Abstract
The incidence of obesity and obesity-related conditions, such as metabolic syndrome and insulin resistance, is on the increase. The effect of obesity on skeletal muscle function, especially the regulation of muscle mass, is poorly understood. In this study we investigated the effect of diet-induced obesity on the ability of skeletal muscle to respond to an imposed growth stimulus, such as increased load. Male C57BL/6 mice were randomized into two diet groups: a low fat, high carbohydrate diet (LFD) and a high fat, low carbohydrate diet (HFD) fed ad libitum for 14 weeks. Mice from each diet group were divided into two treatment groups: sedentary control or bilateral functional overload (FO) of the plantaris muscle. Mice were evaluated at 3, 7, 14 or 30 days following FO. By 14 days of FO, there was a 10% reduction (P < 0.05) in absolute growth of the plantaris in response to overload in HFD mice vs. LFD mice. By 30 days the attenuation in growth increased to 16% in HFD mice compared to LFD mice. Following FO, there was a reduction in the formation of polysomes in the HFD mice relative to the LFD mice, suggesting a decrease in protein translation. Further, activation of Akt and S6K1, in response to increased mechanical loading, was significantly attenuated in the HFD mice relative to the LFD mice. In conclusion, chronic high fat feeding impairs the ability of skeletal muscle to hypertrophy in response to increased mechanical load. This failure coincided with a failure to activate key members of the Akt/mTOR signalling pathway and increase protein translation.
Introduction
Obesity is increasingly prevalent today in both young and old individuals, and has multisystemic patho-physiological consequences. Prolonged obesity can lead to a multitude of conditions and diseases, including diabetes and metabolic syndrome which includes a collection of metabolic risk factors such as high blood pressure, dyslipidaemia, and insulin resistance (Reaven, 1988; Pischon et al. 2008; Silveira et al. 2008). While insulin resistance tends to increase with age, when combined with obesity it can lead to type 2 diabetes, in which individuals have both insulin resistance and hyperglycaemia (Kahn et al. 2006).
The association between obesity and insulin resistance in skeletal muscle is well established (Kraegen & Cooney, 2008; Silveira et al. 2008); however, the mechanism responsible for the reduced insulin sensitivity is still unclear. Many recent studies suggest that the accumulation of triglycerides in muscle with high fat feeding leads to the development of insulin resistance, in part, by interfering with protein phosphorylation along the insulin/IRS-1/PI3-K/Akt signalling pathway (Tremblay & Marette, 2001; Aguirre et al. 2002; Silveira et al. 2008). Disruption of Akt signalling can lead to a reduced capacity for glucose transport and glucose metabolism in peripheral tissues such as skeletal muscle (Tremblay & Marette, 2001; Beeson et al. 2003; Belfort et al. 2005; Pedrini et al. 2005; Casaubon et al. 2006). As a result of decreased insulin activity, the ability of skeletal muscle to maintain normal glucose homeostasis is compromised. However, in addition to activating glucose metabolism, insulin plays an important role in the initiation of protein synthesis in both pre- and postnatal muscle through activation of downstream targets of mTOR (Kimball et al. 1998; Balage et al. 2001; Prod’homme et al. 2005).
Skeletal muscle is a dynamic tissue that plays a critical role in glucose homeostasis; however, its primary role is the development of force. The maximum amount of force produced by a muscle is directly related to its physiological cross-sectional area (Powell et al. 1984), which is a tightly controlled property of skeletal muscle that is regulated by the balance of two processes, protein synthesis and degradation (Rennie et al. 2004; Favier et al. 2008). During periods of growth, the balance favours synthesis over degradation; while loss of muscle mass is associated with a shift in the balance towards protein degradation. Recent evidence shows that activation of mTOR and its downstream targets, S6K1 and 4E-BP1 is critical for skeletal muscle growth, especially under increased loading conditions in adult mammals, through its control of the rate of protein translation (Bolster et al. 2004; Bodine, 2006; Miyazaki & Esser, 2008).
There is increasing evidence showing dysregulation of the Akt/mTOR pathway in models of diet-induced obesity (Eldar-Finkelman et al. 1999), fatty acid infusion (Belfort et al. 2005; Pedrini et al. 2005) and diabetes (Krook et al. 1998; Kim et al. 1999) although it has been primarily studied in the context of glucose homeostasis. Given that skeletal muscle mass is a critical regulator of glucose uptake, it is important to know whether diet-induced obesity has an effect on the ability of skeletal muscle to adapt and respond appropriately to external growth cues. Recent evidence shows a significant deficit in the ability to increase protein synthesis (Anderson et al. 2008) and ATP synthesis (Abdul-Ghani et al. 2008; Yerby et al. 2008) in response to an insulin challenge in diet-induced obesity. These data suggest that other properties of skeletal muscle, such as the ability of skeletal muscle to increase muscle mass in response to growth stimuli, could be impaired following diet-induced obesity. Consequently, the present study was designed to test the hypotheses that following diet-induced obesity: (1) muscle growth in response to an increase in mechanical loading is attenuated, and (2) the attenuation of muscle growth in obese mice is related to a decrease in the activation of the Akt/mTOR pathway resulting in a reduction in protein translation.
Methods
Animals
Male C57BL/6 mice (n= 120), 5 weeks old, were obtained from the Jackson Laboratories and housed in temperature-controlled rooms (19–21°C) with a 12 h:12 h light–dark cycle at the University of California, Davis. The Institutional Animal Use and Care Committee at the University of California, Davis approved all animal protocols. Upon arrival, all animals were given a 2 week acclimation period in which they were fed a low fat diet (D12450B, 10% fat, Research Diets, New Brunswick, NJ, USA). After the 2 week acclimation period, animals were randomly assigned to either a high fat diet (D12451, 45% fat, Research Diets) or a low fat diet for a period of 14 weeks. A small cohort of mice remained on the low fat or high fat diet for a period of 30 weeks. Mice on both diets had unlimited access to both food and water.
