Abstract
Two experiments were conducted to test the hypothesis that cortisol interferes with the positive feedback action of estradiol that induces the luteinizing hormone (LH) surge. Ovariectomized sheep were treated sequentially with progesterone and estradiol to create artificial estrous cycles. Cortisol or vehicle (saline) was infused from 2 h before the estradiol stimulus through the time of the anticipated LH surge in the artificial follicular phase of two successive cycles. The plasma cortisol increment produced by infusion was ∼1.5 times greater than maximal concentrations seen during infusion of endotoxin, which is a model of immune/inflammatory stress. In experiment 1, half of the ewes received vehicle in the first cycle and cortisol in the second; the others were treated in reverse order. All ewes responded with an LH surge. Cortisol delayed the LH surge and reduced its amplitude, but both effects were observed only in the second cycle. Experiment 2 was modified to provide better control for a cycle effect. Four treatment sequences were tested (cycle 1-cycle 2): vehicle-vehicle, cortisol-cortisol, vehicle-cortisol, cortisol-vehicle. Again, cortisol delayed but did not block the LH surge, and this delay occurred in both cycles. Thus, an elevation in plasma cortisol can interfere with the positive feedback action of estradiol by delaying and attenuating the LH surge.
Keywords: cortisol, estradiol, glucocorticoid, LH surge, luteinizing hormone, positive feedback, stress
An elevation in plasma cortisol interferes with the positive feedback action of estradiol in the ewe by delaying and attenuating the induced LH surge.
INTRODUCTION
Various types of stress disrupt the follicular phase of the ovarian cycle and delay or block the preovulatory luteinizing hormone (LH) surge. For example, a delay of the surge has been observed in rhesus monkeys exposed to the combined stress of exercise, food restriction, and exposure to a novel environment [1] or treated with endotoxin [2], which is a commonly used model of immune/inflammatory stress. In sheep, the LH surge can be delayed or blocked by infusion of endotoxin [3] or by transport in a truck [4, 5]. Restraint stress can block the preovulatory LH surge of the rat [6].
Mechanistically, stress could disrupt the LH surge either by interfering with generation of the follicular phase increase in estradiol secretion, which constitutes the ovarian positive feedback signal for inducing the LH surge, or by interfering with the neuroendocrine response to this positive feedback signal. Both disruptive mechanisms are likely called upon during stress. In sheep, for example, endotoxin inhibits both pulsatile gonadotropin-releasing hormone (GnRH) secretion [7, 8] and pituitary responsiveness to GnRH [9], which would reduce gonadotropic drive to the ovary and thus interfere with the follicular-phase rise in estradiol secretion [3]. Endotoxin also delays or blocks estradiol-induced GnRH and LH surges [10, 11] and transport delays and attenuates the LH surge response to estradiol [4, 5]. These stressors, therefore, interfere with the positive feedback action of estradiol.
One signature of the stress response is the close association of reproductive suppression and activation of the hypothalamo-pituitary-adrenal axis leading to enhanced glucocorticoid secretion [12, 13]. In this study, we tested the hypothesis that an elevation in plasma cortisol interferes with LH surge generation by disrupting the neuroendocrine response to the positive feedback action of estradiol. Three observations in sheep prompted this hypothesis. First, a stress-like increase in plasma cortisol delayed [14, 15] or blocked [16] the preovulatory LH surge of nonstressed sheep. That response, however, could have been due to suppression of estradiol synthesis rather than interference with the response to positive feedback because cortisol suppresses pulsatile GnRH and LH secretion and lowers plasma estradiol levels in follicular-phase ewes [14–16]. Second, an infusion of endotoxin that interfered with the estradiol-induced LH surge also caused a large increase in plasma cortisol sustained for the entire latent period between onset of the estradiol stimulus and generation of the LH surge [10]. Thus, elevated cortisol accompanies the impaired positive feedback response. Third, disruption of the estradiol-induced LH surge by endotoxin was not reversed by suppressing the synthesis of prostaglandins [11], and the disruptive effects of transport or restraint on the LH surge were not reversed by blocking the action of endogenous opioids [6, 17]. Because prostaglandins and opioids mediate other reproductive neuroendocrine responses to stress [8, 18–20], those findings implicate a role for a different intermediary molecule. Although cortisol is a likely candidate, its influence on the LH surge response to a fixed estradiol signal has not been fully investigated. Herein we tested whether an increase in plasma cortisol interferes with the positive feedback action of estradiol in ovariectomized ewes treated with estradiol and progesterone to simulate changes in these steroids during the natural estrous cycle (artificial estrous cycle model).
MATERIALS AND METHODS
Experiments were conducted on sexually mature Suffolk ewes maintained at the Sheep Research Facility, Ann Arbor, MI. The animals were fed hay and alfalfa pellets and had free access to water and mineral licks. At least 2 mo before the study, the ewes were ovariectomized using aseptic conditions and general anesthesia. Two experiments were conducted in April and June (nonbreeding season) of successive years; our previous work indicates that season does not affect cortisol-induced inhibition of pulsatile LH secretion [21]. For the experiments, ewes were moved into rooms where the photoperiod was controlled to simulate that of the outdoors. All procedures were approved by the University Committee for the Use and Care of Animals at the University of Michigan.
