation (Experiment 1).
Data from 11 subjects. The abscissa indicates the MEP size induced by test stimulus (S1) alone. The ordinate indicates the amplitude of conditioned MEP (S1–S2 paired-pulse) expressed as a ratio of the MEP amplitude induced by S1 alone. Filled circles indicate short interval intracortical facilitation (SICF)-1 (S1–S2 interval 1.5 ms). Open circles indicate SICF-2 (S1–S2 interval 2.9 ms). Filled triangles indicate SICF-3 (S1–S2 interval 4.5 ms). The ratios are greater than 1, indicating that all interactions are facilitatory. *P < 0.05, **P < 0.01, ***P < 0.001, comparing different S1 intensities.
Experiments 2: Effects of SICI on SICF with PA current direction
Experiment 2 tested the effects of SICI on SICF at the three peaks. The stimulus intensities and MEP amplitudes for conditions 2A and 2E at different S1–S2 ISIs (1.5, 2.9 and 4.5 ms) were listed in Table 3. The MEP amplitudes induced by S1 alone (condition 2A) and those induced by CS3 followed by S1 with adjusted intensity (condition 2E) were matched (∼0.5 mV). In addition, conditions 2Acon and 2A used the same S1 intensity and produced similar MEP size (Table 3). MEPs under condition 2Dcon and 2D were also at a similar size (not shown). This confirmed that S1 used in the main and control experiments recruited a similar number of corticospinal neurons and allowed us to compare the ratio of 2Gcon/2E (obtained from control experiment) to other ratios obtained from the main experiment (2C/2A and 2G/2E). SICI alone for 0.5 mV test MEP (2B/2A) was 0.50 ± 0.19 (n= 8) and SICI alone for the adjust S1 intensity (2E/2D) was 0.24 ± 0.09. This is similar to the results of Experiment 1 (Fig. 1) showing more prominent SICI with larger test MEP.
Table 3.
Stimulus intensities and MEP amplitudes under different stimulus conditions in Experiments 2 to 6
| S1–S2 ISI | Stimulus intensity (% of maximum stimulator output) |
MEP amplitude (mV) under different conditions |
||||||
|---|---|---|---|---|---|---|---|---|
| Threshold | CS | S1 | Adjusted S1 | S1 alone (A) | CS-S1 (E) | S1 alone (Acon) | ||
| Experiment 2 | 1.5 ms | 49.0 ± 6.6 | 0.8RMT | 54.8 ± 6.5 | 74.5 ± 13.4 | 0.50 ± 0.19 | 0.59 ± 0.18 | 0.48 ± 0.25 |
| (n= 8) | 2.9 ms | — | 0.8RMT | 54.8 ± 6.5 | 74.5 ± 13.4 | 0.57 ± 0.12 | 0.59 ± 0.22 | 0.57 ± 0.15 |
| 4.5 ms | — | 0.8RMT | 54.8 ± 6.5 | 74.5 ± 13.4 | 0.56 ± 0.13 | 0.47 ± 0.05 | 0.60 ± 0.17 | |
| Experiment 3 | 1.5 ms | 69.0 ± 9.0 (AP) | 0.8RMT (PA) | 75.2 ± 12.1 | 86.3 ± 13.2 | 0.56 ± 0.15 | 0.48 ± 0.28 | 0.49 ± 0.37 |
| (n= 7) | 48.3 ± 6.7 (PA) | |||||||
| Experiment 4 | 4.5 ms | 39.8 ± 7.7 (AMT) | 0.6AMT | 58.3 ± 12.4 | 60.8 ± 9.5 | 0.58 ± 0.17 | 0.54 ± 0.20 | NT |
| (n= 6) | — | 0.7AMT | 58.3 ± 12.4 | 65.2 ± 10.7 | 0.53 ± 0.13 | 0.48 ± 0.17 | NT | |
| — | 0.8AMT | 58.3 ± 12.4 | 70.8 ± 13.5 | 0.55 ± 0.20 | 0.58 ± 0.13 | NT | ||
| — | 0.9AMT | 58.3 ± 12.4 | 80.3 ± 11.8 | 0.59 ± 0.18 | 0.53 ± 0.22 | NT | ||
| — | 1.0AMT | 58.3 ± 12.4 | 74.8 ± 10.9 | 0.56 ± 0.20 | 0.55 ± 0.18 | NT | ||
| Experiment 5 | 4.0 ms | 47.5 ± 10.4 | 0.8RMT | 58.3 ± 12.4 | 76.8 ± 10.5 | 0.58 ± 0.25 | 0.49 ± 0.18 | NT |
| (n= 6) | 4.5 ms | — | 0.8RMT | 58.3 ± 12.4 | 76.8 ± 10.5 | 0.58 ± 0.25 | 0.49 ± 0.18 | NT |
| Experiment 6 | 1.5 ms | 48.3 ± 6.7 | 0.8RMT | 56.0 ± 7.2 | 52.0 ± 9.1 | 0.49 ± 0.16 | 0.50 ± 0.26 | 0.54 ± 0.22 |
| (n= 7) | 2.9 ms | — | 0.8RMT | 56.0 ± 7.2 | 52.0 ± 9.1 | 0.49 ± 0.19 | 0.53 ± 0.31 | 0.57 ± 0.24 |
| 4.5 ms | — | 0.8RMT | 56.0 ± 7.2 | 52.0 ± 9.1 | 0.63 ± 0.21 | 0.52 ± 0.23 | 0.64 ± 0.21 | |
AMT, active motor threshold; RMT, rest motor threshold; AP, anterior–posterior; PA, posterior–anterior; NT, not tested. AMT was used in Experiment 4. RMT was used in other experiments. RMT both in PA and AP current directions was tested in Experiment 3. For other experiments only RMT in PA current direction was tested. S1 intensity used in condition E was adjusted to produce 0.5 mV MEP in the presence of CS. Values are expressed as means ± standard deviation.