Functional overload and tissue removal
To induce mechanical overload of the plantaris muscle, animals were subjected to surgical ablation of the soleus and gastrocnemius muscles as previously described (Roy et al. 1982; Bodine et al. 2001). Briefly, mice were anaesthetized with 2–4% isoflurane, and using aseptic surgical procedures, an incision was made to the lower hind limb exposing the ankle extensor muscle complex. The soleus and one-third of the lower medial and lateral gastrocnemius were carefully removed with particular attention to preserving the neural and vascular supply to the plantaris muscle. The incision site was irrigated with sterile saline and closed using subcuticular sutures. This procedure was performed on both hind limbs. At 3, 7, 14 and 30 days post surgery, animals were anaesthetized with 2–4% isoflurane and the plantaris muscles were excised, weighed and flash frozen in liquid nitrogen. Epididymal and retroperitoneal fat pads were removed, weighed and flash frozen in liquid nitrogen. Blood samples were collected, and then animals were humanely killed. Tissue samples were collected, in all groups, in the morning following an overnight fast.
Skeletal muscle polysomal aggregation
Sucrose density gradient centrifugation was used to separate the subpolysomal from the polysomal ribosome fractions in the plantaris of control and overloaded animals using an adaptation of a previous published protocol (Kubica et al. 2005). Plantaris muscle was homogenized on ice in buffer containing 50 mm Hepes, 75 mm KCl, 5 mm MgCl2, 250 mm sucrose, 100 μg ml−1 cyclohexamide and 2 mm DTT. Samples were incubated on ice for 5 min before addition of 75 μl of Triton-DOC (1.34 ml of Triton X, 0.66 g of deoxycholate, 18 ml of sterile water). Following a 15 min incubation on ice, samples were centrifuged at 3000 g for 10 min at 4°C. The resulting supernatant (600 μl) was layered on a 20–47% linear sucrose gradient (20 mm Tris (pH 7.5), 250 mm KCl and 10 mm MgCl2) and centrifuged in a Beckman SW41 rotor at 143 000 g for 4 h at 4°C. Following centrifugation, the gradient was displaced upward (3 ml min−1) using Fluorinert (Isco, Lincoln, NE, USA) through a spectrophotometer, and optical density at 254 nm was continuously recorded (chart speed, 150 cm h−1). The individual traces were quantified using Image J software (http://rsb.info.nih.gov/ij).
SDS-PAGE, Western blotting and immunodetection
The plantaris muscle was homogenized on ice in buffer containing 50 mm Hepes (pH 7.4), 4 mm EGTA, 20 mm EDTA, 15 mm sodium pyrophosphate, 100 mmβ-glycerophosphate, 0.1% Triton X-100, 25 mm NaF, 5 mm NaVO4, 10 mg ml−1 leupeptin, 1.75 mg ml−1 aprotinin and 1 mm PMSF as previously described (Sitnick et al. 2006). Following processing, samples were stored at −80°C. Protein concentration was determined in triplicate using the Bradford Method (Bio-Rad Protein Assay, Hercules, CA, USA).
Homogenates were solubilized in sample buffer (250 mm Tris-HCl pH 6.8, 30% glycerol, 8% SDS, 10%β-mercaptoethanol, and 0.02% Bromophenol Blue) and allowed to boil at 100°C for 5 min. For each sample, 75 μg of protein was loaded onto 10% SDS-PAGE gels with the following exceptions: 100 μg of protein was used for total S6K1 kinase and Thr389 phos-S6K1. All gels were run at 150 V for approximately 1 h to allow for ample separation of protein. The proteins then were transferred onto a PVDF membrane (Millipore, Bedford, MA, USA) at 50 V for 1 h. To confirm complete transfer and equal loading of the samples, the membranes were stained in Ponceau S solution (data not shown). Following a successful transfer, membranes were blocked in 5% non-fat dry milk (NFDM) in Tris-buffered saline with 0.1% Tween-20 added (TBS-T) for 1 h with gentle rocking. Blots were then serially washed (3 × 5 min) in TBS-T and incubated overnight with primary antibody, at 4°C. The following day, blots were serially washed in TBS-T and incubated with the appropriate secondary antibody in 5% NFDM for 1 h rocking at room temperature, after which they were serially washed again in TBS-T and incubated with enhanced chemiluminescence reagent (GE Healthcare, Amersham, UK) to detect HRP activity on Kodak XAR-5 auto-radiographic film. Films were exposed long enough to keep integrated optical densities in a linear, non-saturated range for each band on every membrane. Bands were quantified using a BioRad VersaDoc Imaging System and Quantity One software (BioRad Instruments, Hercules, CA, USA).
Antibodies
The primary antibodies for Ser473 phos-Akt, (1:1000 dilution), Akt (1:1000), Thr389 phos-S6K1 (1:500), S6K1 (1:500), phos-GSK-3β (1:1000) were purchased from Cell Signalling Technology (Beverly, MA, USA). Anti-rabbit IgG HRP-conjugated secondary antibodies were also purchased from Cell Signalling Technology.
Insulin assays
Plasma insulin was measured using an Ultra Sensitive Mouse Insulin ELISA Kit (CrystalChem, Downers Grove, IL, USA) according to the provided protocol.
Statistical analysis
All data are expressed as means ±s.d. Statistical significance was determined using an analysis of variance for multiple comparisons (ANOVA) followed by a Tukey's post hoc test. A P value of < 0.05 was considered significant.
Results
Effects of HFD on body composition
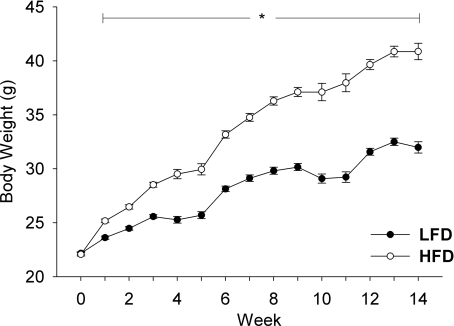
At 7 weeks of age, male mice were randomly assigned to either a low fat (LFD) or a high fat (HFD) ad libitum diet for a period of 14 weeks. After 14 weeks, mice in each diet group were randomly assigned into either a FO or sedentary cage control group and maintained on the selected diet until the termination of the experiment. The growth curves of the mice over the 14 week period on the two diets are shown in Fig. 1. The initial body weights of the mice on the two diets were similar; however, after only 1 week, mice on the high fat diet were significantly heavier than mice on the LFD (Fig. 1). The difference in body weight between the two groups continued to increase over the course of the treatment, increasing to a difference of 20% at 9 weeks. At the end of the experiment, the control HFD mice were 31% heavier than the LFD mice (Fig. 1 and Table 1). Furthermore, following 14 weeks on the high fat diet, mice were hyperinsulinaemic, having a 7.6-fold increase in fasting insulin levels relative to LFD mice (Table 1).
