Abstract
Locus coeruleus (LC) noradrenergic neurons are implicated in a variety of functions including the regulation of vigilance and the modulation of sensory processing. Thyrotropin-releasing hormone (TRH) is an endogenous neuropeptide that induces a variety of behavioural changes including arousal and antinociception. In the present study, we explored whether the activity of LC noradrenergic neurons is modulated by TRH. Using current-clamp recording from isolated rat LC neurons, we found that TRH increased the firing rate of spontaneous action potentials. The TRH action was mimicked by TRH analogues including taltirelin and TRH-gly. In voltage-clamp recording at a holding potential of −50 mV, TRH produced an inward current associated with a decrease in the membrane K+ conductance. This current was inhibited by the TRH receptor antagonist chlordiazepoxide. Following inhibition of the pH-sensitive K+ conductance by extracellular acidification, the TRH response was fully inhibited. The TRH-induced current was also inhibited by the phospholipase C (PLC) inhibitor U-73122, but not by the protein kinase C inhibitor chelerythrine nor by chelation of intracellular Ca2+ by BAPTA. The recovery from the facilitatory action of TRH on the spike frequency was markedly inhibited by a high concentration of wortmannin. These results suggest that TRH activates LC noradrenergic neurons by decreasing an acid-sensitive K+ conductance via PLC-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate. The present findings demonstrate that TRH activates LC neurons and characterize the underlying signalling mechanisms. The action of TRH on LC neurons may influence a variety of CNS functions related to the noradrenergic system which include arousal and analgesia.
Introduction
The locus coeruleus (LC), located at either side of the fourth ventricle in the pontine and medullary brainstem, sends noradrenergic projections to many regions of the central nervous system (CNS) and plays an important role in the regulation of multiple physiological processes including attention, pain control, sleep–wake cycle, learning and memory (Aston-Jones et al. 1991; Stamford, 1995; Nestler et al. 1999; Sara, 2009). The LC noradrenergic neurons are silent during rapid eye movement sleep, fire at a regular slow rate during quiet wakefulness and show bursts of firing in response to arousing stimuli (Sara, 2009). Spontaneous firing activity is also observed in isolated LC neurons (Arima et al. 1998; Koga et al. 2005), and this pacemaker activity can be modulated by neurotransmitters, neuromodulators and intracellular second messengers. For example, noradrenaline via α2-adrenoceptors and enkephalin via μ-receptors inhibit LC neuronal firing by the activation of G-protein-mediated inwardly rectifying K+ (GIRK) channels (Arima et al. 1998; Torrecilla et al. 2002). In contrast, these neurons are activated by orexins acting on non-selective cationic and K+ channels (Ivanov & Aston-Jones, 2000; Murai & Akaike 2005).
Thyrotropin-releasing hormone (TRH), a neuropeptide originally isolated from mammalian hypothalami, is distributed widely throughout the CNS (Winokur & Utiger, 1974; Bayliss et al. 1994). TRH has a variety of direct CNS effects, i.e. actions that are independent of its endocrine action in releasing thyroid-stimulating hormone (Nillni & Sevarino, 1999). These effects implicate this peptide in the regulation of arousal, cognition, circadian rhythm, mood, seizure activity and motor function (Nillni & Sevarino, 1999). TRH also has antinociceptive properties against noxious stimuli (Boschi et al. 1983). There are two subtypes of TRH receptors, TRH-R1 and TRH-R2, and these receptors couple primarily to the Gq/11 subfamily of G-proteins (reviewed by Sun et al. 2003). Consequently, stimulation of TRH receptors results in the activation of phospholipase C (PLC), which mediates phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) hydrolysis to form inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol. These second messengers mediate Ca2+ release from intracellular Ca2+ stores and the activation of protein kinase C.
It has been reported that the TRH-induced locomotor hyperactivity in mice is blocked in part by the α1-adrenergic antagonist (Heal et al. 1987) and that systemic administration of TRH and its related analogues increases the extracellular concentration of noradrenaline in frontal cortex of urethane-anaesthetized rat (Itoh et al. 1994). Recently, Tanabe et al. (2007) have reported that taltirelin, a stable analogue of TRH, exhibits an analgesic effect on mechanical nociception via a descending noradrenergic pathway. Since the LC is the sole source of noradrenaline in the forebrain and also contributes to descending pain inhibitory pathways (Pertovaara, 2006; Sara, 2009), these observations suggest that the LC is a major of site of action of TRH and its related analogues. The LC does indeed contain TRH receptors (Manaker et al. 1985), and TRH-containing fibres have been reported to be localized in the rat and human LC (Eskay et al. 1983; Pammer et al. 1990). Since the action of TRH on the LC noradrenergic neurons remains to be clarified, we investigated the effect of TRH on noradrenergic neurons acutely isolated from rat LC using the patch-clamp recording technique.
Methods
All experimental procedures were approved by the animal research committee of the National Institute for Physiological Sciences, Japan, and complied with the policies of The Journal of Physiology (Drummond, 2009).
Preparation
Wistar rats (10–17 days old, n= 115) were decapitated under pentobarbital sodium anaesthesia (100 mg kg−1, i.p.). The brain was quickly removed and sliced at a thickness of 380 μm using a microslicer (VT1000S; Leica, Nussloch, Germany). Slices were kept in the incubation medium (see below) saturated with 95% O2 and 5% CO2 at room temperature (21–24°C) for at least 1 h before mechanical dissociation. Slices were then transferred into a 35 mm culture dish filled with standard external solution (Primaria 3801; Becton Dickinson, Rutherford, NJ, USA), and the region of the LC was identified under a binocular microscope. Details of the mechanical dissociation have been previously described (Ishibashi et al. 2007). Briefly, mechanical dissociation was accomplished using a custom-built vibration device and a fire-polished glass pipette oscillating at 50–60 Hz. The tip of the fire-polished glass pipette was lightly placed on the surface of the locus coeruleus and was vibrated horizontally (0.1–0.2 mm) for about 5 min. Slices were then removed and the mechanically dissociated neurons allowed to settle and adhere to the bottom of the dish for at least 15 min before recordings commenced. The noradrenergic neurons were easily identified with their morphological features: a large oval-shaped cell body (> 25 μm) with several robust dendrites, as shown in our previous study (Koga et al. 2005).
