Abstract
Dehydration increases vasopressin (antidiuretic hormone) secretion from the posterior pituitary gland to reduce water loss in the urine. Vasopressin secretion is determined by action potential firing in vasopressin neurones, which can exhibit continuous, phasic (alternating periods of activity and silence), or irregular activity. Autocrine κ-opioid inhibition contributes to the generation of activity patterning of vasopressin neurones under basal conditions and so we used in vivo extracellular single unit recording to test the hypothesis that changes in autocrine κ-opioid inhibition drive changes in activity patterning of vasopressin neurones during dehydration. Dehydration increased the firing rate of rat vasopressin neurones displaying continuous activity (from 7.1 ± 0.5 to 9.0 ± 0.6 spikes s−1) and phasic activity (from 4.2 ± 0.7 to 7.8 ± 0.9 spikes s−1), but not those displaying irregular activity. The dehydration-induced increase in phasic activity was via an increase in intraburst firing rate. The selective κ-opioid receptor antagonist nor-binaltorphimine increased the firing rate of phasic neurones in non-dehydrated rats (from 3.4 ± 0.8 to 5.3 ± 0.6 spikes s−1) and dehydrated rats (from 6.4 ± 0.5 to 9.1 ± 1.2 spikes s−1), indicating that κ-opioid feedback inhibition of phasic bursts is maintained during dehydration. In a separate series of experiments, prodynorphin mRNA expression was increased in vasopressin neurones of hyperosmotic rats, compared to hypo-osmotic rats. Hence, it appears that dynorphin expression in vasopressin neurones undergoes dynamic changes in proportion to the required secretion of vasopressin so that, even under stimulated conditions, autocrine feedback inhibition of vasopressin neurones prevents over-excitation.
Introduction
Vasopressin (the antidiuretic hormone) is secreted from the posterior pituitary gland in proportion to plasma osmolarity (Bourque, 2008) to maintain body fluid homeostasis by promoting water reabsorption in the kidneys (Snyder, 2005). Moderate dehydration impairs concentration and co-ordination while severe dehydration can cause seizures, permanent brain damage or death. Hence, vasopressin secretion is increased during dehydration (which increases plasma osmolarity) to reduce water loss in the urine until body water can be replenished by drinking.
Vasopressin secretion is largely determined by action potential (spike) discharge initiated at the cell bodies of magnocellular vasopressin neurones in the hypothalamic supraoptic and paraventricular nuclei. In response to increased plasma osmolarity, some vasopressin neurones exhibit phasic spike discharge characterized by alternating periods of silence and activity, each lasting tens of seconds (Wakerley et al. 1978). Because this activity pattern makes optimal use of the properties of the axon terminals for efficient vasopressin secretion (Leng et al. 1999), the mechanisms that underpin phasic patterning have been extensively studied (Brown, 2004; Brown & Bourque, 2006).
Phasic bursts are initiated when synaptic potentials summate to cross the threshold for spike generation. When spikes occur close enough together, post-spike afterdepolarizations (ADPs) summate, generating a persistent plateau potential close to threshold that sustains continued firing (Andrew & Dudek, 1983; Brown et al. 2006). During bursts, a fast afterhyperpolarization (AHP) enhances the firing rate via activation of a hyperpolarization-activated inward current (Ghamari-Langroudi & Bourque, 2000), and a medium AHP induces spike frequency adaptation (Kirkpatrick & Bourque, 1996; Greffrath et al. 2004; Ruan & Brown, 2009). Burst termination involves activity-dependent κ-opioid inhibition of ADPs (Brown & Bourque, 2004) and activation of a slow AHP to decrease plateau potential amplitude (Greffrath et al. 1998; Ghamari-Langroudi & Bourque, 2004) and thus reduce neuronal excitability (Brown et al. 2006). Because activity-dependent κ-opioid inhibition is important for the expression of phasic activity (Brown et al. 1998), we tested the hypothesis that changes in autocrine κ-opioid feedback inhibition drive changes in activity patterning of vasopressin neurones during dehydration.
Methods
Ethical approval
All experimental procedures were carried out in accordance with a procedure approved by the University of Otago Animal Ethics Committee (electrophysiology) or in accordance with the UK Animals (Scientific Procedures) Act, 1986 and associated guidelines (prodynorphin expression in vasopressin neurones).
Electrophysiology
Virgin female Sprague–Dawley rats (250–350 g) either had access ad libitum to drinking water (euhydrated) or had water removed for 24 h (dehydrated) prior to electrophysiological recording. On the day of electrophysiology the rats were anaesthetised by an intraperitoneal injection of urethane (ethyl carbamate; 1.25 g kg−1) and a catheter was inserted into the right femoral vein for drug injection and blood sampling (for measurement of plasma osmolarity). The pituitary stalk and the right supraoptic nucleus were exposed by transpharyngeal surgery. Following removal of the meninges, a U-shaped microdialysis probe (supplied by Prof. M. Ludwig, University of Edinburgh, total membrane length 2 mm; Spectra/Por RC Hollow Fibers, Spectrum Medical Inc., Houston, TX, USA) was bent to position the loop of the membrane flat onto the exposed ventral surface of the brain over the supraoptic nucleus. A glass recording pipette (15–40 MΩ; filled with 0.9% NaCl) was placed in the supraoptic nucleus through the centre of the dialysis loop. A side-by-side SNEX-200 stimulating electrode (Science Products GmbH, Hofheim, Germany) was placed on the pituitary stalk to elicit antidromic action potentials in supraoptic nucleus neurones. The supraoptic nucleus was continually dialysed with artificial cerebrospinal fluid (aCSF; composition in mm: NaCl 138; KCl 3.36; NaHCO3 9.52; Na2HPO4 0.49; urea 2.16; CaCl2 1.26; MgCl2 1.18; pH 7.2) at 3 μl min−1 and the dialysate changed to include the experimental drug during recording. At the end of the experiments the rats were killed by anaesthetic overdose.
