Abstract
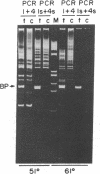
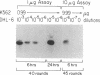
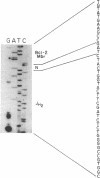
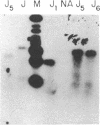
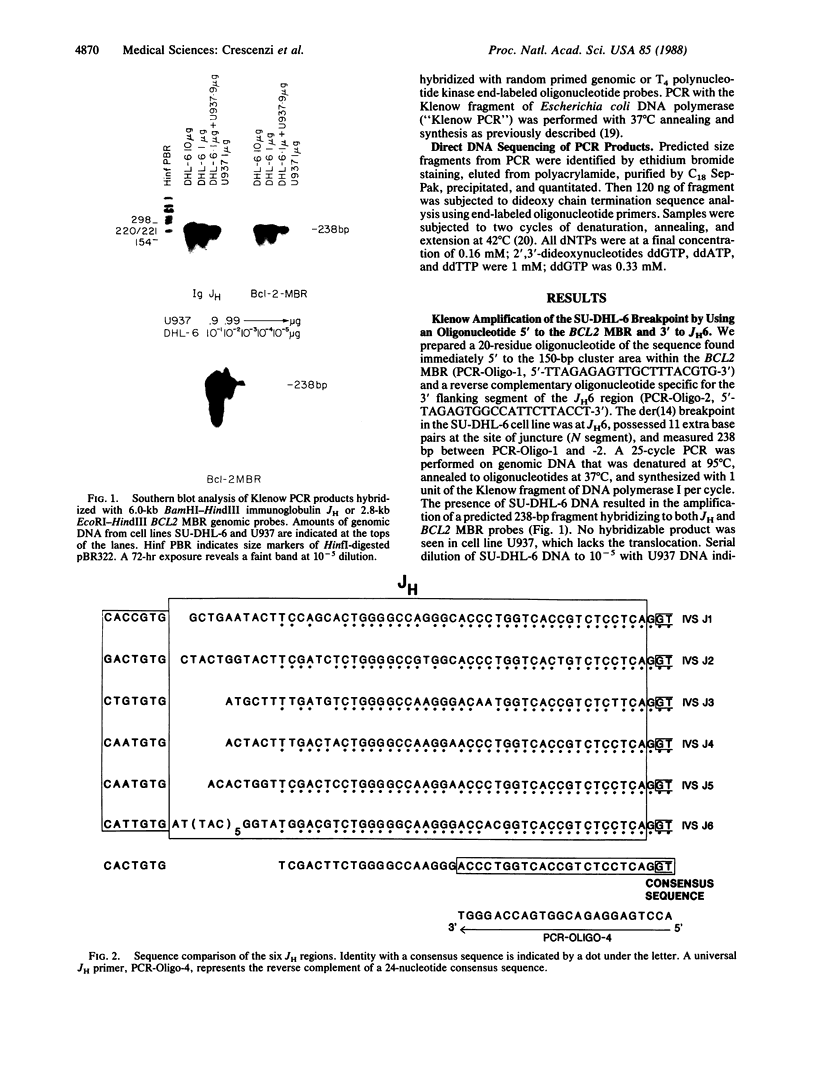
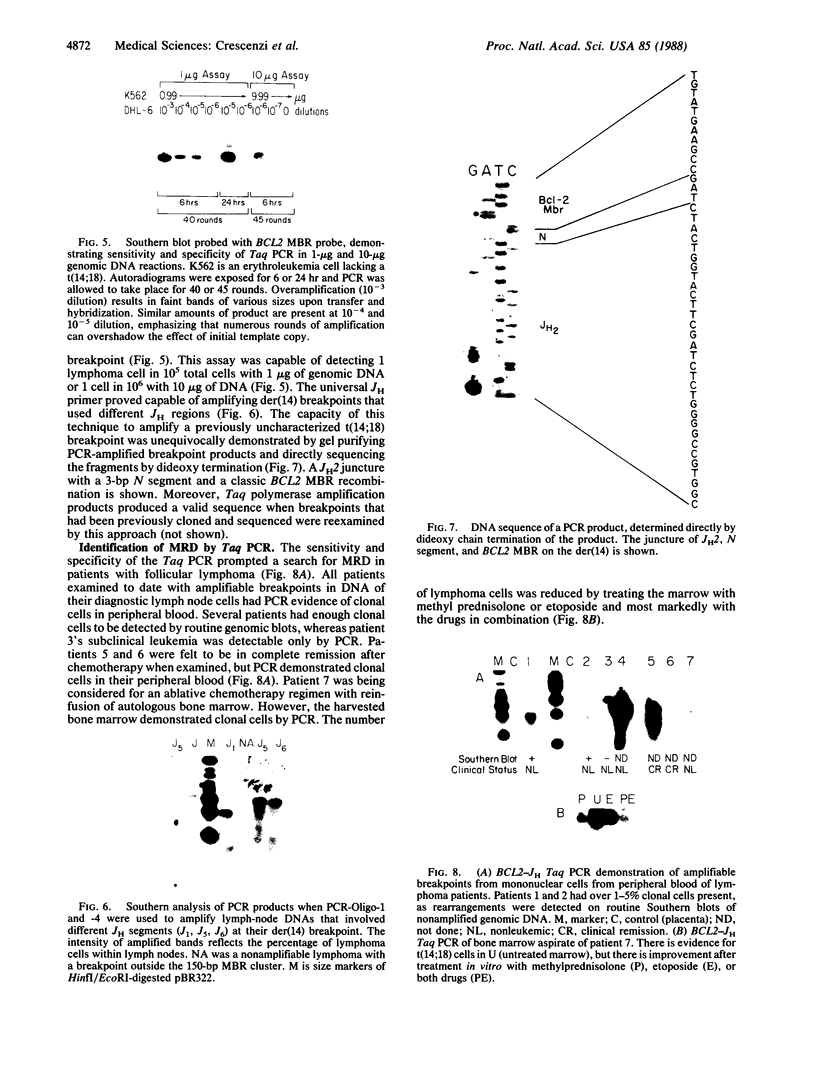
Achieving the capacity to detect minimal numbers of neoplastic cells is a major cancer diagnostic challenge. Chromosomal translocations such as the t(14;18)(q32;q21) found in follicular and some nonfollicular lymphomas provide a tumor-specific molecular marker. The 14;18 breakpoints are focused at one of six immunoglobulin heavy chain joining (JH) regions on chromosome 14 and a small major breakpoint region (MBR) of the BCL2 gene on chromosome 18. We utilized universal oligonucleotide primers of a region 5' to the BCL2 MBR and at the 3' end of JH segments to initiate a DNA polymerase chain reaction that amplified these BCL2-JH junctures. Use of thermostable DNA polymerase enabled annealing and synthesis steps at temperatures approaching the melting point of the primers, providing a sensitive and specific assay capable of detecting 1 lymphoma cell in 10(6) normal cells. This technique identified the subclinical presence of leukemic cells in all seven patients examined, including two in clinical remission. It also assessed the effectiveness of protocols designed to purge malignant cells from marrow. Moreover, this approach enabled the rapid DNA sequencing of chromosomal breakpoints without their molecular cloning. This assay markedly refines the capacity to detect minimal residual disease and should improve the ability to determine the stage of disease, stratify treatment, and evaluate therapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Bakhshi A., Wright J. J., Graninger W., Seto M., Owens J., Cossman J., Jensen J. P., Goldman P., Korsmeyer S. J. Mechanism of the t(14;18) chromosomal translocation: structural analysis of both derivative 14 and 18 reciprocal partners. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2396–2400. doi: 10.1073/pnas.84.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien A., Edgar D. B., Trela J. M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol. 1976 Sep;127(3):1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Graninger W. B., Seto M., Boutain B., Goldman P., Korsmeyer S. J. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest. 1987 Nov;80(5):1512–1515. doi: 10.1172/JCI113235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E., Trela M., Thompson J., Lowder J., Horning S., Levy R., Sklar J. Detection of B-cell lymphoma in peripheral blood by DNA hybridisation. Lancet. 