Electrical measurements
Most electrical measurements were performed using the perforated patch-clamp recording configuration with amphotericin B. In the experiments using BAPTA, conventional whole-cell patch-clamp recordings were used. Membrane voltage was controlled, and currents recorded, with the use of a patch-clamp amplifier (EPC-7plus; List Medical, Darmstadt-Eberstadt, Germany). Patch pipettes were made from borosilicate capillary glass in two stages on a vertical pipette puller (PC-10, Narishige, Tokyo, Japan). The resistance between the recording pipettes filled with internal solution (see below) and the reference electrode in the standard external solution was 4–6 MΩ. Neurons were visualized under phase contrast on an inverted microscope (DMIRB; Leica Microsystems, Wetzlar, Germany). Current and voltage were continuously monitored on an oscilloscope and a pen recorder (WR3320, Graphtec, Tokyo, Japan). Membrane currents were filtered at 3 kHz (E-3201A; NF Electric Instruments, Tokyo, Japan), digitized at 10 kHz, and stored on a computer equipped with pCLAMP8.2 software (Axon Instruments). Only dissociated LC neurons that had resting membrane potentials more negative than −50 mV and action potentials that overshot zero by > 20 mV were studied. In extracellular solutions containing 2.5, 5 and 10 mm K+, the reversal potential of TRH-induced currents was recorded using voltage ramps of −100 mV in amplitude and of 1.5 s duration, applied to the neuron from a holding potential (VH) of −50 mV. In extracellular solution containing 20 mm K+, voltage ramps from −30 mV to −100 mV were used. The reversal potential of U-73122-induced current was recorded using voltage ramps from a VH of −50 mV to +30 mV. All experiments were performed at room temperature (21–24°C).
Solutions
The composition of the incubation solution was (in mm): 124 NaCl, 2.5 KCl, 1.2 KH2PO4, 24 NaHCO3, 2 CaCl2, 1 MgCl2 and 10 glucose saturated with 95% O2 and 5% CO2. The standard external solution consisted of (in mm): 150 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes and 10 glucose. When K+ levels were increased, Na+ was replaced with equimolar K+. When Na+ was removed, Na+ was replaced with equimolar N-methyl-d-glucamine+. These external solutions were adjusted to pH 7.4 with tris(hydroxymethyl)aminomethane (Tris-base). These extracellular solutions routinely contained 5 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 5 μm d-aminophosphonovalerate (AP-5), 3 μm SR-95531 and 1 μm strychnine to prevent glutamatergic, GABAergic and glycinergic synaptic currents. In voltage-clamp experiments, tetrodotoxin (TTX, 0.3 μm) was also added to the extracellular solution. The ionic composition of the internal (patch pipette) solution for perforated patch-clamp recordings was (in mm): 140 potassium methanesulfonate, 10 KCl, 1 MgCl2 and 10 Hepes. The patch-pipette solution for whole-cell patch recording contained (mm): 130 potassium methanesulfonate, 10 KCl, 2 NaCl, 2 MgCl2, 4 ATP-Mg, 0.1 GTP-3Na, 5 BAPTA, 10 Hepes. The pH was adjusted to 7.2 with Tris-base. Amphotericin B was dissolved in dimethylsulfoxide (10 mg ml−1) and this stock solution was add to the internal solution just before use to give a final concentration of 25 μg ml−1. Rapid application of external solution was performed using a ‘Y-tube’ as described previously (Ishibashi et al. 2005). Unless otherwise mentioned, only one recording of a TRH response was obtained from each dish of neurons, to ensure that neurons had not been previously exposed to TRH.
Data analysis
All data are presented as mean ±s.e.m. A two-tailed Student's t test was used to determine significant differences between different conditions. For multiple groups, one-way ANOVA with Dunnett's multiple comparison post hoc test was used. The level of significance was set at P < 0.05 for all tests.
Drugs
Drugs used in the present study were tertiapin, TRH (Peptide Institute, Osaka, Japan), CNQX, SR-95531, taltirelin, U-73122 (Tocris Cookson, Avonmouth, UK), BaCl2, caffeine, noradrenaline, tetrodotoxin (TTX) (Wako, Tokyo, Japan), edelfosine (Enzo Life Sciences, Plymouth Meeting, PA, USA), TRH-gly (Bachem, Torrance, CA, USA), amphotericin B, AP-5, 2-aminoethyl diphenylborate (2-APB), BAPTA, chelerythrine, 2-nitro-4-carboxyphenyl N,N-diphenylcarbamate (NCDC), strychnine, U-73343 and wortmannin (Sigma, St Louis, MO, USA).
Results
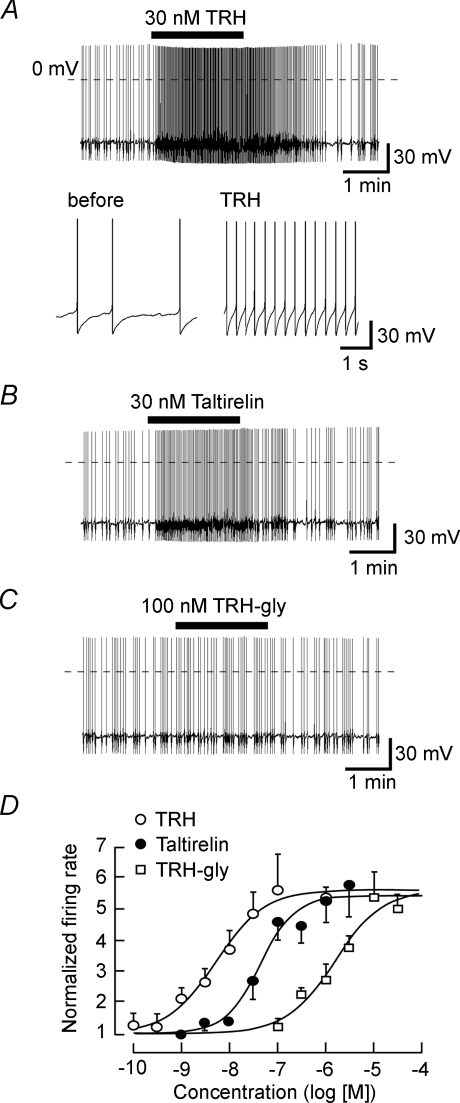
Whole-cell recordings were initially performed on freshly dissociated rat LC noradrenergic neurons using perforated patch-clamp recordings. Figure 1A shows a representative response to TRH when recording in current-clamp mode. Before application of TRH the neuron's resting membrane potential was −54.7 ± 0.60 mV (n= 16), and neurons fired spontaneous action potentials at frequencies between 0.3 and 1.4 Hz (0.68 ± 0.08 Hz, n= 16). The application of 30 nm TRH increased the frequency of action potentials to 3.81 ± 0.46 Hz (n= 7), with the firing rate returning back to lower levels after washing out the peptide. The TRH analogue taltirelin (30 nm) also increased the firing frequency (Fig. 1B). The TRH analogue TRH-gly, at a concentration of 100 nm that fully activates TRH-R2 receptors (Cao et al. 1998), had no significant effect on the frequency of spontaneous action potentials (Fig. 1C). At much higher concentrations, however, TRH-gly did increase firing frequency (Fig. 1D). Figure 1D depicts the concentration–response curves constructed from pooled data for these three ligands, giving apparent EC50 values of 5.46 nm, 44.0 nm and 1.57 μm for TRH, taltirelin and TRH-gly, respectively.