Electrophysiology data analysis
Neuronal activity was recorded onto a computer and analysed off-line using Spike2 software (Cambridge Electronic Design, Cambridge, UK). Neurones that fired less than one spontaneous action potential every 10 s were categorized as silent and were not recorded. Phasic activity was characterized using the ‘bursts’ script in Spike2, with a burst being defined as activity lasting a minimum of 5 s with a minimum of 20 spikes within the burst and at least a 5 s interval between bursts, during which there was less than 1 spike every 5 s; phasic neurones were those for which these parameters partitioned more than 95% of spikes into bursts.
Non-phasic neurones were characterized as oxytocin neurones on the basis of a transient excitation following i.v. cholecystokinin injection (20 μg kg−1, 0.5 ml kg−1 in 0.9% saline; Sigma) (Brown et al. 1996), or as vasopressin neurones by transient inhibition following cholecystokinin injection (Sabatier et al. 2004).
Non-phasic vasopressin neurones for which the bursts script partitioned less than 95% of spikes into bursts were categorized as irregular and active neurones that did not display silent periods were categorized as continuous. Non-phasic vasopressin neurones were confirmed as either irregular or continuously active based upon the variability of spike firing, as shown by the index of dispersion of firing rate (IDFR), which was calculated using the formula: IDFR = SDFR2/MFR, where SDFR is the standard deviation of the firing rate (in 1 s bins) and MFR is the mean firing rate. For non-phasic vasopressin neurones, the IDFR showed a bimodal distribution; regardless of hydration status, all continuously active vasopressin neurones had an IDFR of <1.5, whereas all irregular vasopressin neurones had an IDFR > 1.5.
The mean firing rate, and where appropriate the mean intraburst firing rate, burst duration and interburst interval, of each neurone was calculated before and during drug administration. For phasic neurones, the temporal evolution of firing during bursts was determined by calculating the firing rate of each neurone in 1 s intervals. The peak firing rate within bursts was aligned with that of all other bursts in the same neurone for each condition and mean intraburst firing rate was calculated in 1 s intervals. To represent the group data, the mean firing rate for the 5 s preceding peak intraburst firing rate was then calculated for each neurone in 1 s intervals, as was the mean firing rate in each consecutive 1 s interval from peak intraburst firing rate.
Induction of hyponatraemia and hypernatraemia
Hyponatraemia was induced in male Sprague–Dawley rats (250–350 g) by a nutritionally balanced liquid diet (AIN-76; BioServ, Frenchtown, NJ, USA) combined with chronic systemic administration of a vasopressin receptor agonist to stimulate inappropriate antidiuresis (Verbalis & Drutarosky, 1988). The liquid rat diet formula was dissolved in 14% dextrose at a concentration of 0.54 g ml−1. Rats were caged individually with standard rat chow replaced with 50 ml liquid diet each day with free access to drinking water on days 1 and 2. On day 3, under halothane anaesthesia, rats were implanted subcutaneously (in the inter-scapular space) with an mini-osmotic pump (flow rate 0.5 μl h−1; model 2002, Alzet, Cupertino, CA, USA) filled with desmopressin acetate (Desmospray, 1-desamino-8-d-arginine vasopressin; Ferring Pharmaceuticals, Langley, UK) at 10 μg ml−1 resulting in a continuous infusion of vasopressin agonist at 5 ng h−1. On the day of surgery, for 24 h only, rats were allowed 70 ml of a more dilute diet (0.32 g ml−1), after which rats were given 50 ml of the liquid diet at the original concentration (0.54 g ml−1). Throughout the 7-day drug infusion period the rats were denied access to drinking water.
To induce hypernatraemia, the drinking water of individually caged male Sprague–Dawley rats was replaced with 2% NaCl (w/v) for 7 days with access to standard rat chow ad libitum. Control rats were housed individually for the duration of the experiment, with free access to drinking water and standard rat chow.
Rats were weighed daily to ensure that changes in diet did not affect their general well-being. On the morning of day 8, all rats were killed by conscious decapitation and trunk blood collected for determination of plasma osmolarity and [Na+]. Whole brains were rapidly removed, frozen on dry ice and stored at −70°C until sectioned coronally at 15 μm on a cryostat. Sections were thaw mounted onto electrostatically charged glass microscope slides (VWR, superfrost plus; cat no 406/0179/00) and stored at −70°C until processed for immunocytochemistry and in situ hybridization.
Immunocytochemistry
Two slides per rat were pre-selected to include sections at the level of the supraoptic nucleus. Slides from each rat were thawed at room temperature before being immersed in 4% paraformaldehyde (w/v) in 0.1 m phosphate buffered saline (PBS) solution (pH 7.4) for 5 min followed by three 10 s rinses in 0.1 m PBS. Non-specific binding of immunoglobulin G (IgG) was blocked by a 5 min incubation of slides in a 0.1 m PBS solution containing 5% normal sheep serum (NSS) solution (v/v). Sections were processed for arginine vasopressin immunoreactivity using a polyclonal antibody raised in rabbit (AB1565; Chemicon International, Temecula, CA, USA) diluted at 1: 200 in 0.1 m PBS and 1% NSS (250 μl per slide) and incubated at room temperature for 15 min. Slides were rinsed three times for 10 s each in 0.1 m PBS before incubation in goat horseradish peroxidase anti-rabbit IgG (PI 1000; Vector Laboratories, Burlingame, CA, USA) at 1: 100 (250 μl per slide) for a further 15 min at room temperature. Next, slides were rinsed a further three times for 10 s in 0.1 m PBS before the antibody–antigen complex was visualized using 0.025% diaminobenzidine (w/v) and 0.03% H2O2 (v/v). The reaction was halted after 3 min with three 30 s rinses in 0.1 m PBS.