Figure 1. Growth curves of male C57BL/6 mice fed either a low fat (LFD) or a high fat diet (HFD) for a period of up to 18 weeks.
The average (mean ±s.e.m.) body weight of mice fed either a LFD (•) or HFD (ô) was measured weekly over the 14 weeks of feeding (n= 60/diet). *Statistical difference between LFD and HFD mice at a specific age (P < 0.05).
Table 1.
Body composition of mice on low and high fat diets
| LFD | HFD | |
|---|---|---|
| Body weight (g) | 29.2 ± 3.2 | 38.0 ± 3.8* |
| EPI (mg) | 832 ± 460 | 1810 ± 470* |
| RP (mg) | 193 ± 109 | 397 ± 86* |
| Soleus (mg) | 11.1 ± 1.3 | 11.9 ± 1.9 |
| Gast (mg) | 143 ± 11 | 150 ± 13 |
| Heart (mg) | 141 ± 20 | 148 ± 18 |
| Insulin (ng ml−1) | 0.096 ± 0.01 | 0.73 ± 0.13* |
Male C57BL/6 mice were fed either a low fat (LFD) or a high fat (HFD) for a period of 14–18 weeks. The average (mean ±s.e.m.) body weight, muscle mass and fat pad mass of control mice used in the functional overload experiments (n= 5–10/time point) were calculated upon completion of the experiments. All control mice (n= 30) were pooled together to calculate the means since there was no difference in the means across the groups. Abbreviations are: Gast, gastrocnemius muscle; EPI, epididymal fat pad; RP, retroperitoneal fat pad.
Statistical difference from LFD mice (P < 0.05).
The increase in body weight associated with high fat feeding over the 14 week period was attributed to an expansion of adipose tissue rather than lean muscle mass. High fat feeding resulted in an increase in the mass of both epididymal and retroperitoneal fat pads. After 14 weeks on the high fat diet, the masses of the epididymal and retroperitoneal fat pads were 120% and 105% larger, respectively, in HFD compared to the LFD mice (Table 1). The wet weights of the soleus, gastrocnemius and heart were similar in HFD and LFD mice (Table 1).
Effects of functional overload on plantaris mass
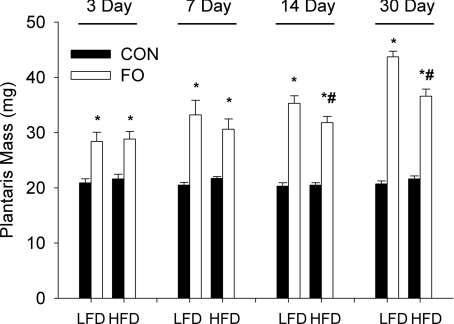
To assess the effect of chronic high fat feeding on the ability of skeletal muscle to hypertrophy in response to increased mechanical loading, we subjected the plantaris muscle from LFD and HFD animals to functional overload for 3, 7, 14 or 30 days. The plantaris mass of LFD and HFD control mice was similar at each of the time points studied (Fig. 2). In both LFD and HFD mice, functional overload led to a significant increase in plantaris mass at all time points studied (Fig. 2); however, significant differences were noted in the absolute growth response between mice in the two diet groups at 14 and 30 days. At 7 days the growth response of the LFD mice (mean LFD – mean HFD/mean LFD × 100) was 8% greater (P > 0.05) relative to the HFD mice and increased to a significant difference of 10 and 16% (P < 0.05) at 14 and 30 days, respectively. At 30 days of FO, the relative difference between control and overloaded muscles (mean FO/mean control) was 2.1- and 1.7-fold in the LFD and HFD mice, respectively. These data reveal that diet-induced obesity resulted in a significant impairment in the response of skeletal muscle to increased mechanical loading.
Figure 2. Effect of diet on load-induced growth of the plantaris muscle following 3, 7, 14 and 30 days of functional overload (FO).
Histograms representing the average (mean ±s.e.m.) wet weight, in milligrams, of the plantaris of mice fed either a low fat diet (LFD) or a high fat diet (HFD) and subjected to normal cage activity (control (CON), filled bars) or FO (open bars) for a period of 3, 7, 14 or 30 days. *Statistical difference between CON and FO groups at a specific time (P < 0.05). #Statistical difference between LFD and HFD FO groups at a specific time (P < 0.05). Sample size: n= 10 mice/group at 3 and 7 days; n= 14, n= 20 mice/group at 14 and 30 days.
Effect of the duration of high fat feeding on muscle growth in response to loading
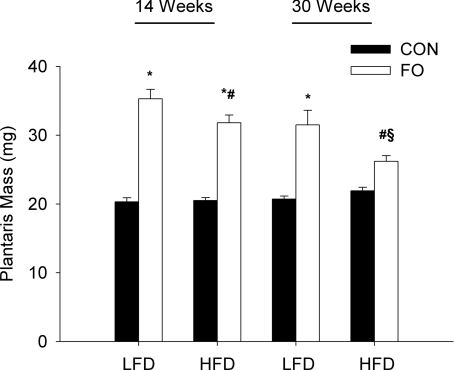
Next, we examined the effect of a longer duration of high fat feeding (14 vs. 30 weeks) on the growth response of the plantaris to functional overload. In these experiments, only one time point (14 days) was examined. In response to the FO, mice on the extended high fat diet showed no significant increase in plantaris mass after 14 days of increased loading (Fig. 3). This was in marked contrast to the mice maintained on the high fat diet for 14 weeks that showed a relative increase of 55% at 14 days of FO compared to a relative increase of 75% in the LFD mice (Fig. 3). No significant differences were observed in the growth response of the LFD groups at 14 and 30 weeks.
Figure 3. Effect of the diet duration on the growth response of the plantaris to 14 days of functional overload (FO).