1985 Nov 16;2(8464):1092–1095. doi: 10.1016/s0140-6736(85)90686-5. [DOI] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Chang K. S., Cabanillas F., Freireich E. J., Trujillo J. M., Stass S. A. Detection of minimal residual cells carrying the t(14;18) by DNA sequence amplification. Science. 1987 Jul 10;237(4811):175–178. doi: 10.1126/science.3110950. [DOI] [PubMed] [Google Scholar]
- Levine E. G., Arthur D. C., Frizzera G., Peterson B. A., Hurd D. D., Bloomfield C. D. There are differences in cytogenetic abnormalities among histologic subtypes of the non-Hodgkin's lymphomas. Blood. 1985 Dec;66(6):1414–1422. [PubMed] [Google Scholar]
- Lipford E., Wright J. J., Urba W., Whang-Peng J., Kirsch I. R., Raffeld M., Cossman J., Longo D. L., Bakhshi A., Korsmeyer S. J. Refinement of lymphoma cytogenetics by the chromosome 18q21 major breakpoint region. Blood. 1987 Dec;70(6):1816–1823. [PubMed] [Google Scholar]
- McMahon G., Davis E., Wogan G. N. Characterization of c-Ki-ras oncogene alleles by direct sequencing of enzymatically amplified DNA from carcinogen-induced tumors. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4974–4978. doi: 10.1073/pnas.84.14.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffeld M., Wright J. J., Lipford E., Cossman J., Longo D. L., Bakhshi A., Korsmeyer S. J. Clonal evolution of t(14;18) follicular lymphomas demonstrated by immunoglobulin genes and the 18q21 major breakpoint region. Cancer Res. 1987 May 15;47(10):2537–2542. [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Identification of the constant chromosome regions involved in human hematologic malignant disease. Science. 1982 May 14;216(4547):749–751. doi: 10.1126/science.7079737. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Roe B. A., Canaani E. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell. 1986 Oct 24;47(2):277–284. doi: 10.1016/0092-8674(86)90450-2. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Wright J. J., Poplack D. G., Bakhshi A., Reaman G., Cole D., Jensen J. P., Korsmeyer S. J. Gene rearrangements as markers of clonal variation and minimal residual disease in acute lymphoblastic leukemia. J Clin Oncol. 1987 May;5(5):735–741. doi: 10.1200/JCO.1987.5.5.735. [DOI] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]
- Zehnbauer B. A., Pardoll D. M., Burke P. J., Graham M. L., Vogelstein B. Immunoglobulin gene rearrangements in remission bone marrow specimens from patients with acute lymphoblastic leukemia. Blood. 1986 Mar;67(3):835–838. [PubMed] [Google Scholar]