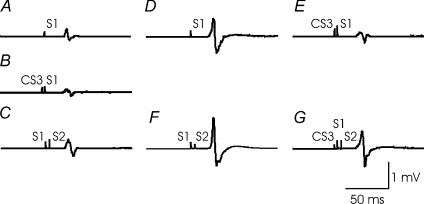
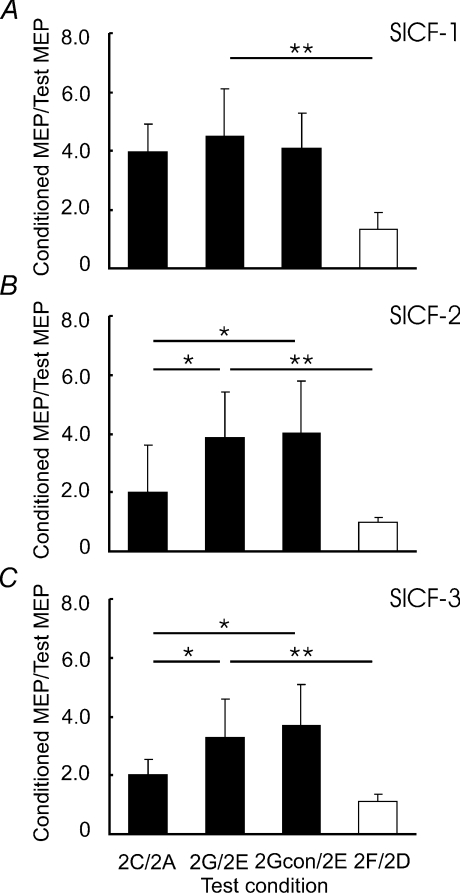
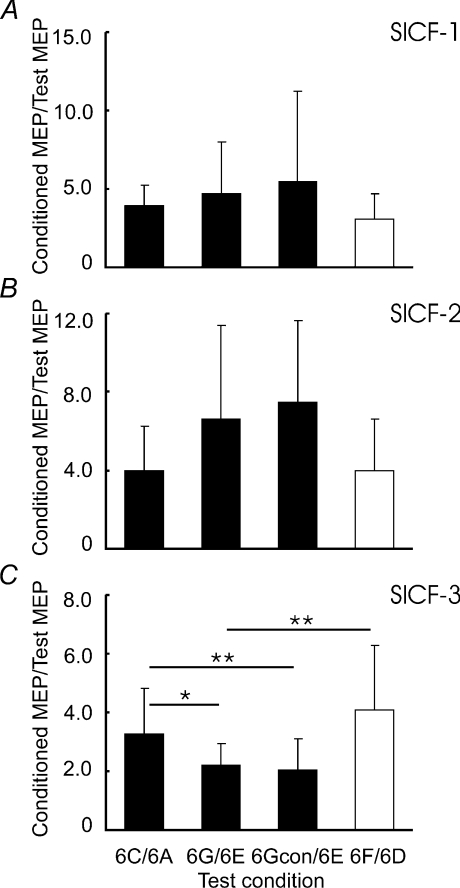
Figure 3 shows the effects of SICI on SICF-3 in one subject. It was found that SICF-3 in the presence of SICI (compare Fig. 3G to Fig. 3E) was stronger than SICF-3 alone (compare Fig. 3C to Fig. 3A), although the test MEP sizes were matched (Fig. 3A and Fig. 3E). Stronger SICF-3 in the presence of SICI was also observed with matched S1 intensity (Fig. 3, comparing F/D and G/E). For the group data analysis, we first confirmed that SICF was elicited by S1–S2 at the three ISIs tested in all subjects. Figure 4 shows that SICF (2C/2A) was clearly above 1 at all three ISIs. Then, SICF at three peaks in the presence of SICI with adjusted S2 intensity (2Gcon/2E) and without adjusted S2 intensity (2G/2E) were compared to SICF alone (2C/2A) matched for MEP amplitude. SICF-1 (Fig. 4A) was not changed by SICI with or without adjustment for S2 intensity (ANOVA, F2,14= 1.48, P= 0.26). Different results were obtained from SICF-2 (Fig. 4B) and SICF-3 (Fig. 4C). ANOVA showed that SICF-2 and SICF-3 were significantly increased in the presence of SICI (SICF-2, F2,14= 8.99, P= 0.003; SICF-3, F2,14= 9.59, P= 0.002). Post hoc tests confirmed this main effect with both adjusted S2 intensity (2Gcon/2E vs. 2C/2A; P= 0.040 for SICF-2 and P= 0.020 for SICF-3) and without adjusted S2 intensity (2G/2E vs. 2C/2A; P= 0.040 for SICF-2 and P= 0.038 for SICF-3). Paired t tests comparing SICF in the presence of SICI to SICF alone matched for S1 intensity (2G/2E vs. 2F/2D) showed that stronger facilitation in the presence of SICI occurred at all three SICF peaks (SICF-1, df = 7, t= 5.41, P= 0.001; SICF-2, df = 7, t= 5.12, P= 0.001; SICF-3, df = 7, t= 4.73, P= 0.002). This effect is likely to be due to the larger test MEP size, for 2F/2D decreased the degree of SICF, similar to the results of Experiment 1 (Fig. 2).