Histograms representing the average (mean ±s.e.m.) wet weight, in milligrams, of the plantaris of mice fed either a low fat diet (LFD) or a high fat diet (HFD) for a total of 16 or 32 weeks, and then subjected to normal cage activity (control (CON), filled bars) or FO (open bars) for a period of 14 days. *Statistical difference between CON and FO groups for a specific diet group (P < 0.05). #Statistical difference between LFD FO and HFD FO groups for a specific diet duration (P < 0.05). §Statistical difference between 16 and 32 weeks HFD FO groups (P < 0.05).
Assessment of protein translation following functional overload in HFD and LFD mice
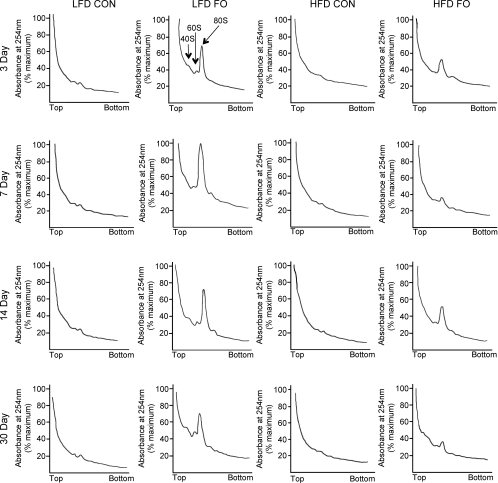
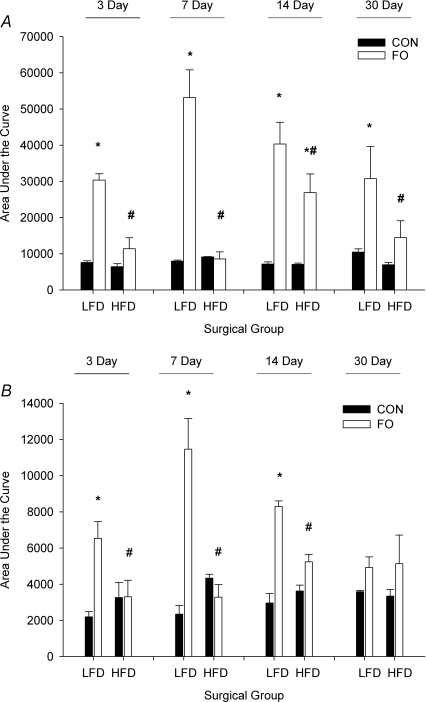
In order to examine the role of protein translation in the load-induced increase in muscle mass, and to delineate the effect of diet on attenuating this increase, muscle homogenates were analysed by sucrose density gradient centrifugation. The sucrose density gradient traces revealed an increase in subpolysomal and polysomal aggregation in the plantaris of LFD mice starting at 3 days of loading compared with basal levels in control muscles (Fig. 4). Individual traces were digitized and the area under the curve was determined for the entire profile (total, Fig. 5A) and the area after the 80S peak (polysomes, Fig. 5B). Comparison of LFD and HFD mice following FO showed significant attenuation in the HFD mice of both the total and polysomal fraction at 3, 7 and 14 days (Fig. 5).
Figure 4. Qualitative analysis of polysome aggregation in the plantaris.
Polysome profiles were produced using sucrose density gradient ultracentrifugation. A representative polysome profile is presented for sedentary control mice (CON) and mice subjected to 3, 7, 14 and 30 days of functional overload (FO) in both the low fat diet (LFD) and high fat diet (HFD) groups.
Figure 5. Quantitative analysis of polysome aggregation in the plantaris.
Histograms representing the average (mean ±s.e.m.) area under the curve for the entire polysome trace (A), and for the region after the 80S peak (B) of mice (n= 3–5/group) fed either a low fat diet (LFD) or a high fat diet (HFD), and then subjected to normal cage activity (control (CON), filled bars) or FO (open bars) for a period of 3, 7, 14 or 30 days. *Statistical difference between CON and FO groups for a specific diet group (P < 0.05). #Statistical difference between LFD FO and HFD FO groups for a specific diet duration (P < 0.05).
Effect of high fat diet on Akt-signalling following functional overload
Phosphorylation of specific kinases in the Akt/mTOR signalling pathway was measured to identify the underlying causes of the diet-induced attenuation in muscle growth and protein translation in response to increased loading.
Akt phosphorylation
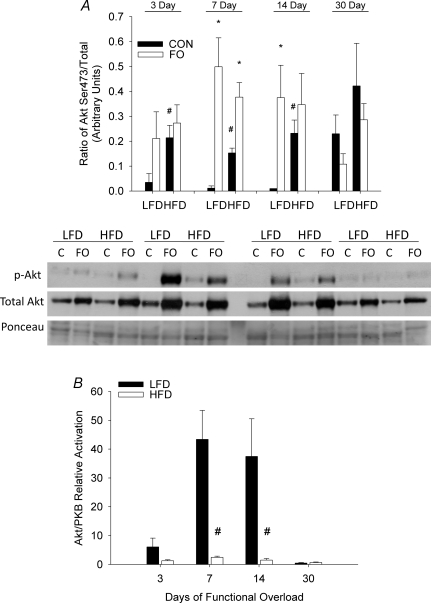
In sedentary, control mice phosphorylation of Akt on Ser473 was significantly higher in HFD mice relative to LFD mice. A significant increase in Akt phosphorylation was observed at 7 and 14 days of FO in LFD mice. In contrast, mice fed a HFD showed a significant increase in Akt phosphorylation only at 7 days following FO (Fig. 6A). As levels of phosphorylated Akt were higher in HFD animals at rest, the relative activation of Akt in response to FO was calculated by taking the ratio of FO phosphorylated Akt to the mean levels of Akt phosphorylation at rest. When calculated as a fold change from control, the difference in the response of the LFD and HFD FO mice was clearly apparent. While activation of Akt was evident in the plantaris of both LFD and HFD mice following FO, there was a significant attenuation in the response seen in HFD (< 2-fold) relative to LFD (> 35-fold) mice (Fig. 6B).
Figure 6. Effect of diet on Akt/PKB activation following functional overload (FO).