Figure 1. Excitatory actions of TRH and its analogues on the firing of locus coeruleus.
A, a typical current-clamp recording showing the excitatory effect of 30 nm TRH on the firing of action potentials. Lower panels show the action potentials with an expanded time scale in the absence (left) and presence (right) of TRH. B and C, effects of 30 nm taltirelin (B) and 100 nm TRH-gly (C) on LC neurons. Traces shown in A–C were obtained from different neurons. Dashed lines in A–C show the 0 mV level. D, concentration–response curves for TRH (○), taltirelin (•) and TRH-gly (□). Each point represents the mean ±s.e.m. from 4–7 neurons.
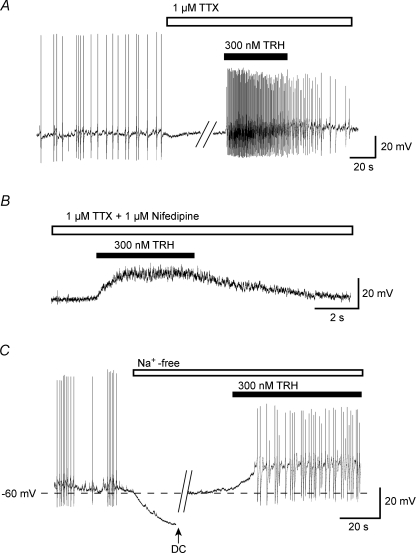
Application of tetrodotoxin (1 μm; TTX) completely inhibited the spontaneous action potentials (n= 5). In the presence of 1 μm TTX, TRH (300 nm) induced a depolarization associated with smaller-amplitude action potentials (Fig. 2A). These action potentials were inhibited by the voltage-dependent Ca2+ channel antagonist nifedipine (1 μm; Fig. 2B), or by Cd2+ (200 μm; not shown), indicating that they are Ca2+ spikes. The depolarization induced by 300 nm TRH in the presence of 1 μm nifedipine or 200 μm Cd2+ was 13.6 ± 1.7 mV (n= 5) and 16.0 ± 3.1 mV (n= 4), respectively. In order to further confirm that these spikes were Ca2+ spikes, we tested the effect of TRH in the absence of extracellular Na+. Removal of extracellular Na+ markedly hyperpolarized the membrane potential (Fig. 2C), which was consistent with a previous study (Alreja & Aghajanian, 1993). After the membrane potential returned to its original level, application of TRH produced a depolarizing response associated with repetitive action potentials (n= 4; Fig. 2C). Hence TRH induces depolarization and Ca2+ spikes in the absence of extracellular Na+. Ca2+ spikes in LC noradrenergic neurons were also reported previously (Horvath et al. 1999).
Figure 2. Depolarization of LC neurons by TRH.
A, effects of 300 nm TRH in the presence of 1 μm tetrodotoxin (TTX). The trace is representative of five experiments. B, effect of 300 nm TRH in the presence of 1 μm TTX and 1 μm nifedipine. C, TRH-induced depolarization in the absence of extracellular sodium. At the arrow (DC), depolarizing current was used to return the cell to its original resting potential.
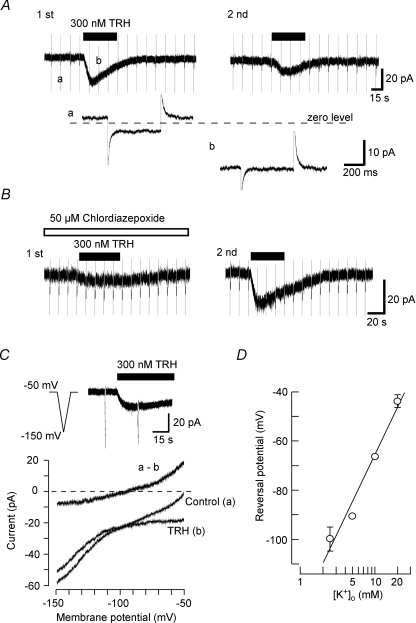
We next performed the voltage-clamp experiments to analyse the depolarizing TRH response. Figure 3A shows a representative response to 300 nm TRH in a voltage-clamped LC neuron at a VH of −50 mV. Hyperpolarizing voltage steps of 10 mV and 400 ms duration were continuously applied to monitor the changes in the membrane conductance. Application of TRH induced an inward current which reached a peak of 19.3 ± 1.5 pA (n= 8) before declining. During the peak response, the membrane conductance was significantly reduced from 0.87 ± 0.11 to 0.33 ± 0.08 nS (n= 8, P < 0.001). Re-application of TRH 20 min after the first application produced a smaller current, with a peak amplitude of 6.4 ± 0.6 pA (n= 8, P < 0.01). The membrane conductance observed in this study was relatively lower than that reported in slice preparations (Williams et al. 1988; Travagli et al. 1996) which is likely to reflect the reduced extent of dendritic processes in these isolated cell preparations, since the conductance is reported to be strongly correlated to the number of distal dendrites in LC neurons (Travagli et al. 1996).
Figure 3. Inward currents induced by TRH.
Voltage-clamp recordings were performed at a holding potential (VH) of −50 mV. A, inward currents produced by application of TRH. Hyperpolarizing step pulses of 400 ms duration from a VH of −50 mV to −60 mV were applied every 15 s. TRH (300 nm, 1 min) was applied twice to the same neuron with a 20 min interval between applications. In the lower panels, selected current responses to the step hyperpolarization are shown on an expanded time scale. B, the effect of chlordiazepoxide on the TRH-induced current. In this experiment, the first application of TRH was performed in the presence of 50 μm chlordiazepoxide and the second application in the same cell in the absence of 50 μm chlordiazepoxide. C, current–voltage (I–V) relationship for the TRH response. Upper panel shows the current induced by 300 nm TRH. Hyperpolarizing ramp commands from −50 mV to −150 mV of 1.5 s duration were applied before and during application of TRH. Lower panel shows the I–V relationships obtained from these voltage ramps in the normal external solution containing 2.5 mm K+. The TRH-sensitive current (a - b) was obtained by subtraction of the TRH I–V curve (b) from control (a). D, the relationship between the external K+ concentration ([K+]o) and the reversal potential of the TRH-induced current. Each point represents the mean ±s.e.m. from 4–6 neurons.
Chlordiazapoxide is known as an antagonist at TRH receptors and has been used at a concentration of 50 μm to block TRH receptors in previous studies (Deng et al. 2006; Parmentier et al. 2009). In the present study, 50 μm chlordiazepoxide itself produced no detectable current (n= 5, not shown) but markedly inhibited the TRH response, being 6.2 ± 2.1 pA in the presence of chlordiazepoxide (n= 5), significantly different (P < 0.001) as compared to the 15.0 ± 3.1 pA TRH response observed in the same neurons after washout of chlordiazepoxide (Fig. 3B).