In situ hybridization
After immunostaining for arginine vasopressin, a synthetic 45-mer oligoprobe (5′-GTT GTC CCA CTT AAG CTT GGG GCG AAT GCG CCG CAG GAA GCC CCC-3′) complementary to bases 865–909 of the rat prodynorphin gene (Civelli et al. 1985) was used to detect prodynorphin mRNA expression (MWG-Biotech, Ebersberg, Germany). Probes were 3′-labelled with [35S]dATP using terminal deoxynucleotidyl transferase and purified using spin columns (QIAquick nucleotide removal kit; Qiagen, Crawley, UK). Sections were hybridized with radiolabelled probe (105 cpm labelled probe per section) in hybridization buffer (1% BSA (w/v), 5% dextran sulphate (w/v), 15 mm diothiothreitol, 2 mm EDTA, 1% Ficoll (w/v), 50% formamide (v/v), 0.1 mg ml−1 PolyA, 1% polyvinylpyrrolidone (w/v), 0.2 mg ml−1 salmon testes DNA, 1.2 m NaCl, 2.5% sodium pyrophosphate (w/v), 20 mm Tris, pH 7.6, 0.1 mg ml−1 yeast tRNA, 0.1 mg ml−1 yeast total RNA) overnight at 37°C in humidified chambers. Slides were rinsed three times in saline sodium citrate (SSC) at room temperature, washed four times for 15 min each in SSC at 55°C, and twice in SSC for 30 min each at room temperature. Slides were air-dried at room temperature overnight before being dipped in liquid autoradiographic emulsion (K5 Gel Emulsion, Ilford Imaging UK Ltd, Knutsford, UK) and stored, with silica gel desiccant, at 4°C. After 8 weeks exposure, the slides were developed (Kodak D-19 developer; Eastman Kodak Co., Rochester, NY, USA) and fixed (Hypam rapid fixer; Ilford Imaging UK Ltd) before being counterstained with haematoxylin. Following dehydration, through an alcohol series, the slides were cover-slipped with DePeX mountant (VWR International Ltd, Poole, UK).
Neurochemistry data analysis
The slides were evaluated using a light microscope under bright-field illumination and a computerized image analysis system (NIH Image version 1.62). To quantify total prodynorphin mRNA expression in the supraoptic nucleus, silver grain area was measured over an average of 10 supraoptic nucleus profiles (over six sections) for each rat (×10 objective). The area of each supraoptic nucleus profile was measured to calculate grain area per supraoptic nucleus (mm2 per mm2). Background measurements were made over areas adjacent to the region of interest and subtracted. To evaluate the expression of prodynorphin mRNA by individual vasopressin neurones, the silver grain area overlying each of 30 immunolabelled cells (×40 objective) was measured over 10–12 supraoptic nucleus profiles. A positively labelled cell was defined when the number of overlying silver grains was greater than twice that of the equivalent area of background. Background measurements were taken from tissue adjacent to the supraoptic nucleus and subtracted from the area per labelled neurone. For quantification of both single cell expression and total expression within the supraoptic nucleus, means were calculated for each rat with these values used to calculate group means.
Statistics
All data are shown as means ±s.e.m. and statistical tests were carried out using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA). Between groups, data were analysed by Student's t test or, where appropriate, by one-way or two-way repeated measures ANOVA; only where the F-ratio was significant, ANOVA was followed by all-pairwise Bonferroni's tests.
Results
Dehydration increases the activity of vasopressin and oxytocin neurones
In vivo extracellular single-unit recordings were obtained from 135 identified supraoptic nucleus neurones; 87 vasopressin neurones (38 from non-dehydrated (euhydrated) rats and 49 from 24 h dehydrated rats; e.g. Fig. 1A) and 49 oxytocin neurones (14 from euhydrated rats and 35 from 24 h dehydrated rats). Under urethane anaesthesia, the mean plasma osmolarities of euhydrated rats and dehydrated rats were 301.4 ± 2.3 and 310.7 ± 3.7 mosmol l−1, respectively (P= 0.04).
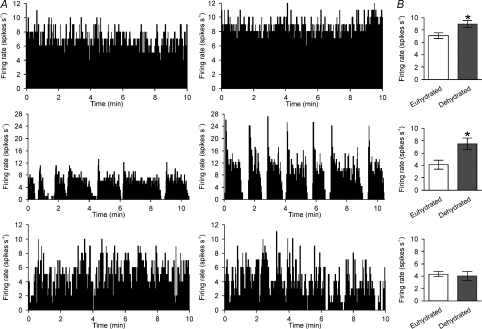
Figure 1. Dehydration increases the firing rate of continuously active and phasic, but not irregular, vasopressin cells.
A, ratemeter records (in 1 s bins) of the firing rates of supraoptic nucleus vasopressin neurones in euhydrated rats (left) and 24 h dehydrated rats (right), displaying spontaneous continuous activity (top), phasic activity (middle) and irregular activity (bottom). B, the mean firing rates (±s.e.m.) of continuous (top), phasic (middle) and irregular (bottom) vasopressin neurones in euhydrated rats and dehydrated rats (*P < 0.05, unpaired t test).
As previously shown by others (Dyball & Pountney, 1973; Walters & Hatton, 1974; Wakerley et al. 1978), the mean basal firing rate of vasopressin neurones recorded from dehydrated rats (7.7 ± 0.5 spikes s−1) was greater than in euhydrated rats (5.5 ± 0.4 spikes s−1, P= 0.003, Student's t test). Similarly, the mean basal firing rate of oxytocin neurones was greater in dehydrated rats (6.2 ± 0.5 spikes s−1) than in euhydrated rats (4.2 ± 0.5 spikes s−1; P= 0.02).
The proportion of vasopressin neurones displaying continuous (euhydrated: n= 16 of 38; dehydrated: 28 of 49), phasic (euhydrated: 9 of 38; dehydrated: 10 of 49) and irregular (euhydrated: 13 of 38; dehydrated: 11 of 49) activity patterns were not different between euhydrated and dehydrated rats (P= 0.36, chi-square test).