The phosphorylation status of Akt on Ser473 was measured in the plantaris muscle of mice fed either a low fat diet (LFD) or high fat diet (HFD), and then subjected to functional overload (FO) for 3, 7, 14 or 30 days or sedentary control (CON). Data expressed as mean ±s.e.m., n= 3–5/group. A, Akt phosphorylation on residue Ser473 expressed as level of phosphorylation per total protein for FO (open bars) and CON (filled bars) mice in each diet group and at each time point. B, relative activation of Akt on Ser473 in response to FO, expressed as fold change in Akt phosphorylation relative to control, for LFD (filled bars) and HFD (open bars) groups. *Statistical difference between CON and FO at a specific time point (P < 0.05). #Statistical difference between LFD and HFD groups (P < 0.05). Western blots of phosphorylated and native proteins from the plantaris of a single mouse in each group and time point (C, control).
S6K1 phosphorylation
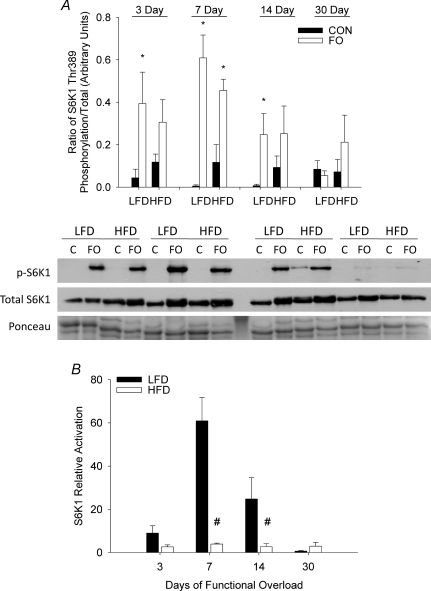
The phosphorylation status of S6K1 on Thr389 followed similar trends to that seen with Akt phosphorylation on Ser473 (Fig. 7A). A significant increase in Thr389 phosphorylation was noted at 3, 7 and 14 days of functional overload in LFD mice, whereas in HFD mice a significant increase in phosphorylated S6K1 was observed only at 7 days. Calculation of fold change in phosphorylation from control showed that both LFD and HFD mice were able to significantly activate S6K in response to loading; however, there was a significant attenuation in the response seen in HFD (< 2-fold) relative to LFD (> 25-fold) mice (Fig. 7B).
Figure 7. Effect of diet on S6K1 activation following functional overload (FO).
The phosphorylation status of S6K1 on Thr389 was measured in the plantaris muscle of mice fed either a low fat diet (LFD) or high fat diet (HFD), and then subjected to functional overload (FO) for 3, 7, 14 or 30 days or sedentary control (CON). Data expressed as mean ±s.e.m., n= 3–5/group. A, S6K1 phosphorylation on residue Thr389 expressed as level of phosphorylation per total protein for FO (open bars) and CON (filled bars) mice in each diet group and at each time point. B, relative activation of S6K1 on Thr389 in response to FO, expressed as fold change in S6K1 phosphorylation relative to control, for LFD (filled bars) and HFD (open bars) groups. *Statistical difference between CON and FO at a specific time point (P < 0.05). #Statistical difference in FO between LFD and HFD groups (P < 0.05). Western blots of phosphorylated and native proteins from the plantaris of a single mouse in each group and time point (C, control).
GSK-3β phosphorylation
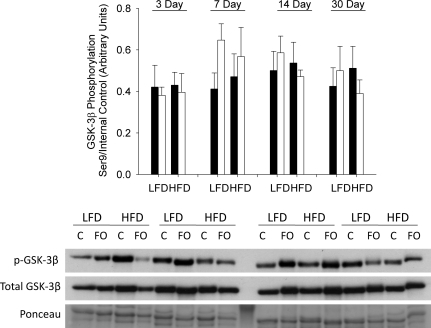
GSK-3β is phosphorylated and inactivated by Akt on the Ser9 residue. With respect to GSK-3β phosphorylation, there were no significant differences between the response of LFD and HFD mice to FO. There was a general trend for greater phosphorylation and inactivation of GSK-3β in response to increased loading in the LFD compared to the HFD mice (Fig. 8).
Figure 8. Effect of diet on GSK-3β inactivation following functional overload (FO).
The phosphorylation status of GSK-3β on Ser9 was measured in the plantaris muscle of mice fed either a low fat diet (LFD) or high fat diet (HFD), and then subjected to functional overload (FO) for 3, 7, 14 or 30 days or sedentary control. Data expressed as mean ±s.e.m., n= 3–5/group. GSK-3β phosphorylation on residue Ser9 expressed as level of phosphorylation per total protein for FO (open bars) and control (filled bars) mice in each diet group and at each time point. Western blots of phosphorylated and native proteins from the plantaris of a single mouse in each group and time point (C, control).
Discussion
Here we demonstrate for the first time that chronic feeding of a high fat diet in young mice results in the attenuation of skeletal muscle growth in response to an imposed increase in load. Specifically, we show that the plantaris muscle, a predominantly fast glycolytic muscle, of mice fed a high fat diet for durations of 14 weeks had a significantly impaired growth response to functional overload, or synergist ablation, especially after 14 and 30 days of chronic increased loading. Moreover, mice maintained on a high fat diet for a longer duration, i.e. 30 vs. 14 weeks, and then subjected to functional overload showed a complete failure to grow in response to the increased load. The attenuated growth response in the HFD mice was associated with a reduction in the activation of Akt and its downstream target, S6K1, following FO. Furthermore, sucrose density gradient centrifugation analysis revealed an increase in polysome aggregation at 3, 7, 14 and 30 days following overload in LFD mice that was prevented or markedly attenuated in mice fed a high fat diet. These data suggest that feeding mice a chronic high fat diet interferes with the cellular signalling involved in the muscle response to increased loading leading to a decrease in the activation of protein synthetic pathways. The mechanisms responsible for the altered cellular response to loading are unknown.
Our data demonstrate a strong inhibition of muscle growth in response to increased loading in a rodent model of diet-induced obesity. Attenuation of Akt/mTOR signalling and a reduction in polysome aggregation suggest that in the HFD mice there was a failure to adequately activate protein translation initiation, leading to a decrease in protein synthesis and a reduction in muscle growth in response to an increased load. The time course data reveal that both LFD and HFD mice were able to initiate a growth response; however, the size of the response was significantly blunted in the HFD mice as reflected by the growth curves (Fig. 2). Comparison of the polysome profiles for the FO groups reveals marked differences in the polysomal fraction between the LFD and HFD mice, which correspond nicely to the divergence seen in the growth curves at 7 days and beyond.