The ionic base of the TRH-mediated current was investigated using voltage ramps during voltage-clamp recordings. The pipette (internal) solution contained 150 mm K+, and neurons were initially perfused with a normal external solution containing 2.5 mm K+. Current responses to voltage ramps from a VH of −50 mV to −150 mV, before and during 300 nm TRH application, intersected each other at a membrane potential of −99.8 ± 4.1 mV (n= 5, Fig. 3C). The TRH-sensitive current, obtained by subtracting current in the presence of TRH from that under control conditions, showed outward rectification (Fig. 3C). As the extracellular K+ concentration was increased to 5, 10 and 20 mm, the reversal potential shifted to −90.5 ± 0.4 mV (n= 4), −66.3 ± 1.3 mV (n= 6) and −43.7 ± 2.6 mV (n= 5), respectively (Fig. 3D). From this relationship, the reversal potential of the TRH-induced current for a 10-fold change in the extracellular K+ concentration was estimated as 63 mV, close to the shift in the Nernst potential for an ideally selective K+ channel of 58.7 mV per 10-fold shift in the extracellular K+ concentration. This indicates that the TRH-induced current is due to a decrease in K+ conductance.
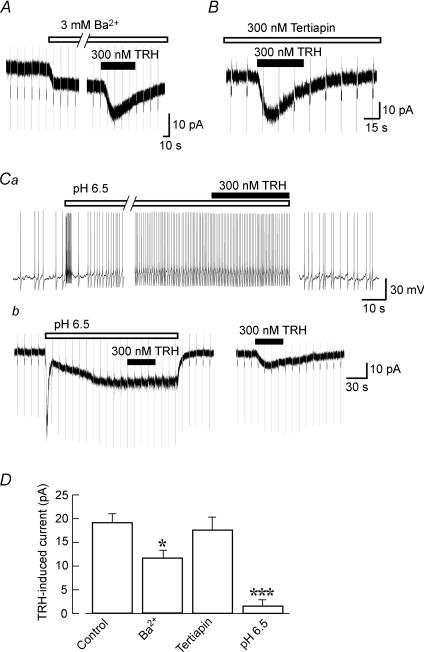
As shown in Fig. 3C, and as reported in a previous study (Williams et al. 1988), the resting membrane conductance of LC neurons showed weak inward rectification. The LC noradrenergic neurons have GIRK currents (Arima et al. 1998), and the GIRK channels can be constitutively active in hippocampal neurons (Chen & Johnston, 2005). Thus, we tested the effects of GIRK channel inhibitors on the TRH-induced currents. As shown in Fig. 4A, application of 3 mm Ba2+, a concentration which fully inhibits GIRK currents in LC neurons (Arima et al. 1998), caused an inward current of 6.5 ± 2.6 pA (n= 6). In the presence of 3 mm Ba2+, TRH still induced an inward current (n= 6), although this current was smaller than that observed under control conditions (Fig. 4D). In contrast, another inhibitor of inwardly rectifying K+ channels, tertiapin (300 nm), failed to cause any inhibition of the TRH-induced inward currents (n= 5; Fig. 4B and D). It was also noted that tertiapin (300 nm) per se produced no detectable current response and that it fully inhibited the noradrenaline-induced GIRK currents (n= 4, not shown).
Figure 4. Effect of K+ channel inhibitors on the TRH response.
Voltage-clamp recordings were performed at a VH of −50 mV. Hyperpolarizing step pulses to −60 mV and of 400 ms duration were applied every 10–15 s. A, the effect of 3 mm Ba2+ on the TRH-induced currents. Ba2+ itself induced an inward current which was accompanied by a decrease in the membrane conductance. B, the effect of the GIRK channel inhibitor tertiapin on the TRH-induced current. C, the TRH response under conditions designed to inhibit the pH-sensitive conductance. Application of an acidified solution (pH 6.5) increased action potential frequency under current-clamp conditions (a) and evoked an initial transient inward current, followed by slowly activating inward current, under voltage-clamp conditions (b). The TRH-responses on membrane potential (a) and membrane current (b) were recorded from different cells. After wash-out of pH 6.5 solution, TRH still induced an inward current in the same cell. Note that the TRH-induced current was fully blocked at an external pH 6.5. D, summary of the mean currents elicited by 300 nm TRH at a VH of −50 mV in the presence of different solutions and drugs. *P < 0.05, ***P < 0.001 compared with control. Each bar corresponds to 5–7 neurons.
TRH-induced inhibition of leak-like K+ currents, probably representing acid-sensitive TASK channels, has been reported in motor neurons (Bayliss et al. 1994) and hippocampal interneurons (Deng et al. 2006). LC neurons also express high levels of TASK channels (Talley et al. 2001). Thus, we next examined the effect of the TASK channel inhibition by acid on the TRH response. Application of a pH 6.5 solution caused an initial transient increase in the frequency of action potentials followed by a sustained increase in frequency to 429 ± 95% of control (n= 5). Under these conditions TRH (300 nm) had no significant effect on the action potential frequency (116 ± 13% of pH 6.5 conditions, P > 0.1; Fig. 4Ca). After wash-out of pH 6.5 solution, action potential frequency recovered to control levels. As shown in Fig. 4Cb, under voltage-clamp conditions, the reduction of pH from 7.4 to 6.5 activated a rapid initial transient inward current followed by a slower activating and sustained inward current of 17.4 ± 3.8 pA, n= 5) associated with a decreased membrane conductance. The initial transient current was accompanied by an increase in the membrane conductance (not shown), suggesting that it was mediated by acid-sensing cationic channels. In the low pH solution, the TRH response was markedly inhibited (being 3.0 ± 2.0 pA, n= 5; Fig. 4D). After returning to the control solution, TRH still elicited an inward current of 7.4 ± 3.4 pA, which was similar to the current amplitude that was evoked by a second application of TRH under control conditions. This also suggests that the low pH solution application did not cause any irreversible damage or run down in activity in the LC neuron. These data suggest that TRH depolarizes LC neurons by inhibiting acid-sensitive TASK channels.