Figure 3. Short interval intracortical facilitation in the presence of short interval intracortical inhibition in one representative subject (Experiment 2).
Changes in the third phase of short interval intracortical facilitation (SICF-3) in the presence of short interval intracortical inhibition (SICI) in one subject are shown. Each trace represents averaged MEPs of 10 trials. A, test stimulus (S1) alone, able to generate MEP of about 0.5 mV in amplitude (S10.5mV). B, SICI induced by a subthreshold conditioning stimulus (CS3) preceding the S1 by 3 ms. C, SICF-3 elicited by a threshold conditioning stimulus (S2) following S1 by 4.5 ms. D, S1 alone, but able to generate 0.5 mV MEP in the presence of CS3 (S10.5mVCS3). E, CS3– S10.5mVCS3 pulse combination, the test MEP amplitude in the presence of CS3 was matched to 0.5 mV, similar to A. F, S10.5mVCS3–S2 combination, SICF-3 with adjusted S1 intensity (S10.5mVCS3). G, effect of SICI on SICF-3. The stimulus conditions correspond to Table 2. C/A (MEP amplitude in C divided by that in A) represents SICF-3 alone. Similarly, F/D represents SICF-3 alone with adjusted S1 intensity (S10.5mVCS3). G/E indicates SICF-3 in the presence of SICI with same test MEP amplitude as C/A.
Figure 4. Changes in the three different phases of short interval intracortical facilitation in the presence of short interval intracortical inhibition (Experiment 2).
Means and standard deviations (n= 8) of short interval intracortical facilitation (SICF) under different experimental conditions. SICF was normalized as a ratio of conditioned MEP to the test MEP. 2C/2A indicates SICF alone. 2G/2E indicates SICF in the presence of short interval intracortical inhibition (SICI) with same test MEP size as 2C/2A. 2Gcon/2E indicates SICF in the presence of SICI, but with adjusted S2 intensity (motor threshold in the presence of CS3). These three ratios are shown in filled columns. They are matched by test MEP size. Open columns indicate 2F/2D (SICF alone, but with adjusted S1 intensity). 2F/2D and 2G/2E used same S1 intensity. A, interaction between SICI and SICF-1. B, interaction between SICI and SICF-2. C, interaction between SICI and SICF-3. *P < 0.05, **P < 0.01, comparing SICF under different experimental conditions. SICF-2 and SICF-3 but not SICF-1 were increased by SICI with matched test MEP amplitude.
Experiment 3: Effects of SICI on SICF with AP current direction
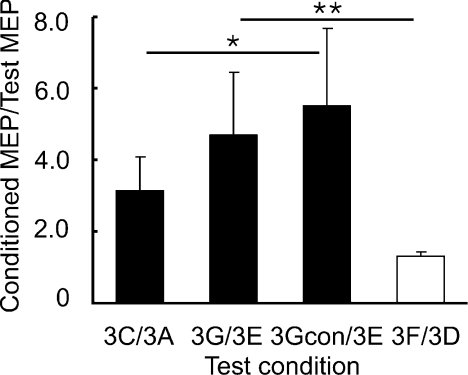
In Experiment 3, we tested the effects of SICI on SICF at 1.5 ms with stimulation current for S1 and S2 in the AP instead of PA direction. The averaged MEP amplitudes for S1 alone (condition 3A) and for S1 with preceding CS3 (condition 3E) were matched (Table 3). In addition, conditions 3Acon and 3A induced similar MEP amplitudes (Table 3). Similar to Experiment 2, we compared 3Gcon/3E and 3G/3E to 3C/3A, but the results were different from Experiment 2 (Fig. 5). SICF at ISI of 1.5 ms was increased by SICI (ANOVA, F2,12= 5.44, P= 0.021). Post hoc tests showed that with adjusted S2 intensity (3Gcon/3E), SICF in the presence of SICI was stronger than SICF alone (3C/3A) (P= 0.016). Without adjustment for S2 intensity (3G/3E), SICF showed a trend for increase (P= 0.085). Moreover, SICF in the presence of SICI (3G/3E) compared to SICF alone matched for S1 intensity (3F/3D) also showed a strong increase of facilitation (df = 6, t= 5.06, P= 0.002).
Figure 5. Changes in short interval intracortical facilitation in the presence of short interval intracortical inhibition with reversed stimulation current (Experiment 3).