Polysome profiles give a representative snapshot of the distribution of ribosomes during the process of translation initiation, elongation and termination. The status of ribosomal assembly can be assessed from the separation of muscle extracts on a sucrose gradient where polysomes (mRNA, multiple ribosomes and nascent polypeptides) migrate to the denser fractions and monosomes (40S, 60S and 80S RNA) remain in the less dense fractions. A shift in the distribution of ribosomes from monosomes to polysomes can imply changes in the rate of protein translation (Baar & Esser, 1999; Kubica et al. 2005). Functional overload in the LFD mice produced a dramatic increase in the 80S peak and an increase in the relative amount of RNA associated with the polysome pool. Given that increases in protein synthesis have been measured in functional overload (Goldspink et al. 1983), the present data are interpreted to mean that there was an increase in the rate of translation initiation and possibly elongation following FO in the LFD mice. In contrast, following FO in the HFD mice there was a much smaller increase in the 80S peak and little increase in RNA associated with polysomes suggesting a decrease in protein translation initiation and/or elongation. Based on these data, we hypothesize that protein synthesis, especially protein initiation, was not increased to the same level in the HFD mice as the LFD mice following functional overload. It is clear, however, that protein translation was not completely inhibited in the HFD mice since the amount of some proteins, such as Akt and S6K1, increased in these mice following FO. This finding suggests that the translation of specific mRNA may be prevented in the HFD mice in response to loading.
The alteration in polysome profiles in the HFD mice was associated with decreases in the activation of Akt and S6K1, as measured by phosphorylation levels. Signalling downstream of Akt can potentially regulate protein synthesis through changes in the rate of translation initiation and the abundance of ribosomes (Bolster et al. 2004; Baar et al. 2006). Translation initiation is regulated primarily at two steps: (1) the binding of the initiator tRNA to the 40S ribosomal subunit to make the 43S pre-initiation complex which is regulated by eukaryotic initiation factor (eIF)2, and (2) the cap-dependent binding of mRNA to the 43S pre-initiation complex which is regulated by eIF4E and its repressor the eIF4E binding protein (4E-BP), which are controlled by the Akt/GSK-3/mTOR signalling pathways. Ribosomal biogenesis is critical for increasing translation efficiency and is regulated, in part, through mTOR and the phosphorylation/activation of S6K1 leading to hyperphosphorylation of the ribosomal protein S6 that is associated with enhanced translation of mRNAs that encode ribosomal proteins and elongation factors (Fingar et al. 2004).
In response to increases in external loading, as occur with functional overload, reloading following disuse, and resistance exercise, skeletal muscle adapts by increasing the cross-sectional area of individual fibres (Ishihara et al. 1998; Bodine et al. 2001; Kosek et al. 2006). Muscle growth following periods of increased loading is associated with activation of the Akt/mTOR signalling pathway with increases being observed in the phosphorylation of Akt, mTOR, 4E-BP1 and S6K1 (Baar & Esser, 1999; Bolster et al. 2003; Childs et al. 2003; Drummond et al. 2009), as well as an increase in the activity of eIF2 (Kubica et al. 2005, 2008). Further, skeletal muscle growth can be induced by the transgenic over-expression of both Akt1 and Akt2, leading to activation of mTOR and S6K1, and increases in muscle fibre size (Bodine et al. 2001; Cleasby et al. 2007; Izumiya et al. 2008). Growth induced by Akt over-expression is probably due to the activation of mTOR since it is inhibited by rapamycin (Bodine et al. 2001).
In the present study, the response of the plantaris in the LFD mice was consistent with previous reports (Bodine et al. 2001) showing an increase in the phosphorylation of Akt, GSK-3β and S6K1 (Figs 6–8), and a significant increase in muscle growth over a 30 day period of increased loading. In contrast, the HFD mice had attenuated growth associated with a decrease in the activation of Akt and its downstream targets. While 14 weeks on a high fat diet only partially blocked the growth response, 30 weeks on a high fat diet resulted in a nearly complete block of growth. The response of the HFD mice, especially the mice on the extended high fat diet, is reminiscent of the attenuated growth that occurs with inhibition of mTOR (Bodine et al. 2001) and its downstream target S6K1 (Ohanna et al. 2005). The extent to which the growth of individual fibre types (especially oxidative vs. non-oxidative) was affected by the high fat diet was not examined in this study; however, it is possible that the growth of fast, glycolytic fibres was more impacted than the growth of slow and fast oxidative fibres, especially in those mice fed a high fat diet for a period of 14 weeks, given the differential responses observed in glycolytic and oxidative muscles to high fat diets of relatively short (6–8 weeks) duration (Levin et al. 2007). The reason for the much greater growth attenuation in the 30 versus 14 weeks duration diets is unknown and requires further study.
Attenuation of growth and a decrease in Akt/mTOR signalling in response to increased load has been noted in other models (Sitnick et al. 2006; Thomson & Gordon, 2006; Drummond et al. 2008). The reason for the attenuated Akt/mTOR signalling in response to loading in the diet-induced obese mice is unknown. One potential contributor to the attenuated response to loading is a decrease in the activity level of the HFD mice following the FO surgery. In this study, we did not directly measure activity levels following FO. A previous study (Bjursell et al. 2008) shows that following the switch from a low fat to a high fat diet there is an acute (3–5 h) decrease in voluntary activity; however, after the first 24 h on the high fat diet locomotor activity increases and by 21 days there is no significant difference in the voluntary activity of mice on a high fat or low fat diet. Daily observations of our mice did not reveal any obvious difference in the behaviour of the LFD and HFD mice following the functional overload. Further, there was no significant change in food intake in the HFD and LFD mice following the surgery. While we cannot rule out differences in the activity of the LFD and HFD mice following FO, it does not appear to be a major contributor to the attenuated growth response. Interestingly, the initial growth response to the increased loading (3 days) was similar in the LFD and HFD mice. Direct measurement of activity levels will be necessary in future studies to address this question.