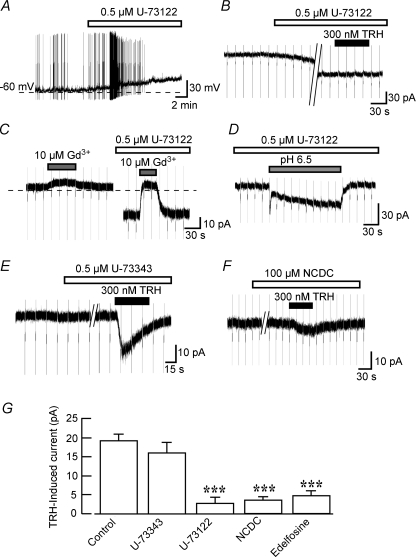
The TRH receptors are well known to increase the intracellular Ca2+ concentration via activation of PLC (Sun et al. 2003). We therefore next investigated whether PLC is involved in the TRH response. Under current-clamp conditions, application of 0.5 μm U-73122, a PLC inhibitor, caused a gradual depolarization that initially increased the firing frequency and finally blocked the occurrence of action potentials (n= 4; Fig. 5A). This U-73122 action was irreversible after 30 min wash-out. Consequently, the effect of U-73122 on the TRH response was examined under voltage-clamp conditions. U-73122 (0.5 μm) induced a slowly activating inward current at a VH of −50 mV (33.8 ± 5.2 pA, n= 7; Fig. 5B). After 10 min pretreatment with U-73122, the TRH-induced current was markedly inhibited (Fig. 5B). The U-73122-induced inward current reversed at −7.3 ± 2.9 mV (n= 4, not shown), and this current was blocked by the non-selective cation channel blocker Gd3+ (10 μm, n= 5; Fig. 5C), suggesting the involvement of non-selective cation channels. In the presence of U-73122, on the other hand, application of pH 6.5 solution still induced the sustained inward current of 15.2 ± 2.8 pA (n= 5; Fig. 5D), indicating that U-73122 did not occlude the acid-sensitive K+ channels. When neurons were pretreated for 10 min with U-73343 (0.5 μm), an inactive analogue of U-73122, TRH still induced an inward current of 16.0 ± 2.7 pA (n= 5) which was not significantly different from that observed under control conditions (Fig. 5E and G). The TRH-induced current was also inhibited by pretreatment (10 min) with further PLC inhibitors NCDC (100 μm, n= 5; Fig. 5F and G) and edelfosine (10 μm, n= 4; Fig. 5G). In contrast to U-73122, NCDC and edelfosine produced no detectable changes in the holding current. These results indicate that PLC contributes to the TRH response.
Figure 5. Contribution of PLC to the TRH response.
A, the PLC inhibitor U-73122 elicited a membrane depolarization. The dashed line represents a membrane potential of −60 mV. The trace is representative of four experiments. B, the TRH response in the presence of U-73122 measured in voltage clamp at VH of −50 mV. U-73122 itself induced an inward current, and after this current had reached a steady level, TRH was applied. C, inhibition of the U-73122-induced current by Gd3+. Gd3+ was applied before and during application of U-73122. Horizontal dashed line shows the zero current level. D, effect of pH 6.5 solution in the presence of U-73122. E, the TRH-induced current was unaffected in the presence of U-73343, an inactive isomer of U-73312. F, inhibition of the TRH-induced current by NCDC, a PLC inhibitor. G, summary of the mean TRH-induced current in the presence of 0.5 μm U-73122, 0.5 μm U-73343, 100 μm NCDC or 10 μm edelfosine. ***P < 0.01 compared with control.
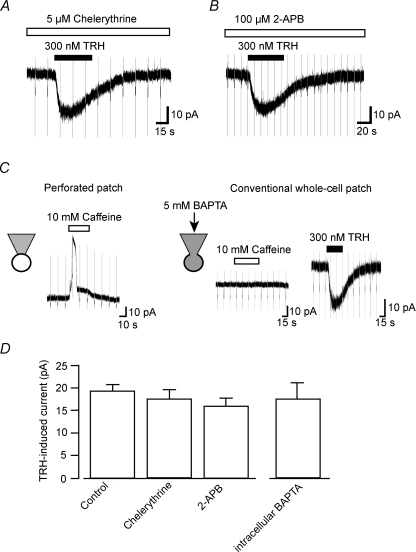
To further elucidate the possible signal transduction mechanisms for the TRH response, we next examined the effects of chelerythrine, a protein kinase C (PKC) inhibitor, and 2-APB, a membrane-permeable IP3 receptor inhibitor. In the continued presence (following preincubation for 20 min) of 5 μm chelerythrine or 100 μm 2-APB, 300 nm TRH still elicited robust inward currents of 17.3 ± 5.9 pA (n= 6) and 15.8 ± 4.9 pA (n= 5), respectively. We also investigated the TRH response using the conventional whole-cell patch-clamp recording configuration which allowed us to dialyse the LC neurons with BAPTA and hence prevent any increases in intracellular Ca2+ concentration that may contribute to the TRH response. To confirm effective intracellular Ca2+ chelation, we examined the response to caffeine which is known to evoke Ca2+-activated K+ currents in LC neurons (Murai et al. 1997). Caffeine (10 mm) produced such outward currents in all neurons tested when recorded using the perforated patch-clamp technique (n= 8; Fig. 6C). When neurons were dialysed with a pipette solution containing 5 mm BAPTA for 30 min, caffeine (10 mm) failed to produce any detectable outward current (n= 4), while TRH still induced an inward current, with a peak amplitude of 17.5 ± 3.2 pA (n= 4), similar to that observed with the perforated-patch method.
Figure 6. Effects of chelerythrine, 2-APB and BAPTA on the TRH-induced currents.
A, the TRH-induced current in the presence of PKC inhibitor chelerythrine. B, the TRH-induced current in the presence of the IP3 receptor-inhibitor 2-APB. C, effect of intracellular Ca2+ chelation by BAPTA on the TRH response. Caffeine responses recorded using perforated patch-clamp recordings (left panel) and conventional whole-cell patch-clamp recordings (right panel). Although the caffeine response was abolished by intracellular dialysis of BAPTA, the TRH response was unaffected. Hyperpolarizing step pulses of 500 ms duration from a VH of −50 mV to −60 mV were applied every 10–15 s. D, summary of the mean TRH-induced currents recorded under the various conditions as indicated. Each column shows the mean ±s.e.m. from 4–8 neurons.
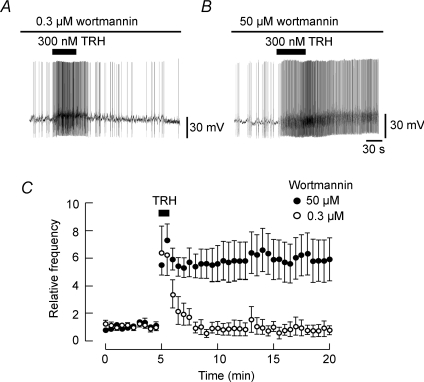
The PLC-mediated hydrolysis of PI(4,5)P2 may cause a local reduction of PI(4,5)P2 levels in the plasma membrane. Since phosphatidylinositol 4-kinase (PI 4-kinase) is required for re-synthesis of PI(4,5)P2, its inhibition is expected to disrupt the recovery of PI(4,5)P2 levels. Micromolar, but not nanomolar, concentrations of wortmannin have been reported to inhibit PI 4-kinase (Sorensen et al. 1998). Therefore, we examined whether the inhibition of re-synthesis and restoration of PI(4,5)P2 by wortmannin affects the TRH-induced increase of spike frequency and its recovery. Pretreatment of neurons with a high concentration of wortmannin (50 μm) for 10 min had no significant effect on the basal frequency of action potentials (96.5 ± 6.4% of control, P= 0.5, n= 6). Under these conditions, application of 300 nm TRH still increased the frequency of spontaneous action potentials, but the enhancement of action potential frequency was virtually irreversible. A similar pretreatment of neurons with 0.3 μm wortmannin did not result in such an irreversible TRH effect, indicating that degradation of PI(4,5)P2 was probably responsible for the depolarizing effect of TRH.