Means and standard deviations (n= 7) of the first phase of short interval intracortical facilitation (SICF-1) under different experimental conditions. SICF was normalized as a ratio of conditioned MEP to the test MEP. 3C/3A indicates SICF alone. 3G/3E indicates SICF in the presence of short interval intracortical inhibition (SICI) with same test MEP size as 3C/3A. 3Gcon/3E indicates SICF in the presence of SICI, but with adjusted S2 intensity (motor threshold in the presence of CS3). These three ratios are shown in filled columns and they are matched by test MEP size. Open columns represent 3F/3D (SICF alone, but with adjusted S1 intensity). 3F/3D and 3G/3E used same S1 intensity. *P < 0.05, **P < 0.01, comparing SICF under different experimental conditions. SICF-1 is increased in the presence of SICI with reversed (anterior–posterior) stimulation current.
Experiment 4: CS intensity recruitment curve for the effects of SICI on SICF
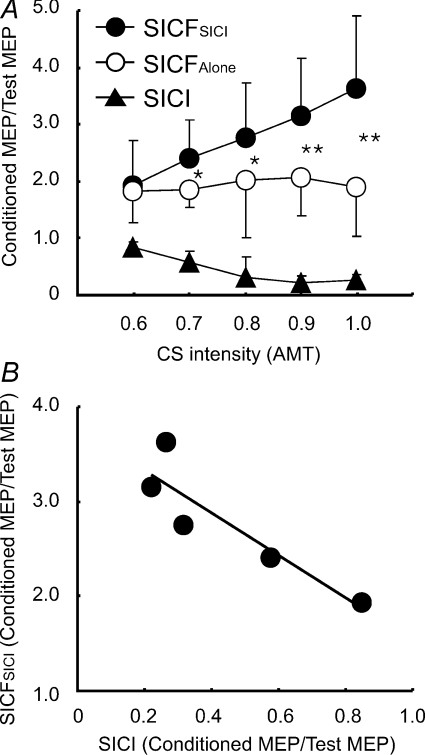
We compared SICF in the presence of SICI (SICFSICI) to the SICF alone (SICFAlone) at different CS intensities. S1 intensity was adjusted to match a MEP amplitude of ∼0.5 mV for each CS intensity step (Table 3). SICI increased with CS intensity from 0.6 to 0.9 AMT and slightly decreased with CS intensity from 0.9 to 1.0 AMT (Fig. 6A). ANOVA showed that SICF was stronger in the presence of SICI than SICF alone (F1,20= 49.54, P < 0.001). The effect of CS intensity on SICF (F4,20= 2.99, P= 0.044) and the interaction between two main effects were also significant (F1,20= 5.15, P= 0.005). In particular, SICF in the presence of SICI increased with CS intensity while SICF alone did not (no CS was applied in this condition) (Fig. 6A). Post hoc tests confirmed that increase for SICF in the presence of SICI occurred at CS intensities higher than 0.7 AMT (0.7 AMT, P= 0.037; 0.8 AMT, P= 0.030; 0.9 AMT, P= 0.009; 1.0 AMT, P= 0.005). Correlation analysis (Fig. 6B) showed that SICF in the presence of SICI increased linearly with the strength of SICI (R2= 0.816, F1,3= 13.29, P= 0.036).
Figure 6. Effects of different conditioning stimulus intensities for interaction between short interval intracortical inhibition and short interval intracortical facilitation (Experiment 4).
Data from 6 subjects. A, the effects of different conditioning stimulus (CS) intensities for short interval intracortical inhibition (SICI) on short interval intracortical facilitation (SICF) in the presence of SICI, and for SICI and SICF alone. The abscissa indicates CS intensities expressed as a fraction of active motor threshold (AMT). The ordinate indicates conditioned MEP, including SICF in the presence of SICI (SICFSICI, filled circles), SICF alone (SICFAlone, open circles) and SICI alone (filled triangles). They are normalized as ratios of conditioned MEP to the test MEP. *P < 0.05, **P < 0.01, comparing SICFSICI to SICFAlone. B, relationship between SICFSICI and SICI. The abscissa indicates the strength of SICI, with one being no inhibition. Ordinate indicates SICF in the presence of SICI. SICI and SICF were normalized as ratios of conditioned MEP to the test MEP. Each point represents the averaged value of six subjects at a given CS intensity.