Another potential contributor to the attenuated response to loading could be an elevation in intramyocellular triglycerides (IMTG) in the muscle following the high fat feeding. In response to HFD in rodents, others have noted an increase in IMTG that is strongly correlated with the development of insulin resistance and a decrease in oxidative metabolism (Corcoran et al. 2007; Moro et al. 2008; Silveira et al. 2008). The lipids themselves are not thought to be the problem, but rather the increase in the lipid intermediates, fatty acyl coenzyme A and ceramide, associated with fat metabolism, and possibly an increase in oxidative stress within the tissue (Silveira et al. 2008). Interestingly, the effects of a high fat diet on lipid accumulation and insulin signalling appear to be greater in predominantly glycolytic than in oxidative muscles (Levin et al. 2007). In the present study, diet-induced obesity appears to have had a significant effect on the plantaris, a muscle composed of predominantly type IIb, fast glycolytic fibres. Whether the negative effect of a high fat diet on load-induced growth is similar in the soleus, an oxidative muscle, as in the plantaris remains to be investigated.
The majority of diet-induced obesity studies have focused on the development of insulin resistance, with few, if any, examining the effects of obesity on other properties of muscle such as force production or growth capacity. Interestingly, obesity has been associated with decrements in protein synthesis in response to insulin. Anderson et al. (2008) noted a significant attenuation in the ability of muscles from HFD mice to stimulate protein synthesis following a meal as compared to LFD mice. In addition, Yerby et al. (2008) examined ATP synthesis in response to insulin in rats fed a high fat diet for 8 weeks and found that under basal (i.e. low insulin) conditions, the muscle ATP synthesis rates of HFD rats were approximately 30% lower than chow-fed rats. Further, when given an insulin challenge, chow-fed rats showed a significant increase in muscle ATP synthesis while HFD rats showed no increase. Interestingly, Chanseaume et al. (2007) measured an increase in the basal fractional synthesis rates of mixed and mitochondrial proteins in the soleus and tibialis anterior of rats fed a high fat diet for 6 weeks. In the present study, we made no measurements of basal protein synthesis rates; however, comparison of the polysome profiles of control LFD and HFD mice did not show a significant increase in translational activity in the HFD mice under control conditions.
The mechanisms responsible for the reduction in the ability to activate protein synthetic pathways in response to insulin or other growth stimuli, such as loading, following diet-induced obesity are unknown. Multiple factors could be involved, such as a decrease in energy balance (ATP/ADP ratio) or an increase in oxidative stress that could negatively affect the ability to activate mTOR and its downstream targets. Recent findings suggest that mTOR plays a key role in both growth and metabolism (Dann et al. 2007). While growth factor and insulin activation of mTOR is through tyrosine receptor activation of a class 1 PI3K-Akt pathway; activation of mTOR by mechanical loading and amino acids appears to be through alternate pathways that may not require Akt (Hornberger et al. 2006; Dann et al. 2007). In the present study, both Akt and S6K1 activation were attenuated following loading in the HFD mice; however, the extent to which the decrease in Akt activation is linked to the decrease in S6K1 activation is unknown. It is possible that the decrease in S6K1 activation is occurring through inhibition of mTOR in an Akt-independent manner.
The present study represents the first investigation to show that diet-induced obesity has a negative impact on the ability of skeletal muscle to adapt to growth signals such as mechanical loading. A decrease in the ability of muscle to respond to growth signals could adversely affect glucose homeostasis, as well as prevent recovery from injuries and accelerate the effects of ageing. Additional studies are required to investigate the mechanisms by which obesity interferes with the response of skeletal muscle to growth stimuli.
Acknowledgments
We would like to thank Scot Kimball and his group for their assistance with the polysome aggregation analysis. We also thank Robin Altman for her assistance. This work was supported by the Richard A. and Nora Eccles Harrison Endowed Chair in Diabetes Research and NIH grant HL 55667.
Glossary
Abbreviations
- FO
functional overload
- HFD
high fat diet
- IMTG
intramyocellular triglycerides
- LFD
low fat diet
Author contributions
M.S. is a doctoral student and contributed to the conception and design of the experiments, data collection, analysis and interpretation, and to the writing of the manuscript. S.C.B. contributed to the conception and design of the experiments, data analysis and interpretation, and to the writing, critical revisions and final approval of the manuscript. J.R. contributed to the conception and design of the experiments, and to critical revisions.
References
- Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, Chang Z, Tripathy D, Jani R, Molina-Carrion M, Monroy A, Folli F, Van Remmen H, DeFronzo RA. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E678–E685. doi: 10.1152/ajpendo.90287.2008. [DOI] [PubMed] [Google Scholar]
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Anderson SR, Gilge DA, Steiber AL, Previs SF. Diet-induced obesity alters protein synthesis: tissue-specific effects in fasted versus fed mice. Metabolism. 2008;57:347–354. doi: 10.1016/j.metabol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem. 2006;42:61–74. doi: 10.1042/bse0420061. [DOI] [PubMed] [Google Scholar]
- Balage M, Sinaud S, Prod’Homme M, Dardevet D, Vary TC, Kimball SR, Jefferson LS, Grizard J. Amino acids and insulin are both required to regulate assembly of the eIF4E · eIF4G complex in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281:E565–E574. doi: 10.1152/ajpendo.2001.281.3.E565. [DOI] [PubMed] [Google Scholar]
- Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO43 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, DeFronzo RA, Cusi K. Dose–response effect of elevated plasma free fatty acid on insulin signalling. Diabetes. 2005;54:1640–1648. doi: 10.2337/diabetes.54.6.1640. [DOI] [PubMed] [Google Scholar]
- Bjursell M, Gerdin AK, Lelliott CJ, Egecioglu E, Elmgren A, Tornell J, Oscarsson J, Bohlooly YM. Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2008;294:E251–E260. doi: 10.1152/ajpendo.00401.2007. [DOI] [PubMed] [Google Scholar]