Previous studies of vasopressin neurone responses to chronic osmotic stimulation have limited detailed analysis of activity to that of neurones displaying phasic activity (Dyball & Pountney, 1973; Walters & Hatton, 1974; Wakerley et al. 1978). Because vasopressin secretion depends on the activity of the whole population of vasopressin neurones, we also analysed the activity of continuously active neurones and irregularly active neurones in euhydrated rats and dehydrated rats. The mean basal firing rate of continuously active vasopressin neurones was greater in dehydrated rats (9.0 ± 0.6 spikes s−1) than in euhydrated rats (7.1 ± 0.5 spikes s−1, P= 0.04; Fig. 1B), as was the mean basal firing rate in phasic neurones (4.2 ± 0.7 spikes s−1 in euhydrated rats and 7.8 ± 0.9 spikes s−1 in dehydrated rats, P= 0.02; Fig. 1B). However, there was no difference between the mean basal firing rate of irregularly active vasopressin neurones in euhydrated rats (4.3 ± 0.4 spikes s−1) and dehydrated rats (4.1 ± 0.8 spikes s−1, P= 0.75; Fig. 1B).
Dehydration increases intraburst firing rate of phasic neurones
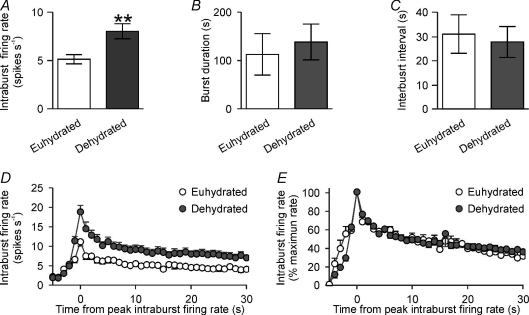
The mean intraburst firing rate of phasic neurones was greater in dehydrated rats (8.0 ± 0.8 spikes s−1) than in euhydrated rats (5.1 ± 0.5 spikes s−1, P= 0.009; Fig. 2A), but there were no significant differences in burst duration (P= 0.66; Fig. 2B) or interburst interval (P= 0.75; Fig. 2C). Detailed analysis of the phasic bursts from eight neurones in euhydrated rats and nine in dehydrated rats (by calculating the firing rate in 1 s bins relative to the peak intraburst firing rate) revealed that the dehydration-induced increase in intraburst firing rate was evident from the onset of bursts and was maintained throughout bursts (Fig. 2D and E).
Figure 2. Dehydration increases intraburst firing rate of phasic vasopressin neurones.
A–C, the mean intraburst firing rate (A), burst duration (B) and interburst interval (C) of 17 phasic vasopressin neurones from Fig. 1, recorded from euhydrated rats (n= 9) and dehydrated rats (n= 8) rats (**P < 0.05 compared to euhydrated, unpaired t test). D and E, intraburst firing rate (in 1 s bins, and aligned to peak intraburst firing) expressed in spikes per second (D) and as the percentage of maximum firing rate (E), showing that dehydration causes a proportionately similar increase in intraburst firing rate throughout bursts.
Intra-supraoptic nucleus κ-opioid receptor antagonist administration increases phasic activity
We have previously shown that phasic bursts are under endogenous activity-dependent κ-opioid receptor restraint (Brown et al. 1998, 2004, 2006; Brown & Bourque, 2004). Here, we recorded the activity of phasic cells from five euhydrated rats and four dehydrated rats during microdialysis administration of nor-BNI (200 μg ml−1 (0.27 mm) at 3 μl min−1 over 60 min) into the supraoptic nucleus (Fig. 3A and B). Nor-BNI increased the firing rate of all phasic neurones tested (P= 0.005), inducing continuous activity in two neurones from euhydrated rats and two neurones from dehydrated rats. The firing rate of the phasic neurones in dehydrated rats was greater than in euhydrated rats (P= 0.01, two-way repeated measures ANOVA followed by Bonferroni's post hoc test; Fig. 3C), but there was no significant interaction between the hydration status and the effect of nor-BNI on firing rate (P= 0.51; Fig. 3C), indicating that endogenous κ-opioid inhibition of phasic activity is maintained during dehydration.
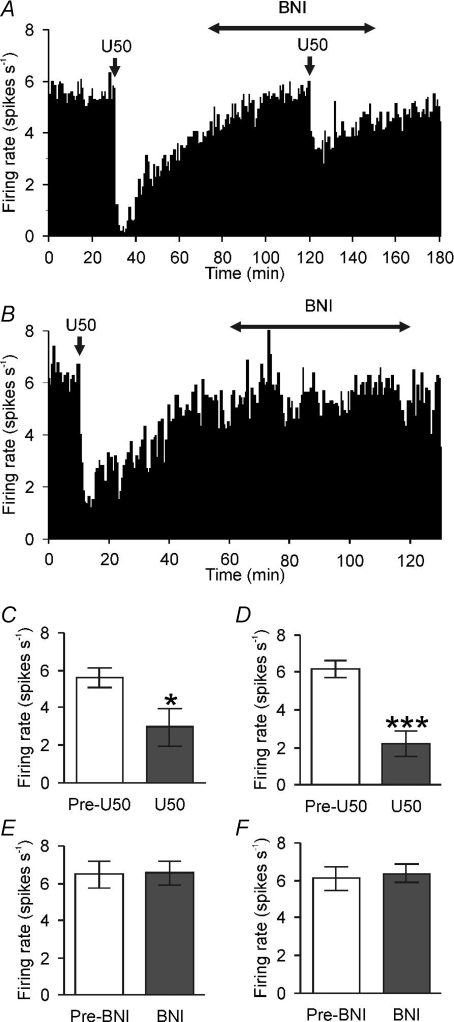
Figure 3. κ-Opioid receptor antagonism increases the activity of phasic vasopressin neurones in euhydrated and dehydrated rats.