Experiment 5: Effects of SICI on SICF at SICF trough 2 and peak 3
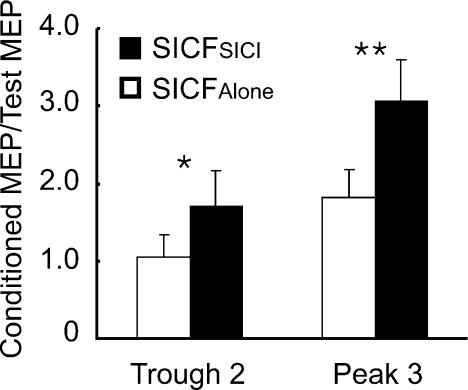
The test MEP sizes at both ISIs of 4.0 and 4.5 ms were matched to ∼0.5 mV (Table 3). Figure 7 shows that SICF was facilitated by SICI at both trough 2 and peak 3. The effect of ISI (trough 2 vs. peak 3) was significant (F1,5= 40.22, P= 0.001). As expected, at trough 2 there was no facilitation with SICF alone (MEP ratio ∼1) whereas SICF alone was evident at peak 3 (MEP ratio ∼ 2). There was also a significant main effect of SICI on SICF (SICFSICIvs. SICFAlone, F1,5= 22.52, P= 0.005). Post hoc tests showed that SICF in the presence of SICI was stronger than SICF alone at both trough 2 (P= 0.017) and peak 3 (P= 0.006). The interaction between SICI and ISI was significant (F1,5= 11.75, P= 0.019). Figure 7 shows that the absolute increase in SICF in the presence of SICI was slightly greater for peak 3 than trough 2. However, the ratios of SICFSICI to SICFAlone were similar for trough 2 (1.64 ± 0.36) and peak 3 (1.76 ± 0.54) (paired t test, df = 5, t= 0.82, P= 0.451). Thus, the degrees of facilitation elicited by CS3 were similar at SICF trough 2 and peak 3.
Figure 7. Short interval intracortical facilitation at the second trough and third peak in the presence of short interval intracortical inhibition (Experiment 5).
Means and standard deviations (n= 6) of short interval intracortical facilitation (SICF) in the presence of short interval intracortical inhibition (SICI) and SICF alone at the trough 2 and peak 3 of SICF. The ordinate indicates SICF. SICF was normalized as a ratio of conditioned MEP to the test MEP. Filled columns indicate SICF in the presence of SICI (SICFSICI). Open columns indicate SICF alone (SICFAlone). *P < 0.05, **P < 0.01, comparing SICFSICI to SICFAlone.
Experiment 6: Effects of ICF on SICF
The MEP amplitudes for conditions 6A and 6E at different S1–S2 ISIs (1.5, 2.9 and 4.5 ms) were listed in Table 3. MEP amplitudes for S1 alone (6A) and CS10 followed by adjusted S1 intensity (6E) were matched. Conditions 6Acon and 6A induced the same MEP size (Table 3). Similar to Experiment 2, we compared the ratios of 6Gcon/6E and 6G/6E to 6C/6A. SICF-1 (Fig. 8A) and SICF-2 (Fig. 8B) were not changed by ICF (ANOVA; SICF-1, F2,12= 0.39, P= 0.684; SICF-2, F2,12= 2.10, P= 0.166). On the other hand, SICF-3 (Fig. 8C) was weaker in the presence of ICF (ANOVA; F2,12= 5.63, P= 0.019). Post hoc tests showed that both with adjusted S2 intensity (6Gcon/6E, P= 0.008) and without adjusted S2 intensity (6G/6E, P= 0.014), SICF-3 was weaker in the presence of ICF than SICF-3 alone (6C/6A). A separate t test comparing 6G/6E vs. 6F/6D showed that SICF-3 in the presence of ICF was weaker than SICF-3 alone with matched S1 intensity (df = 6, t= 3.18, P= 0.007).
Figure 8. Changes in the three different phases of short interval intracortical facilitation in the presence of intracortical facilitation (Experiment 6).
Means and standard deviations (n= 7) of short interval intracortical facilitation (SICF) under different experimental conditions. SICF was normalized as a ratio of conditioned MEP to the test MEP. 6C/6A indicates SICF alone. 6G/6E indicates SICF in the presence of intracortical facilitation (ICF) with same test MEP size as 6C/6A. 6Gcon/6E indicates SICF in the presence of ICF, but with adjusted S2 intensity (motor threshold in the presence of CS10). These three ratios are shown in filled columns and they are matched by test MEP size. Open columns indicate 6F/6D (SICF alone, but with adjusted S1 intensity). 6F/6D and 6G/6E used same S1 intensity. A, interaction between ICF and SICF-1. B, interaction between ICF and SICF-2. C, interaction between ICF and SICF-3. *P < 0.05, **P < 0.01, comparing SICF under different experimental conditions. Only SICF-3 was suppressed by ICF.
Discussion
Experiment 1 showed that SICI increases whereas facilitatory circuits (ICF and SICF) decrease with increasing strength of the test stimulus (S1). Experiments 2 and 3 suggest that SICI increases SICF mediated by late I waves with both AP and PA directed currents. Experiments 4 and 5 showed that the increase of SICF in the presence of SICI occurs at both SICF peaks and troughs, and it correlates with the strength of SICI. Experiment 6 suggests that ICF suppresses SICF mediated by late I waves.