Discussion
The present study reveals the excitatory effect of TRH and the cellular mechanisms of this excitatory response in rat locus coeruleus noradrenergic neurons. We show a direct depolarizing action of TRH with an EC50 of 5.5 nm (Fig. 1D), resulting in an increase in the spontaneous firing rate. This EC50 value is comparable to those observed in GH3 cells (McDermott et al. 1990), GH4C1 cells (Cao et al. 1998) and orexin/hypocretin neurons (González et al. 2009). The TRH analogue taltirelin also increased the spike frequency with an EC50 of 44 nm. The results were consistent with a previous report which showed that taltirelin had a lower binding affinity than TRH (Asai et al. 1999). Inhibition of the TRH response by chlordiazepoxide (Fig. 3B) also supports the contribution of TRH receptors. To date, two TRH receptors, TRH-R1 and TRH-R2, have been identified. Cao et al. (1998) reported that TRH-gly was more potent at functionally activating the TRH-R2 receptors (EC50= 42 nm) than the TRH-R1 receptors (EC50= 1.8 μm). In the present study, TRH-gly induced an excitatory response only at relatively high concentrations (> 100 nm). However, we could not further explore the receptor subtype because of the absence of reliable and specific antagonists. The future development of selective agonists and antagonists for each subtype will enable their specific functions to be investigated.
It has been suggested that members of the twin-pore-domain K+ channel family, including the TASK group of K+ channels, contribute to the resting conductance in various neurons (Lesage, 2003). LC noradrenergic neurons are reported to express high levels of TASK-1 and TASK-3 K+ channels (Talley et al. 2001), which are inhibited by extracellular acidification and activated by alkalization (Talley et al. 2000). In the present study, the TRH-sensitive current showed outward rectification (Fig. 3C), consistent with outward rectification of macroscopic whole-cell TASK currents (Rajan et al. 2000; Talley et al. 2000). While the conductance of single TASK channels shows weak inward rectification, the increased open probability of these channels at positive potentials confers a similar macroscopic rectification pattern (Kim et al. 2000; Rajan et al. 2000).
In addition to the inhibitory effect of Ba2+ on GIRK channels, Ba2+ is known to modulate TASK-1 and TASK-3 channels, with some preferential blocking of inward current relative to outward currents; the Ba2+ IC50 values for block of inward TASK-3 currents are about 0.3 mm, and about 3 mm for block of outward TASK-3 currents (Kim et al. 2000; Vega-Saenz de Miera et al. 2001). In the present study, Ba2+ at a concentration of 3 mm elicited an inward current which was probably due to Ba2+ reducing the outward resting K+ currents carried by TASK channels. The proposal that Ba2+ and TRH inhibit the same TASK currents is supported by the TRH-induced current in the presence of Ba2+ being smaller than that observed under control conditions (Fig. 4D). Furthermore, application of pH 6.5 solution caused a slowly activated inward current associated with a decrease in the membrane conductance, and the TRH response was fully inhibited under these acidic conditions (Fig. 4C). Although reducing the extracellular pH may reduce the apparent affinity of TRH binding to its receptors, the pKa value of TRH is about 6.25 and a supramaximal concentration of 300 nm TRH in pH 6.5 solution should still cause receptor activation (Grant et al. 1972; Perlman et al. 1992). Overall, the present data strongly indicate that TRH inhibits acid-sensitive TASK K+ channels in LC neurons. Inhibition of resting K+ currents, probably mediated by acid-sensitive TASK-like K+ channels, by TRH has also been reported in hypoglossal motoneurons (Bayliss et al. 1994) and hippocampal interneurons (Deng et al. 2006).
TRH receptors are known to couple to the Gq/11-type of G-proteins. In LC noradrenergic neurons, Gq/11-coupled receptors include orexin and tachykinin receptors, whose activation causes membrane depolarization by both augmentation of a non-selective cationic conductance and by inhibition of a K+ conductance (Shen & North, 1992; Murai & Akaike, 2005). Recently, Parmentier et al. (2009) reported that TRH activates histaminergic tuberomamillary neurons via enhancing the activity of an electrogenic Na+/Ca2+ exchanger, in addition to activation of non-selective cationic channels. Since this TRH-induced depolarization was not accompanied by an obvious change in membrane resistance, these authors assumed that the origin of the TRH response was in distal dendrites. In addition, they could not observe the TRH-induced activation of the Na+/Ca2+ exchanger current at room temperature. The present study was conducted at room temperature and used acutely isolated neurons that only have proximal dendrites, and hence we found no evidence for these other actions of TRH. Instead, the TRH-induced depolarization could be completely described by the inhibition of K+ channels, although this does not exclude the possibilities that TRH may modulate cationic conductance or Na+/Ca2+ exchangers in LC noradrenergic neurons in vivo. These potential targets should be investigated in future studies.
The PLC inhibitor U-73122 slowly depolarized LC neurons under current-clamp conditions resulting in a gradual cessation of action potential firing (Fig. 5A). Although U-73122 is widely used in physiological studies to prevent PLC activity, this drug has been reported to have unfavourable side effects. In pancreatic acinar cells, U-73122 directly activates cation channels (Mogami et al. 1997). The present results also show that U-73122 may activate cation channels in LC neurons (Fig. 5). However, we did not further characterize the U-73122-induced inward currents. In the presence of U-73122, but not U-73343, TRH failed to induce any inward current under voltage-clamp conditions (Fig. 5). Further PLC inhibitors NCDC and edelfosine also inhibited the TRH response. These results suggest a contribution of PLC to the TRH signalling pathway. PLC cleaves PI(4,5)P2 into IP3 and diacylglycerol, with IP3 releasing internal Ca2+ stores and with diacylglycerol activating PKC. However, the PKC inhibitor chelerythrine at a concentration (5 μm) reported to inhibit PKC-mediated responses in LC neurons (Bailey et al. 2004) failed to inhibit the TRH response (Fig. 6A). Similarly, the membrane-permeable IP3 receptor inhibitor 2-APB had no effect on the TRH-induced current (Fig. 6B). Furthermore, intracellular Ca2+ chelation by inclusion of BAPTA in the whole-cell pipette solution did not prevent the TRH-induced current (Fig. 6C), indicating little, if any, involvement of intracellular Ca2+ concentration in the TRH-induced inward current. However, suppression of PI(4,5)P2 replenishment by high concentrations of wortmannin markedly inhibited the recovery from the facilitatory action of TRH on spontaneous spike frequency (Fig. 7). Together, these results are consistent with a model where TRH activates PLC to result in PI(4,5)P2 hydrolysis, which then couples to inhibition of TASK channels in a manner that does not involve IP3 or PKC.