- Bodine SC. mTOR signalling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38:1950–1957. doi: 10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc. 2004;63:351–356. doi: 10.1079/PNS2004355. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol. 2003;553:213–220. doi: 10.1113/jphysiol.2003.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaubon L, Sajan MP, Rivas J, Powe JL, Standaert ML, Farese RV. Contrasting insulin dose-dependent defects in activation of atypical protein kinase C and protein kinase B/Akt in muscles of obese diabetic humans. Diabetologia. 2006;49:3000–3008. doi: 10.1007/s00125-006-0471-5. [DOI] [PubMed] [Google Scholar]
- Chanseaume E, Giraudet C, Gryson C, Walrand S, Rousset P, Boirie Y, Morio B. Enhanced muscle mixed and mitochondrial protein synthesis rates after a high-fat or high-sucrose diet. Obesity (Silver Spring) 2007;15:853–859. doi: 10.1038/oby.2007.582. [DOI] [PubMed] [Google Scholar]
- Childs TE, Spangenburg EE, Vyas DR, Booth FW. Temporal alterations in protein signalling cascades during recovery from muscle atrophy. Am J Physiol Cell Physiol. 2003;285:C391–C398. doi: 10.1152/ajpcell.00478.2002. [DOI] [PubMed] [Google Scholar]
- Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW. Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol Endocrinol. 2007;21:215–228. doi: 10.1210/me.2006-0154. [DOI] [PubMed] [Google Scholar]
- Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr. 2007;85:662–677. doi: 10.1093/ajcn/85.3.662. [DOI] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signalling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signalling. J Appl Physiol. 2009;106:1374–1384. doi: 10.1152/japplphysiol.91397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG. Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes. 1999;48:1662–1666. doi: 10.2337/diabetes.48.8.1662. [DOI] [PubMed] [Google Scholar]
- Favier F, Benoit H, Freyssenet D. Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflugers Archiv. 2008;456:587–600. doi: 10.1007/s00424-007-0423-z. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink DF, Garlick PJ, McNurlan MA. Protein turnover measured in vivo and in vitro in muscles undergoing compensatory growth and subsequent denervation atrophy. Biochem J. 1983;210:89–98. doi: 10.1042/bj2100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signalling in skeletal muscle. Proc Natl Acad Sci U S A. 2006;103:4741–4746. doi: 10.1073/pnas.0600678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara A, Roy RR, Ohira Y, Ibata Y, Edgerton VR. Hypertrophy of rat plantaris muscle fibres after voluntary running with increasing loads. J Appl Physiol. 1998;84:2183–2189. doi: 10.1152/jappl.1998.84.6.2183. [DOI] [PubMed] [Google Scholar]
- Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, Hamilton JA, Ouchi N, LeBrasseur NK, Walsh K. Fast/glycolytic muscle fibre growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–172. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kim Y-B, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest. 1999;104:733–741. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Horetsky RL, Jefferson LS. Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am J Physiol Cell Physiol. 1998;274:C221–C228. doi: 10.1152/ajpcell.1998.274.1.C221. [DOI] [PubMed] [Google Scholar]
- Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofibre hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Cooney GJ. Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol. 2008;19:235–241. doi: 10.1097/01.mol.0000319118.44995.9a. [DOI] [PubMed] [Google Scholar]
- Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes. 1998;47:1281–1286. doi: 10.2337/diab.47.8.1281. [DOI] [PubMed] [Google Scholar]
- Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bɛ mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem. 2005;280:7570–7580. doi: 10.1074/jbc.M413732200. [DOI] [PubMed] [Google Scholar]
- Kubica N, Crispino JL, Gallagher JW, Kimball SR, Jefferson LS. Activation of the mammalian target of rapamycin complex 1 is both necessary and sufficient to stimulate eukaryotic initiation factor 2Bɛ mRNA translation and protein synthesis. Int J Biochem Cell Biol. 2008;40:2522–2533. doi: 10.1016/j.biocel.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MC, Monetti M, Watt MJ, Sajan MP, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, Farese RV., Jr Increased lipid accumulation and insulin resistance in transgenic mice expressing DGAT2 in glycolytic (type II) muscle. Am J Physiol Endocrinol Metab. 2007;293:E1772–E1781. doi: 10.1152/ajpendo.00158.2007. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Esser KA. Cellular mechanisms regulating protein synthesis and skeletal muscle hypertrophy in animals. J Appl Physiol. 2008;106:1367–1373. doi: 10.1152/japplphysiol.91355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab. 2008;294:E203–E213. doi: 10.1152/ajpendo.00624.2007. [DOI] [PubMed] [Google Scholar]
- Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M. Atrophy of S6K1−/− skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol. 2005;7:286–294. doi: 10.1038/ncb1231. [DOI] [PubMed] [Google Scholar]
- Pedrini MT, Kranebitter M, Niederwanger A, Kaser S, Engl J, Debbage P, Huber LA, Patsch JR. Human triglyceride-rich lipoproteins impair glucose metabolism and insulin signalling in L6 skeletal muscle cells independently of non-esterified fatty acid levels. Diabetologia. 2005;48:756–766. doi: 10.1007/s00125-005-1684-8. [DOI] [PubMed] [Google Scholar]
- Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol. 1984;57:1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- Prod’homme M, Balage M, Debras E, Farges M-C, Kimball S, Jefferson L, Grizard J. Differential effects of insulin and dietary amino acids on muscle protein synthesis in adult and old rats. J Physiol. 2005;563:235–248. doi: 10.1113/jphysiol.2004.068841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- Roy RR, Meadows ID, Baldwin KM, Edgerton VR. Functional significance of compensatory overloaded rat fast muscle. J Appl Physiol. 1982;52:473–478. doi: 10.1152/jappl.1982.52.2.473. [DOI] [PubMed] [Google Scholar]
- Silveira LR, Fiamoncini J, Hirabara SM, Procopio J, Cambiaghi TD, Pinheiro CH, Lopes LR, Curi R. Updating the effects of fatty acids on skeletal muscle. J Cell Physiol. 2008;217:1–12. doi: 10.1002/jcp.21514. [DOI] [PubMed] [Google Scholar]
- Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol. 2006;100:286–293. doi: 10.1152/japplphysiol.00869.2005. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Gordon SE. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J Physiol. 2006;574:291–305. doi: 10.1113/jphysiol.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Marette A. Amino acid and insulin signalling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- Yerby B, Deacon R, Beaulieu V, Liang J, Gao J, Laurent D. Insulin-stimulated mitochondrial adenosine triphosphate synthesis is blunted in skeletal muscles of high-fat-fed rats. Metabolism. 2008;57:1584–1590. doi: 10.1016/j.metabol.2008.06.015. [DOI] [PubMed] [Google Scholar]