Nature of cortical interneurons mediating SICI, ICF and SICF
We found that SICI increases and ICF decreases with higher test stimulus (S1) intensity, consistent with previous studies (Sanger et al. 2001; Daskalakis et al. 2002). It is well established that SICI and ICF are mediated by different groups of cortical interneurons. Increased SICI with higher S1 intensity can be explained by recruitment of late I waves in the descending corticospinal volleys (Di Lazzaro et al. 1998a) and these late I waves are more sensitive to SICI (Di Lazzaro et al. 1998b). Decrease in ICF with higher S1 intensities may be caused by the recruitment of corticospinal neurons which are less sensitive to ICF or spatially away from the centre of TMS. Additionally, spinal activities may contribute to this mechanism because it has been reported that ICF was not associated with increase in descending corticospinal volleys (Di Lazzaro et al. 2006). SICF is considered to occur at the cortical level (Ziemann et al. 1998b; Di Lazzaro et al. 1999; Ziemann & Rothwell, 2000). Hanajima et al. (2002) suggested that S1 alone depolarizes corticospinal neurons to various extents. Some of them depolarize to the firing threshold and generate MEPs to S1 alone and some corticospinal neurons are subliminally depolarized. When a subsequent weak S2 is applied, temporal summation occurs and the subliminally depolarized neurons reach their threshold, leading to the MEP facilitation. We found that with increasing S1 intensity, SICF at all three peaks decreased. With higher S1 intensities, it is possible that most corticospinal neurons would be discharged by S1 alone. Therefore, fewer subliminally depolarized corticospinal neurons are available to be activated by the subsequent S2, similar to a ceiling effect.
SICI facilitates SICF
Based on the findings that SICF is caused by the summation of I waves on the corticospinal neurons (Ziemann et al. 1998b; Ziemann & Rothwell, 2000) and that SICI inhibits the corticospinal neurons (Kujirai et al. 1993), we hypothesized that SICI inhibits SICF. However, the results of Experiment 2 are opposite to our hypothesis, indicating that a more complex mechanism may be involved in the interactions between SICI and SICF. It may be argued that facilitation of SICF in the presence of SICI is simply caused by the contamination of SICI by SICF. We chose ISI of 3 ms for SICI because this is the shortest time between CS and S1 that allowed us to use the current reversal device. Since this ISI is within the second peak of SICF, SICI may be contaminated by SICF-2 (Peurala et al. 2008) and this may affect the results. We performed Experiment 4 to investigate this possibility. SICF in the presence of SICI was increased even at low CS intensities of 0.7 and 0.8 AMT, which were below the threshold for evoking SICF (Peurala et al. 2008). There was also a strong correlation between the degree of SICF in the presence of SICI and the strength of SICI itself (Fig. 6B), consistent with the hypothesis that the increase in SICF in the presence of SICI was due to SICI. In addition, we found similar results for increased SICF-2 and SICF-3 in Experiment 2 whereas SICI at ISI of 3 ms may be contaminated by SICF-2 but not by SICF-3 (Peurala et al. 2008). Therefore, it is unlikely that our results can be accounted for by contamination of SICF-2 with SICI.
We found SICF at the three ISIs tested, consistent with previous studies (Ziemann et al. 1998b; Chen & Garg, 2000; Ziemann & Rothwell, 2000). Additionally, it was reported that there is no facilitation at intermediary intervals between the three peaks. A rapid spontaneous decay of EPSP in the corticospinal neurons is unlikely to explain these troughs between SICF peaks because the duration of EPSP is at least 10–15 ms (Deuchars et al. 1994). A possible mechanism for the absence of facilitation at the intermediary intervals is the existence of inhibitory interneurons interconnected to I wave generating neurons which disable the excitatory input to corticospinal neurons (McCormick et al. 1985). One possible explanation of our findings that SICF increases in the presence of SICI is that the neurons mediating SICI not only inhibit the I wave generating neurons leading to SICI, but also inhibit the inhibitory interneurons surrounding the I wave generating neurons. We performed Experiment 5 to further test this possibility. It was found that not only the SICF peaks but also the troughs were facilitated by a preceding CS3. This result is consistent with the possible existence of the inhibitory interneurons surrounding the I wave generating neurons. Namely, besides the direct inhibitory effect on the I wave generating neurons, neurons mediating SICI may have facilitatory effect on these I wave generating neurons through the inhibitory interneuron mediated disynaptic disinhibition. Because this facilitatory effect is separate from the mechanism of temporal summation at the corticospinal neurons that are thought to mediate SICF (Hanajima et al. 2002), it exists at both the SICF peaks and troughs. The hypothesis that inhibitory interneurons are crucial in generating I waves is also supported by the observation that benzodiazepines which increase GABAA transmission decreased SICF (Ziemann et al. 1998c). Besides the well-known mechanism that GABAA mediated SICI neurons inhibit the corticospinal output (Ziemann, 2004), the present results suggested that these SICI neurons also inhibit a group of GABAA mediated inhibitory interneurons surrounding the I wave generating neurons, causing facilitation of the corticospinal output.
Experiment 2 showed that SICI increased SICF-2 and SICF-3 but not SICF-1 (Fig. 4), confirming our second hypothesis that SICI will have greater influence on the late I wave mediated SICF peaks than the early I wave mediated SICF peaks. This is consistent with the previous observation that SICI decreases the late I waves more than the early I waves (Nakamura et al. 1997; Hanajima et al. 1998; Di Lazzaro et al. 1998b). In Experiment 3, the AP current direction was used to elicit S1 and S2. The results confirmed our third hypothesis that SICI will have greater influence on the SICF-1 induced by the AP current direction than that induced by the PA current direction. It was reported that AP directed current predominantly produces late I (I3) waves (Sakai et al. 1997) although the neuronal circuits mediating the various descending waves for AP and PA current directions may be different (Di Lazzaro et al. 2001). Importantly, if S1 and S2 for eliciting SICF-1 are in the PA direction, the summation may be due to the I2 wave recruited by S1 and the I1 wave recruited by S2. If the two stimuli are in the AP direction, the summation may be due to the I3 wave recruited by S1 and I2 wave recruited by S2 (Deletis et al. 2001; Hanajima et al. 2002). The findings strongly suggest that increased SICF in the presence of SICI is due to the interaction occurring at the late I wave generating neurons.