Figure 7. Effect of wortmannin on the TRH response.
A and B, current-clamp recordings showing the TRH-induced increase in spike frequency in the presence of 0.3 μm (A) or 50 μm (B) wortmannin. C, time course of the effect of TRH (300 nm) on the relative frequency of spontaneous action potentials. The number of spikes occurring every 30 s was normalized to that obtained before application of wortmannin and then plotted over time, in the presence of 0.3 μm (○) or 50 μm (•) wortmannin. The number of action potentials in the presence of 50 μm wortmannin between 8 and 20 min in this panel was significantly greater than that in 0.3 μm wortmannin (n= 6; P < 0.05).
As discussed previously (Aston-Jones et al. 1991; Stamford, 1995; Nestler et al. 1999; Sara, 2009), the LC noradrenergic neurons play a major role in the control of arousal and regulation of pain processing. These neurons receive dense innervation from orexin-containing fibres and orexins are known to induce wakefulness by activating LC neurons (Hagan et al. 1999; Horvath et al. 1999). Recently, orexin neurons have been reported to be activated by TRH (González et al. 2009; Hara et al. 2009). In concert with its excitatory action on orexin neurons, therefore, TRH may also produce a potent arousal-promoting action by directly activating LC neurons. In addition to the robust arousal effect (Hara et al. 2009; Parmentier et al. 2009), the TRH analogue taltirelin is also reported to induce potent analgesia of mechanical nociception via the central noradrenergic systems (Tanabe et al. 2007). Since the descending noradrenergic pathways from the LC participates in this endogenous pain-inhibitory system, taltirelin was proposed to activate these descending pathways to release noradrenaline. This mechanism is supported in the present study, as taltirelin was shown to directly activate LC neurons. Thus, our present results demonstrate that LC noradrenergic neurons are one of the physiological targets of TRH and its analogues.
In summary, the present study clearly demonstrates a direct excitatory effect of TRH and its analogues on rat locus coeruleus neurons. The activation of LC neurons was caused by a decrease in the acid-sensitive resting K+ conductance via PLC-mediated hydrolysis of PI(4,5)P2. Our present study provides a physiological basis for the role for TRH as an activator of LC noradrenergic neurons.
Acknowledgments
This work was supported by KAKENHI (21600019 to H.I., 18077009 to J.N.). We thank Dr A. Moorhouse for critical reading of the manuscript. We also thank Miki Yoshitomo for technical assistance.
Glossary
Abbreviations
- GIRK
G-protein-mediated inwardly rectifying K+
- IP3
inositol 1,4,5-trisphosphate
- LC
locus coeruleus
- NCDC
2-nitro-4-carboxyphenyl N,N-diphenylcarbamate
- PI 4-kinase
phosphatidylinositol 4-kinase
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PKC
protein kinase C
- PLC
phospholipase C
- TRH
thyrotropin-releasing hormone
- VH
holding potential
Author contributions
H.I. and Y.N. contributed to study design, data collection and analysis, as well as manuscript preparation. K.E. carried out the experiments and contributed to data collection. J.N. was responsible for study conception and design as well as manuscript preparation. All authors have given approval of the final manuscript. This study was performed at the National Institute for Physiological Sciences in Japan.
References
- Alreja M, Aghajanian GK. Opiates suppress a resting sodium-dependent inward current and activate an outward potassium current in locus coeruleus neurons. J Neurosci. 1993;13:3525–3532. doi: 10.1523/JNEUROSCI.13-08-03525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima J, Kubo C, Ishibashi H, Akaike N. α2-Adrenoceptor-mediated potassium currents in acutely dissociated rat locus coeruleus neurones. J Physiol. 1998;508:57–66. doi: 10.1111/j.1469-7793.1998.057br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai H, Kinoshita K, Yamamura M, Matsuoka Y. Diversity of thyrotropin-releasing hormone receptors in the pituitary and discrete brain regions of rats. Jpn J Pharmacol. 1999;79:313–317. doi: 10.1254/jjp.79.313. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Kelly E, Henderson G. Protein kinase C activation enhances morphine-induced rapid desensitization ofμ-opioid receptors in mature rat locus coeruleus neurons. Mol Pharmacol. 2004;66:1592–1598. doi: 10.1124/mol.104.004747. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Kanter RK, Szymeczek-Seay CL, Berger AJ, Millhorn DE. Early postnatal development of thyrotropin-releasing hormone (TRH) expression, TRH receptor binding, and TRH responses in neurons of rat brainstem. J Neurosci. 1994;14:821–833. doi: 10.1523/JNEUROSCI.14-02-00821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschi G, Desiles M, Reny V, Rips R, Wrigglesworth S. Antinociceptive properties of thyrotropin releasing hormone in mice: comparison with morphine. Br J Pharmacol. 1983;79:85–92. doi: 10.1111/j.1476-5381.1983.tb10499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, O’Donnell D, Vu H, Payza K, Pou C, Godbout C, Jakob A, Pelletier M, Lembo P, Ahmad S, Walker P. Cloning and characterization of a cDNA encoding a novel subtype of rat thyrotropin-releasing hormone receptor. J Biol Chem. 1998;273:32281–32287. doi: 10.1074/jbc.273.48.32281. [DOI] [PubMed] [Google Scholar]
- Chen X, Johnston D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:3787–3792. doi: 10.1523/JNEUROSCI.5312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P-Y, Porter JE, Shin HS, Lei S. Thyrotropin-releasing hormone increases GABA release in rat hippocampus. J Physiol. 2006;577:497–511. doi: 10.1113/jphysiol.2006.118141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskay RL, Long RT, Palkovits M. Localization of immunoreactive thyrotropin releasing hormone in the lower brainstem of the rat. Brain Res. 1983;277:159–162. doi: 10.1016/0006-8993(83)90919-8. [DOI] [PubMed] [Google Scholar]