A and B, ratemeter recordings (in 1 s bins) of the firing rates of phasic vasopressin neurones in a euhydrated rat (A) and a dehydrated rat (B) before and during microdialysis administration of nor-binaltorphimine (BNI, 200 μg ml−1). The insets show a 2 min period of firing before and during nor-BNI administration. C–F, the mean firing rate (C), intraburst firing rate (D), burst duration (E) and interburst interval (F) of nine phasic vasopressin neurones recorded from euhydrated rats (n= 5) and dehydrated (n= 4) rats. Note that the nor-BNI induced changes in activity were independent of the hydration status of the animals: two-way repeated measures ANOVA showed that dehydration increased firing rate (P= 0.01) as did nor-BNI (P= 0.005), but with no interaction between hydration status and nor-BNI (P= 0.51); dehydration (P= 0.009), but not nor-BNI (P= 0.08), increased intraburst firing rate; nor-BNI (P= 0.003), but not dehydration (P= 0.73), increased burst duration; nor-BNI (P= 0.04), but not dehydration (P= 0.30), decreased interburst interval; (*P≤ 0.05 and **P≤ 0.01). G, the mean intraburst firing rates (in 5 s bins, and aligned to peak intraburst firing) of the nine phasic neurones (euhydrated and dehydrated), recorded before and during administration of nor-BNI. H, the difference in intraburst firing rate of the cells from G, showing a progressive increase in the nor-BNI-induced difference in firing rate over the first 50 s of bursts (Pearson product moment correlation coefficient = 0.67, P= 0.03).
As first shown by Walters & Hatton (1974), dehydration increased intraburst firing rate in phasic cells (P= 0.009; Fig. 3D). Nor-BNI did not alter intraburst firing rate (P= 0.08, Fig. 3D), but increased burst length (P= 0.003; Fig. 3E) and decreased interburst interval (P= 0.04; Fig. 3F) in all phasic cells tested, with a similar response in euhydrated and dehydrated rats (P= 0.85 and P= 0.61, respectively, Fig. 3E and F).
We have previously shown that κ-opioid receptor antagonist enhancement of intraburst firing rate emerges as phasic bursts progress (Brown et al. 2004); here, nor-BNI induced a similar progressive increase in intraburst firing rate in phasic neurones from euhydrated rats (to 1.3 ± 0.4 spikes s−1 at 45–50 s into the bursts) and dehydrated rats (to 0.9 ± 0.7 spikes s−1 at 45–50 s; P= 0.67) Because hydration status did not alter the effect of nor-BNI on phasic neurones, to illustrate the temporal evolution of activity over the course of the bursts we combined data from euhydrated and dehydrated rats. The nor-BNI-induced increase in intraburst firing rate was not evident at burst onset, but emerged as the bursts progressed (Fig. 3G and H). Nor-BNI increased firing rate over the course of bursts from 0.2 ± 0.3 spikes s−1 at 0–5 s to 1.0 ± 0.4 spikes s−1 at 45–50 s (P= 0.03, Pearson product moment correlation; Fig. 3H).
Antagonism of κ-opioid receptors does not alter the firing rate of continuously active vasopressin neurones
We recorded from five euhydrated rats and nine dehydrated rats during intravenous (i.v.) administration of the κ-opioid agonist U50,488H (Fig. 4A and B). U50,488H (1 mg kg−1) decreased the firing rate of all continuously active vasopressin neurones tested (averaged over 10 min before and 10 min after U50,488H injection), from 5.6 ± 0.5 spikes s−1 to 3.0 ± 1.0 spikes s−1 in euhydrated rats (P= 0.04; Fig. 4C) and from 6.2 ± 0.5 spikes s−1 to 2.2 ± 0.6 spikes s−1 in dehydrated rats (P= 0.0007; Fig. 4E). Two-way repeated measures ANOVA showed no significant difference in the effectiveness of U50,488H in dehydrated rats and euhydrated rats (P= 0.89).
Figure 4. Continuously active vasopressin neurones express functional κ-opioid receptors in euhydrated rats and dehydrated rats.
A and B, ratemeter records (in 10 s bins) of the activity of continuously active vasopressin neurones in a euhydrated rat (A) and a dehydrated rat (B). Injection of the κ-opioid receptor agonist U50,488H (U50; 1 mg kg−1, i.v.) profoundly inhibited the firing rate of both neurones. C and E, the mean firing rates of continuously active vasopressin neurones recorded from euhydrated rats (C, n= 5) and dehydrated rats (E, n= 9) before (Pre-U50) and after injection of U50,488H (*P < 0.05 and ***P < 0.001, paired t test). D and F, the mean firing rates of continuously active vasopressin neurones recorded from euhydrated (D, n= 5) and dehydrated rats (F, n= 2) before (Pre-BNI) and during intra-supraoptic nucleus administration of the κ-opioid receptor antagonist nor-BNI over 60 min (BNI, 200 μg ml−1).
Intra-supraoptic nucleus administration of the κ-opioid receptor antagonist nor-BNI (200 μg ml−1 at 3 μl min−1 over 60 min) reduced the inhibition induced by U50,488H (e.g. Fig. 4A) but had no effect on the spontaneous firing rate of five continuously active vasopressin neurones from euhydrated rats (6.5 ± 0.7 spikes s−1 and 6.5 ± 0.6 spikes s−1 before and during nor-BNI, respectively; Fig. 4D), or on two continuously active vasopressin neurones from dehydrated rats (firing rates of the two neurones before and during nor-BNI were 5.4 and 5.9 spikes s−1 and 6.8 and 6.9 spikes s−1, respectively; Fig. 4F). Thus, continuously active vasopressin neurones, unlike phasic vasopressin neurones, do not appear to be under tonic inhibition by endogenous κ-opioid peptides, although they are sensitive to exogenous κ-opioid receptor agonists.
Antagonism of κ-opioid receptors increases the firing rate of irregular vasopressin neurones
We recorded the firing rate of six irregular vasopressin neurones from euhydrated rats, during intra-supraoptic nucleus administration of nor-BNI for 60 min; the mean firing rate was increased by nor-BNI from 4.2 ± 0.7 spikes s−1 to 5.5 ± 0.7 spikes s−1 (P= 0.01). We were able to maintain recording from only one irregular vasopressin neurone from dehydrated rats for 55 min of nor-BNI administration; the firing rate of this neurone was 4.1 spikes s−1 and 4.9 spikes s−1 before and during nor-BNI administration, respectively.