There are several other factors that may potentially influence our results. First, it may be argued that for the effect of SICI on SICF-3, the ISI between CS3 and S2 was 7.5 ms and this ISI falls into the time of ICF and may produce facilitation. This is unlikely to explain our findings because we controlled for the effects of SICI on S2 in conditions Acon to Gcon (Table 2) and the results are similar to the main experiment. Moreover, ICF decreases rather than increases SICF-3 (results of Experiment 6; Fig. 8). Second, since SICI predominately inhibits late I waves, condition 2E is likely to have produced more early I waves and fewer late I waves than 2A even though they produced similar MEP amplitudes. However, we found that SICI increased SICF mediated by late I waves and not those mediated by early I waves. Therefore, changes in the number of I waves due to increased S1 intensity cannot account for our results. Third, the precise timing of the SICF peaks may vary among individuals and we did not test for the precise peaks and troughs in each subject. However, there was significant facilitation at three SICF peaks (1.5, 2.9 and 4.5 ms) tested (Fig. 2 and ratio C/A in Figs 4, 5 and 8), indicating that SICF was clearly present at these ISIs. Since we found that SICI facilitated SICF at both peaks and troughs, it is unlikely that testing specific SICF peaks in individual subjects will yield different results. Previous studies showed that at trough 2 there was no inhibition or facilitation (MEP ratio ∼1) (Ziemann et al. 1998b; Chen & Garg, 2000). The results for trough 2 tested in our study (SICFAlone∼1, Fig. 7) are similar to these previous studies, confirming that the ISI of 4.0 ms represented the trough 2 in our subjects. We did not test SICF at trough 1 between peak 1 and peak 2. It is not known whether SICI facilitates SICF at that ISI.
ICF inhibits SICF-3
We found that ICF inhibits SICF-3, opposite to the effect of SICI. ICF appears to be mediated by a neuronal population separate from SICI since it requires slightly higher CS intensity than SICI (Ziemann et al. 1996; Chen et al. 1998), the current direction of the CS has different effects on SICI and ICF (Ziemann et al. 1996), and in a patient with an array of subdural electrodes over the M1, inhibition and facilitation are elicited at different sites (Ashby et al. 1999). Moreover, ICF and SICI are associated with different patterns of cerebral blood flow response as measured by PET (Strafella & Paus, 2001). Since administration of the N-methyl-d-aspartic acid receptor antagonist dextrometorphan reduces ICF (Ziemann et al. 1996), glutamate may be involved in mediating ICF. One study reported that ICF slightly increased the I4 wave (Nakamura et al. 1997) but another study found that ICF induced no change in I waves (Di Lazzaro et al. 2006). The present results suggested that ICF has cortical effects because it is unlikely that SICF-3 occurs at the spinal level. This is also supported by the observation that ICF is not associated with changes in spinal reflexes (Ziemann et al. 1998a). However, the inhibition of SICF-3 cannot explain MEP facilitation caused by ICF. This is consistent with the suggestion that ICF may not be explained by changes in I waves (Di Lazzaro et al. 2006).
Physiology of SICI/ICF and SICF interaction
The mechanism of I wave generation is still unknown. During voluntary movement, the firing rate of corticospinal neurons in monkey M1 rarely exceeds 100 Hz (Evarts et al. 1983) but the frequency of I waves observed during non-physiological stimulation is ∼600 Hz. A population of cortical interneurons known as chattering cells may play a crucial role in its generation (Gray & McCormick, 1996). It may also be the consequence of a neuronal membrane intrinsic property in response to a large synchronous excitatory input (Rothwell, 1997).
By investigating SICI under different stimulus intensities and muscle activation levels, Ortu et al. (2008) suggested that active SICI may be associated with concurrently recruited SICF. Peurala et al. (2008) extensively investigated CS intensity recruitment curves of SICI at different ISIs and suggested that SICI may be contaminated by SICF at their peaks. These studies supported the opinion that SICF and SICI/ICF are independent neuronal systems and the balance between them determines the final outcome of specific TMS protocols (Ni & Chen, 2008; Rothwell et al. 2009). Our present results showed that there are also interactions between these neuronal systems with SICI facilitating SICF-2 and SICF-3. Therefore, the simultaneous presence of SICI and SICF (Ortu et al. 2008; Peurala et al. 2008) may not be a simple summation of the individual effects of these systems but also reflect interactions between them. In summary, I wave generating neurons are arranged on different chains where each of them is responsible for generating a specific I wave (e.g. the I4 wave was generated by a chain consisting of four facilitatory interneurons which have their final synaptic connection to the corticospinal neuron) (Sakai et al. 1997; Roshan et al. 2003). A suprathreshold S1 is able to activate these I wave generating neurons to depolarize corticospinal neurons and produce a test MEP. S2 activates I1 wave generating neurons (with AP current direction, S2 may activate I2 wave generating neurons) and fires corticospinal neurons which are subliminally depolarized by S1, leading to SICF (Ziemann et al. 1998a; Ziemann & Rothwell, 2000). I3 and I4 wave generating neurons receive inhibitory inputs from neurons mediating SICI, which can be activated by a preceding CS3 (Nakamura et al. 1997; Hanajima et al. 1998; Di Lazzaro et al. 1998b). We propose that these I wave generating neurons are also influenced by a different group of inhibitory interneurons, which are activated by S1. SICI inhibits both I wave generating neurons and this separate group of inhibitory interneurons. Therefore, the presence of CS3 can facilitate a subsequent MEP evoked in the SICF paradigm through disynaptic disinhibition. On the other hand, the cortical neurons mediating ICF may facilitate the inhibitory interneurons surrounding I4 wave generating neurons and suppress SICF-3.