- González JA, Horjales-Araujo E, Fugger L, Broberger C, Burdakov D. Stimulation of orexin/hypocretin neurones by thyrotropin-releasing hormone. J Physiol. 2009;587:1179–1186. doi: 10.1113/jphysiol.2008.167940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G, Ling N, Rivier J, Vale W. Orientation restrictions of the peptide hormone, thyrotropin-releasing factor, due to intramolecular hydrogen bonding. Biochemistry. 1972;11:3070–3073. doi: 10.1021/bi00766a020. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Gerashchenko D, Wisor JP, Sakurai T, Xie X, Kilduff TS. Thyrotropin-releasing hormone increases behavioural arousal through modulation of hypocretin/orexin neurons. J Neurosci. 2009;29:3705–3714. doi: 10.1523/JNEUROSCI.0431-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Stoodley N, Elliott JM, Marsden CA, Bennett GW, Youdim MB. Behavioural and biochemical evidence for the release of noradrenaline in mouse brain by TRH and some of its biologically stable analogues. Neuropharmacology. 1987;26:313–322. doi: 10.1016/0028-3908(87)90183-3. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, Van Den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- Ishibashi H, Eto K, Kajiwara M, Noda M. Facilitation of spontaneous glutamate release by antidepressant drugs in rat locus coeruleus. Neurosci Lett. 2005;374:152–156. doi: 10.1016/j.neulet.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Jang IS, Nabekura J. High potassium-induced facilitation of glycine release from presynaptic terminals on mechanically dissociated rat spinal dorsal horn neurons in the absence of extracellular calcium. Neuroscience. 2007;146:190–201. doi: 10.1016/j.neuroscience.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Ogasawara T, Yamazaki A, Ukai Y, Miura A, Kimura K. Enhancement of noradrenaline release from rat frontal cortex by thyrotropin releasing hormone and its analog, (3R,6R)-6-methyl-5-oxo-3- thiomorpholinylcarbonyl-L-histidyl-L-prolinamide, as studied by intracerebral microdialysis. J Pharmacol Exp Ther. 1994;268:255–261. [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport. 2000;11:1755–1758. doi: 10.1097/00001756-200006050-00031. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Koga H, Ishibashi H, Shimada H, Jang IS, Nakamura TY, Nabekura J. Activation of presynaptic GABAA receptors increases spontaneous glutamate release onto noradrenergic neurons of the rat locus coeruleus. Brain Res. 2005;1046:24–31. doi: 10.1016/j.brainres.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- McDermott AM, Wilkin GP, Dickinson SL. Pharmacological and biochemical comparison of thyrotropin releasing hormone (TRH) and di-methyl proline-TRH on pituitary GH3 cells. Br J Pharmacol. 1990;101:615–620. doi: 10.1111/j.1476-5381.1990.tb14129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaker S, Winokur A, Rostene WH, Rainbow TC. Autoradiographic localization of thyrotropin-releasing hormone receptors in the rat central nervous system. J Neurosci. 1985;5:167–174. doi: 10.1523/JNEUROSCI.05-01-00167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H, Lloyd Mills C, Gallacher DV. Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5)P3-mediated Ca2+ release and directly activates ion channels in mouse pancreatic acinar cells. Biochem J. 1997;324:645–651. doi: 10.1042/bj3240645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai Y, Akaike T. Orexins cause depolarization via nonselective cationic and K+ channels in isolated locus coeruleus neurons. Neurosci Res. 2005;51:55–65. doi: 10.1016/j.neures.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Murai Y, Ishibashi H, Koyama S, Akaike N. Ca2+-activated K+ currents in rat locus coeruleus neurons induced by experimental ischemia, anoxia, and hypoglycemia. J Neurophysiol. 1997;78:2674–2681. doi: 10.1152/jn.1997.78.5.2674. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol Psychiatry. 1999;46:1131–1139. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- Nillni EA, Sevarino KA. The biology of pro-thyrotropin-releasing hormone-derived peptides. Endocr Rev. 1999;20:599–648. doi: 10.1210/edrv.20.5.0379. [DOI] [PubMed] [Google Scholar]
- Pammer C, Görcs T, Palkovits M. Peptidergic innervation of the locus coeruleus cells in the human brain. Brain Res. 1990;515:247–255. doi: 10.1016/0006-8993(90)90603-9. [DOI] [PubMed] [Google Scholar]
- Parmentier R, Kolbaev S, Klyuch BP, Vandael D, Lin JS, Selbach O, Haas HL, Sergeeva OA. Excitation of histaminergic tuberomamillary neurons by thyrotropin-releasing hormone. J Neurosci. 2009;29:4471–4483. doi: 10.1523/JNEUROSCI.2976-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman JH, Nussenzveig DR, Osman R, Gershengorn MC. Thyrotropin-releasing hormone binding to the mouse pituitary receptor does not involve ionic interactions. A model for neutral peptide binding to G protein-coupled receptors. J Biol Chem. 1992;267:24413–24417. [PubMed] [Google Scholar]
- Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Xin Liu G, Preisig-Müller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histidine as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Shen KZ, North RA. Substance P opens cation channels and closes potassium channels in rat locus coeruleus neurons. Neuroscience. 1992;50:345–353. doi: 10.1016/0306-4522(92)90428-5. [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Linseman DA, McEwen EL, Heacock AM, Fisher SK. A role for a wortmannin-sensitive phosphatidylinositol-4-kinase in the endocytosis of muscarinic cholinergic receptors. Mol Pharmacol. 1998;53:827–836. [PubMed] [Google Scholar]
- Stamford JA. Descending control of pain. Br J Anaesth. 1995;75:217–227. doi: 10.1093/bja/75.2.217. [DOI] [PubMed] [Google Scholar]
- Sun Y, Lu X, Gershengorn MC. Thyrotropin-releasing hormone receptors – similarities and differences. J Mol Endocrinol. 2003;30:87–97. doi: 10.1677/jme.0.0300087. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe M, Tokuda Y, Takasu K, Ono K, Honda M, Ono H. The synthetic TRH analogue taltirelin exerts modality-specific antinociceptive effects via distinct descending monoaminergic systems. Br J Pharmacol. 2007;150:403–414. doi: 10.1038/sj.bjp.0707125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci. 2002;22:4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Wessendorf M, Williams JT. Dendritic arbor of locus coeruleus neurons contributes to opioid inhibition. J Neurophysiol. 1996;75:2029–2035. doi: 10.1152/jn.1996.75.5.2029. [DOI] [PubMed] [Google Scholar]
- Vega-Saenz de Miera E, Lau DH, Zhadina M, Pountney D, Coetzee WA, Rudy B. KT3.2 and KT3.3, two novel human two-pore K+ channels closely related to TASK-1. J Neurophysiol. 2001;86:130–142. doi: 10.1152/jn.2001.86.1.130. [DOI] [PubMed] [Google Scholar]
- Williams JT, North RA, Tokimasa T. Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci. 1988;8:4299–4306. doi: 10.1523/JNEUROSCI.08-11-04299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winokur A, Utiger RD. Thyrotropin-releasing hormone: regional distribution in rat brain. Science. 1974;185:265–267. doi: 10.1126/science.185.4147.265. [DOI] [PubMed] [Google Scholar]