Chronic hypo-osmotic and hyperosmotic stimuli modulate prodynorphin mRNA expression in vasopressin neurones
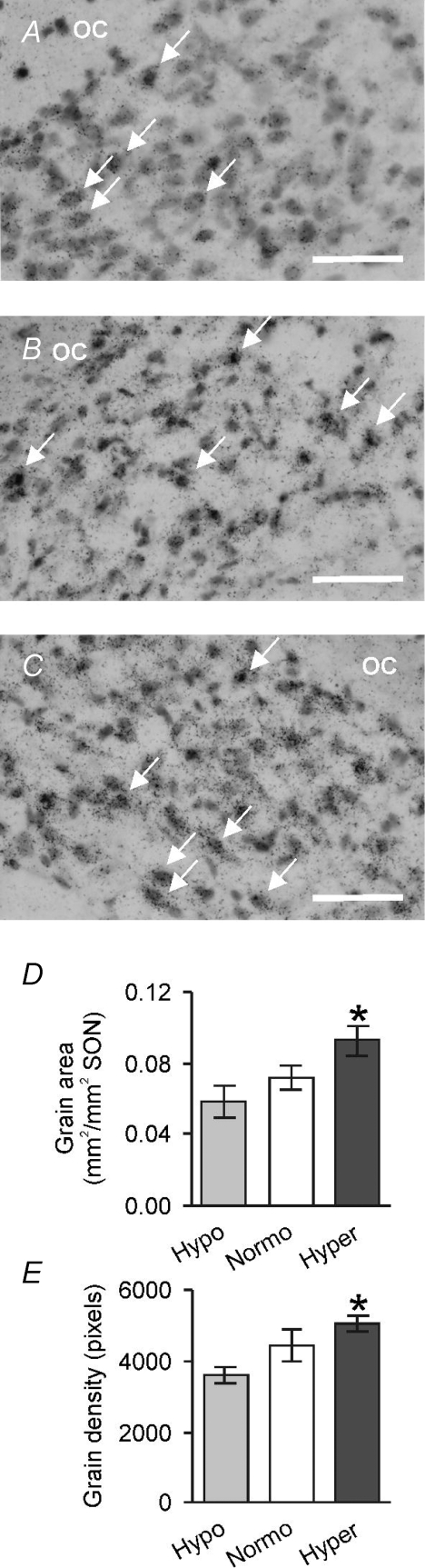
Finally, we determined whether prodynorphin mRNA expression is modulated by osmotic status in rats (Fig. 5) using a different model of dehydration, which permits a more prolonged development of plasma hyperosmolarity, as well as a protocol to induce chronic hypo-osmolarity. The mean plasma osmolarities of hypo-osmotic rats, normo-osmotic rats and hyperosmotic rats were 237 ± 11 mosmol l−1 (n= 7), 294 ± 2 mosmol l−1 (n= 5) and 305 ± 5 mosmol l−1 (n= 6), respectively. One-way repeated measures ANOVA showed that supraoptic nucleus prodynophin mRNA expression was modulated by osmotic status (P= 0.04; Fig. 5D). As dynorphin is expressed by both oxytocin neurones and vasopressin neurones, we went on to measure expression in cells identified as expressing vasopressin, confirming a significant effect of osmotic status on prodynophin mRNA expression in identified vasopressin neurones (P < 0.05, Fig. 5E).
Figure 5. Hypo-osmotic and hyperosmotic stimulation modulate prodynorphin mRNA expression in supraoptic nucleus vasopressin neurones.
A–C, photomicrographs of the supraoptic nucleus showing prodynorphin mRNA expression in vasopressin neurones from rats in hypo-osmotic (A), normo-osmotic (B) and hyperosmotic conditions (C). D and E, mean prodynorphin mRNA expression within the supraoptic nuclei (D) and supraoptic nucleus vasopressin neurones (E) of rats in hypo-osmotic, normo-osmotic and hyperosmotic conditions (*P < 0.05 and compared to hypo-osmotic stimuli, one-way ANOVA followed by Bonferroni's post hoc tests).
Discussion
Here, we found, as expected, that 24 h dehydration increases the average spike discharge rate of vasopressin (and oxytocin) neurones recorded from urethane-anaesthetized rats. It is generally believed that efficient vasopressin secretion depends on the evolution of phasic firing by vasopressin neurones, and that intrinsic mechanisms linked to autoregulation of activity by the κ-opioid agonist dynorphin are critical for the generation of phasic firing (Brown, 2004; Brown & Bourque, 2006). Here we looked in particular at the effects of dehydration on the distribution of firing patterns exhibited by vasopressin cells, and at the changing influence of dynorphin. We found that endogenous κ-opioid receptor modulation of phasic activity is maintained during dehydration.
The persistence of κ-opioid modulation of phasic activity (and perhaps irregular activity) does not reflect a lack of response to osmotic stimulation. Rather, we show that there is dynamic osmotic regulation of prodynorphin mRNA expression within vasopressin neurones, such that prodynorphin mRNA expression is increased in vasopressin neurones during hyperosmotic stimulation, presumably to prevent over-excitation and perhaps protect vasopressin neurones excitotoxicity while defending the body from water loss.
Firing patterns of vasopressin neurones in dehydration
Vasopressin neurones display a range of spontaneous spike discharge patterns, even in conscious rats (Summerlee, 1981; Summerlee & Lincoln, 1981). While acute osmotic stimulation increases the spike discharge of most individual vasopressin neurones, it is unlikely that these responses are sustained in every neurone during chronic osmotic stimulation (Leng et al. 2008). Indeed, it has recently been demonstrated that even acute hyperosmotic stimulation can have variable effects on the activity of phasic vasopressin neurones (Bhumbra et al. 2005). We found no evidence of a change in the proportions of active vasopressin neurones displaying irregular, phasic or continuous spike discharge between euhydrated and dehydrated virgin female rats, similar to the effects of 2% saline drinking in male rats (Dyball & Pountney, 1973). Hence, some vasopressin cells are highly active, while others are relatively inactive during chronic dehydration. Because most vasopressin neurones respond to acutely administered hyperosmotic stimuli (Brimble & Dyball, 1977; Leng et al. 2001; Bhumbra et al. 2005) during chronically maintained hyperosmotic challenge there are probably mechanisms that permit individual neurones to cycle between periods of sustained high spike discharge and periods of lower spike discharge. Such mechanisms might dynamically distribute the increased secretory load across the population of vasopressin neurones (Leng et al. 2008).