Acknowledgments
The study was supported by the Canadian Institutes of Health Research (CIHR) Operating Grant to R.C. (MOP 62917). Z.N. is a recipient of Canadian Institutes of Health Research Fellowship Award in the Area of Dystonia (DFF 88348). R.C. is supported by a CIHR-Industry Partnered Investigator Award and by the Catherine Manson Chair in Movement Disorders.
Glossary
Abbreviations
- AMT
active motor threshold
- ANOVA
analysis of variance
- AP
anterior–posterior
- CS
conditioning stimulus
- D wave
direct wave
- EMG
electromyogram
- FDI
first dorsal interosseous
- GABA
γ-aminobutyric acid
- I wave
indirect wave
- ICF
intracortical facilitation
- ISI
interstimulus interval
- M1
primary motor cortex
- MEP
motor evoked potential
- PA
posterior–anterior
- RMT
rest motor threshold
- S1
first stimulus
- S2
second stimulus
- SICF
short interval intracortical facilitation
- SICI
short interval intracortical inhibition
- TMS
transcranial magnetic stimulation
Author contributions
Conception and design: A.W.-S., Z.N. and R.C. Data collection, analysis and interpretation: all authors. Drafting of manuscript: A.W.-S., Z.N. and R.C. All authors revised the intellectual content and approved the final version of the manuscript.
References
- Ashby P, Reynolds C, Wennberg R, Lozano AM, Rothwell J. On the focal nature of inhibition and facilitation in the human motor cortex. Clin Neurophysiol. 1999;110:550–555. doi: 10.1016/s1388-2457(98)00082-0. [DOI] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di LV, Lefaucheur JP, Magistris MR, Mills K, Rosler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Chen R, Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. J Neurophysiol. 2000;83:1426–1434. doi: 10.1152/jn.2000.83.3.1426. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deletis V, Isgum V, Amassian VE. Neurophysiological mechanisms underlying motor evoked potentials in anaesthetized humans. Part 1. Recovery time of corticospinal tract direct waves elicited by pairs of transcranial electrical stimuli. Clin Neurophysiol. 2001;112:438–444. doi: 10.1016/s1388-2457(01)00461-8. [DOI] [PubMed] [Google Scholar]
- Deuchars J, West DC, Thomson AM. Relationships between morphology and physiology of pyramid–pyramid single axon connections in rat neocortex in vitro. J Physiol. 1994;478:423–435. doi: 10.1113/jphysiol.1994.sp020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol. 1998a;109:397–401. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, Profice P, Tonali P, Rothwell JC. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 2001;138:268–273. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates inhibitory circuits. Exp Brain Res. 1998b;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res. 1999;129:494–499. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Fromm C, Kroller J, Jennings VA. Motor Cortex control of finely graded forces. J Neurophysiol. 1983;49:1199–1215. doi: 10.1152/jn.1983.49.5.1199. [DOI] [PubMed] [Google Scholar]
- Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–113. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired- pulse magnetic stimulation in humans. J Physiol. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalogr Clin Neurophysiol. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Chen R. Short-interval intracortical inhibition: a complex measure. Clin Neurophysiol. 2008;119:2175–2176. doi: 10.1016/j.clinph.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol. 2008;586:5147–5159. doi: 10.1113/jphysiol.2008.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton H, Amassian VE. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Muller-Dahlhaus JF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Paired-pulse investigations of short-latency intracortical facilitation using TMS in humans. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:113–119. [PubMed] [Google Scholar]
- Rothwell JC, Day BL, Thompson PD, Kujirai T. Short latency intracortical inhibition: one of the most popular tools in human motor neurophysiology. J Physiol. 2009;587:11–12. doi: 10.1113/jphysiol.2008.162461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T. Cerebral blood-flow changes induced by paired-pulse transcranial magnetic stimulation of the primary motor cortex. J Neurophysiol. 2001;85:2624–2629. doi: 10.1152/jn.2001.85.6.2624. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clinical Neurophysiology. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998a;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998b;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wischer S, Hildebrandt J, Paulus W. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalogr Clin Neurophysiol. 1998c;109:321–330. doi: 10.1016/s0924-980x(98)00023-x. [DOI] [PubMed] [Google Scholar]