By contrast to our results, it has previously been reported that 24 h dehydration markedly increases the proportion of vasopressin neurones that display phasic spike discharge in lactating rats (Wakerley et al. 1978). In these lactating rats, the plasma osmolarity was much higher after 24 h dehydration (∼340 mosmol l−1) than in our virgin rats (∼310 mosmol l−1) and so 24 h dehydration might constitute a stronger stimulus for vasopressin secretion in lactating rats than in virgin rats. Also, supraoptic nucleus prodynorphin mRNA expression is doubled during lactation (Lightman & Young, 1987), which might promote the adoption of phasic activity by vasopressin neurones via markedly increased endogenous dynorphin inhibition of spike discharge (Brown & Bourque, 2006).
Phasic spike discharge in vasopressin neurones during dehydration
Phasic spike discharge is the most efficient activity pattern for secretion of vasopressin from the posterior pituitary gland (Bicknell & Leng, 1981; Brown et al. 2007, 2008). The increased firing rate of phasic vasopressin neurones observed during dehydration was underpinned by an increase in intraburst firing rate with no change in burst duration or interburst interval. Because the increased firing rate during bursts was evident from burst onset, it is unlikely to be driven by an activity-dependent mechanism, such as potentiation of ADPs. Rather, increased synaptic drive (Di & Tasker, 2004) and/or increased activation of stretch-inactivated cation channels (Zhang & Bourque, 2003) would appear to be a more likely mechanism. Our analysis of post-spike excitability (data not shown), indicating an increase in the probability of spike firing that was independent of spike firing (i.e. no change in the shape of the hazard function; Sabatier et al. 2004) in dehydration, is consistent with such mechanisms driving increased firing rate during phasic activity in dehydrated rats.
We have previously shown that, under basal conditions, spike discharge is restrained during phasic bursts by activity-dependent autocrine inhibition of the ADP by dendritic dynorphin released from vasopressin neurones (Brown & Bourque, 2004; Brown et al. 2006), which reduces intraburst firing rate and burst duration (Brown et al. 1998, 2004). It has recently been shown that dendritically released dynorphin might also regulate vasopressin neurone excitability via retrograde inhibition of excitatory synaptic transmission (Iremonger & Bains, 2009). Here, the κ-opioid receptor antagonist nor-BNI induced a similar marked increase in burst duration in vasopressin neurones in dehydrated rats as it did in euhydrated rats, indicating that κ-opioid restraint of phasic spike discharge is maintained (and perhaps potentiated) during dehydration.
Osmotic regulation of prodynorphin expression in vasopressin neurones
For these experiments, we used a more prolonged method of hyperosmotic stimulation (2% saline-drinking for 7 days) to permit more time for any changes in prodynorphin mRNA expression to be more robustly expressed than might be likely after 24 h of water deprivation. In our hands, 2% saline-drinking induced a similar, but presumably longer-lasting, increase in plasma osmolarity to that induced by 24 h of water deprivation. Similar to our observations after 24 h of water deprivation, 3 days of saline drinking does not increase the proportion of supraoptic nucleus neurones that display phasic activity (Dyball & Pountney, 1973). Here, prodynorphin mRNA expression was up-regulated in hyperosmotic conditions, similar to previous work demonstrating that drinking 2% NaCl progressively increases supraoptic nucleus dynorphin mRNA expression over 1–12 days of 2% saline drinking (Lightman & Young, 1987).
We have previously shown that the spontaneous firing rates of supraoptic nucleus neurones in hyponatraemic rats are lower than those in euhydrated rats and that no vasopressin neurones exhibit phasic firing in hyponatraemic rats, even during acute hypertonic saline infusion (Leng et al. 2001). Here, we show for the first time that prodynorphin mRNA expression is down-regulated in hypo-osmotic conditions. Hence, it appears likely prodynorphin mRNA expression is modulated in response to the changes in overall activity within the population of vasopressin neurones as the osmotic status of the organism changes.
Concluding remarks
Over the years, much work has focused on the causes and consequences of phasic activity in vasopressin neurones because this activity pattern is the most efficient for the secretion of vasopressin from an individual vasopressin neurone (Brown, 2004; Brown & Bourque, 2006). However, the secretion from each individual vasopressin neurone is only of importance in the context of its contribution to the overall secretion of vasopressin from the population as a whole (Leng et al. 2008). Here we show that, even under stimulated conditions such as dehydration, the population of vasopressin neurones display a range of activity patterns, including irregular, phasic and continuous patterns; neurones displaying irregular and phasic activity are under endogenous κ-opioid inhibition, regardless of the osmotic status of the organism while continuously active neurones might constitute a sub-population that have (at least temporarily) escaped endogenous κ-opioid inhibition of firing. Hence, changes in κ-opioid feedback inhibition might contribute to the determination of the activity patterns of individual vasopressin neurones, to set the appropriate population output for the prevailing physiological conditions and protect individual vasopressin neurones from over-excitation during periods of increased demand for vasopressin.
Acknowledgments
This work was supported by a New Zealand Lottery Health Research Grant (no. 223744).
Glossary
Abbreviations
- ADP
afterdepolarization
- AHP
afterhyperpolarization
- nor-BNI
nor-binaltorphimine
- IDFR
index of dispersion of firing rate
- MFR
mean firing rate
- SDFR
standard deviation of firing rate
Author contributions
All authors were involved in the conception and design, or analysis and interpretation of data included in the manuscript, drafting the article or revising it critically for important intellectual content and final approval of the version to be published. V.S. and C.H.B. designed the electrophysiological experiments, which V.S. completed at the University of Otago (including data analysis and interpretation). G.L. designed the in situ experiments, which were completed and analysed by V.R.B. at the University of Edinburgh.
References
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Bhumbra GS, Inyushkin AN, Syrimi M, Dyball RE. Spike coding during osmotic stimulation of the rat supraoptic nucleus. J Physiol. 2005;569:257–274. doi: 10.1113/jphysiol.2005.097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell RJ, Leng G. Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocrinology. 1981;33:295–299. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- Brimble MJ, Dyball RE. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol. 1977;271:253. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH. Rhythmogenesis in vasopressin cells. J Neuroendocrinol. 2004;16:727–739. doi: 10.1111/j.1365-2826.2004.01227.x. [DOI] [PubMed] [Google Scholar]
- Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Bourque CW. Mechanisms of rhythmogenesis: insights from hypothalamic vasopressin neurons. Trends Neurosci. 2006;29:108–115. doi: 10.1016/j.tins.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Brown CH, Leng G, Ludwig M, Bourque CW. Endogenous activation of supraoptic nucleus κ-opioid receptors terminates spontaneous phasic bursts in rat magnocellular neurosecretory cells. J Neurophysiol. 2006;95:3235–3244. doi: 10.1152/jn.00062.2006. [DOI] [PubMed] [Google Scholar]
- Brown CH, Ludwig M, Leng G. κ-Opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. J Neurosci. 1998;18:9480–9488. doi: 10.1523/JNEUROSCI.18-22-09480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Ludwig M, Leng G. Temporal dissociation of the feedback effects of dendritically co-released peptides on rhythmogenesis in vasopressin cells. Neuroscience. 2004;124:105–111. doi: 10.1016/j.neuroscience.2003.11.038. [DOI] [PubMed] [Google Scholar]
- Brown CH, Munro G, Murphy NP, Leng G, Russell JA. Activation of oxytocin neurones by systemic cholecystokinin is unchanged by morphine dependence or withdrawal excitation in the rat. J Physiol. 1996;496:787–794. doi: 10.1113/jphysiol.1996.sp021727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Ruan M, Scott V, Tobin VA, Ludwig M. Multi-factorial somato-dendritic regulation of phasic spike discharge in vasopressin neurons. Prog Brain Res. 2008;170:219–228. doi: 10.1016/S0079-6123(08)00419-6. [DOI] [PubMed] [Google Scholar]
- Brown CH, Scott V, Ludwig M, Leng G, Bourque CW. Somatodendritic dynorphin release: orchestrating activity patterns of vasopressin neurons. Biochem Soc Trans. 2007;35:1236–1242. doi: 10.1042/BST0351236. [DOI] [PubMed] [Google Scholar]
- Civelli O, Douglass J, Goldstein A, Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985;82:4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004;145:5141–5149. doi: 10.1210/en.2004-0702. [DOI] [PubMed] [Google Scholar]
- Dyball RE, Pountney PS. Discharge patterns of supraoptic and paraventricular neurones in rats given a 2 per cent NaCl solution instead of drinking water. J Endocrinol. 1973;56:91–98. doi: 10.1677/joe.0.0560091. [DOI] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Excitatory role of the hyperpolarization-activated inward current in phasic and tonic firing of rat supraoptic neurons. J Neurosci. 2000;20:4855–4863. doi: 10.1523/JNEUROSCI.20-13-04855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Muscarinic receptor modulation of slow afterhyperpolarization and phasic firing in rat supraoptic nucleus neurons. J Neurosci. 2004;24:7718–7726. doi: 10.1523/JNEUROSCI.1240-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greffrath W, Magerl W, Disque-Kaiser U, Martin E, Reuss S, Boehmer G. Contribution of Ca2+-activated K+ channels to hyperpolarizing after-potentials and discharge pattern in rat supraoptic neurones. J Neuroendocrinol. 2004;16:577–588. doi: 10.1111/j.1365-2826.2004.01204.x. [DOI] [PubMed] [Google Scholar]
- Greffrath W, Martin E, Reuss S, Boehmer G. Components of after-hyperpolarization in magnocellular neurones of the rat supraoptic nucleus in vitro. J Physiol. 1998;513:493–506. doi: 10.1111/j.1469-7793.1998.493bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. Retrograde opioid signalling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29:7349–7358. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. J Physiol. 1996;494:389–398. doi: 10.1113/jphysiol.1996.sp021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Brown CH, Bull PM, Brown D, Scullion S, Currie J, Blackburn-Munro RE, Feng J, Onaka T, Verbalis JG, Russell JA, Ludwig M. Responses of magnocellular neurons to osmotic stimulation involves coactivation of excitatory and inhibitory input: an experimental and theoretical analysis. J Neurosci. 2001;21:6967–6977. doi: 10.1523/JNEUROSCI.21-17-06967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol. 1999;57:625–655. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Leng G, Brown CH, Sabatier N, Scott V. Population dynamics in vasopressin cells. Neuroendocrinology. 2008;88:160–172. doi: 10.1159/000149827. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WS., III Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol. 1987;394:23–39. doi: 10.1113/jphysiol.1987.sp016858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan M, Brown CH. Feedback inhibition of action potential discharge by endogenous adenosine enhancement of the medium afterhyperpolarization. J Physiol. 2009;587:1043–1056. doi: 10.1113/jphysiol.2008.167239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Brown CH, Ludwig M, Leng G. Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J Physiol. 2004;558:161–180. doi: 10.1113/jphysiol.2004.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM. Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology. 2005;146:5079–5085. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- Summerlee AJ. Extracellular recordings from oxytocin neurones during the expulsive phase of birth in unanaesthetized rats. J Physiol. 1981;321:1–9. doi: 10.1113/jphysiol.1981.sp013967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerlee AJ, Lincoln DW. Electrophysiological recordings from oxytocinergic neurones during suckling in the unanaesthetized lactating rat. J Endocrinol. 1981;90:255–265. doi: 10.1677/joe.0.0900255. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Drutarosky MD. Adaptation to chronic hypoosmolality in rats. Kidney Int. 1988;34:351–360. doi: 10.1038/ki.1988.188. [DOI] [PubMed] [Google Scholar]
- Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978;148:425–440. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- Walters JK, Hatton GI. Supraoptic neuronal activity in rats during five days of water deprivation. Physiol Behav. 1974;13:661–667. doi: 10.1016/0031-9384(74)90237-6. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Bourque CW. Osmometry in osmosensory neurons. Nat Neurosci. 2003;6:1021–1022. doi: 10.1038/nn1124. [DOI] [PubMed] [Google Scholar]