Artificial estrous cycles were created as previously described [11, 22, 23] by treatment with a 1-cm s.c. estradiol implant and two intravaginal progesterone-releasing devices (controlled internal drug release; DEC International, Hamilton, New Zealand) to simulate plasma concentrations of these steroids during the luteal phase. After 7–9 days, which approximates the duration of maximal progesterone secretion in the luteal phase [24], progesterone was withdrawn by removal of controlled internal drug release devices, and four 3-cm estradiol implants were inserted s.c. 16 h later to simulate the follicular-phase rise in plasma estradiol to ∼6 pg/ml. During this artificial follicular phase, surges of GnRH and LH are reliably induced, with peaks occurring ∼24 h after onset of the estradiol rise [11, 23, 25]. Plasma concentrations of estradiol and progesterone have been extensively characterized in this model [10, 11, 22, 26] and were not monitored in this study.
Two indwelling jugular vein catheters were inserted the day before the surge-inducing estradiol stimulus, one for sampling blood and the other for infusing cortisol or vehicle. Cortisol (325 μg/kg/h, Solu-cortef; Pharmacia & Upjohn, Kalamazoo, MI) or vehicle (saline) was infused using backpack infusion pumps (AutoSyringe, model AS2BH; AutoSyringe, Inc., Hooksett, NH), allowing the sheep to move freely around the room throughout the experimental period. This dose of cortisol was calculated to produce a rise in the plasma cortisol concentration to ∼150 ng/ml, within the upper range of values observed during infusion of endotoxin [3, 10]. Infusions started 2 h before the surge-inducing estradiol stimulus (four 3-cm estradiol implants) and continued for 42 h, well beyond the expected time of the LH surge in control ewes.
Experiment 1
Experiment 1 was conducted according to a crossover design on two groups of ovariectomized ewes in which two successive artificial estrous cycles were produced, separated by a 4-day period of no hormone replacement. One group (n = 5) received vehicle during the first artificial follicular phase and cortisol in the second; the other group (n = 5) received the same treatments in reverse sequence. Blood was sampled hourly from 2 h before to 4 h after onset of the estradiol stimulus (0 h) and again from 12–48 h to encompass the time of the LH surge. Luteinizing hormone was assayed in all samples; cortisol was measured from −4 to +1 h and at 12, 18, 22, 34, and 48 h. Data for one animal in which cortisol was infused in cycle 1 and vehicle in cycle 2 were excluded from the analysis because of technical difficulties with the cortisol infusion.
Experiment 2
Experiment 2 was conducted using a similar approach, but two additional groups were included to control for a cycle effect because experiment 1 suggested that cortisol interfered with the LH surge only in the second cycle. One additional group was treated with vehicle during the artificial follicular phase of both cycles, and the other received cortisol on both occasions. Thus, there were four groups (six ewes per group) treated as follows in the first and second cycles (cycle 1-cycle 2): vehicle-vehicle, cortisol-cortisol, vehicle-cortisol, and cortisol-vehicle. This expanded design strengthened comparison of LH surge parameters between vehicle and cortisol treatments within a given cycle and provided a better control to test for treatment effects within ewes across both cycles. All other procedures were identical to those of experiment 1. Because of technical difficulties, data were excluded for both cycles of one ewe that received cortisol in cycle 1 and vehicle in cycle 2, as well as for cycle 2 of one ewe treated with vehicle in cycle 1 and cortisol in cycle 2.
Assays
Luteinizing hormone was assayed in duplicate aliquots (5–200 μl) of plasma using a modification [24] of a previously described radioimmunoassay [27, 28]. Assay sensitivity averaged 0.56 ng/ml (20 assays), and intraassay and interassay coefficients of variation averaged 4.9% and 8.1%, respectively. Cortisol was assayed in duplicate 50-μl aliquots of unextracted plasma using the Coat-a-Count Cortisol Assay Kit (Diagnostic Products Corp., Los Angeles, CA), validated for use in sheep [7]. Sensitivity averaged 0.62 ng/ml (eight assays), and intraassay and interassay coefficients of variation averaged 2.3% and 10.3%, respectively.
Data Analysis
As in our previous investigations (10), the LH surge was defined as a rise in plasma LH concentration exceeding 2 SD above the presurge baseline (surge onset) and maintenance of that elevated level for at least 4 h. A mean ± SD presurge baseline was calculated for each ewe using values from 2 h before to 12 h after onset of the surge-inducing estradiol stimulus. Four aspects of the LH surge were examined: percentage of ewes expressing the LH surge, interval from onset of the estradiol stimulus to peak of the LH surge (latent period), average of the three highest contiguous hourly values during the LH surge (amplitude), and interval from the surge onset to the time when LH fell to 10% of the surge peak (duration). Data for the latent period, amplitude, and duration during cycles 1 and 2 were compared using paired t-test or Student t-test (see Results).
RESULTS
Cortisol
The mean ± SEM plasma concentrations of cortisol during infusion of saline were 11.3 ± 1.4 ng/ml in experiment 1 and 13.0 ± 1.6 ng/ml in experiment 2, values at or near the level we observe in nonstressed ewes [29, 30]. The mean ± SEM values were maintained at 172.5 ± 6.9 ng/ml during infusion of cortisol in experiment 1 and did not differ between cycles 1 and 2. In experiment 2, the mean ± SEM plasma cortisol concentration during cortisol infusion in cycle 1 exceeded that in cycle 2 (168.0 ± 11.6 vs. 136.0 ± 7.7 ng/ml; P < 0.05). Overall, plasma cortisol levels during infusion were ∼1.5 times greater than the mean values seen in ewes during infusion of endotoxin in the natural or artificial follicular phase [3, 10], but values during cortisol infusion were within the range of maximal values induced by endotoxin.
Experiment 1
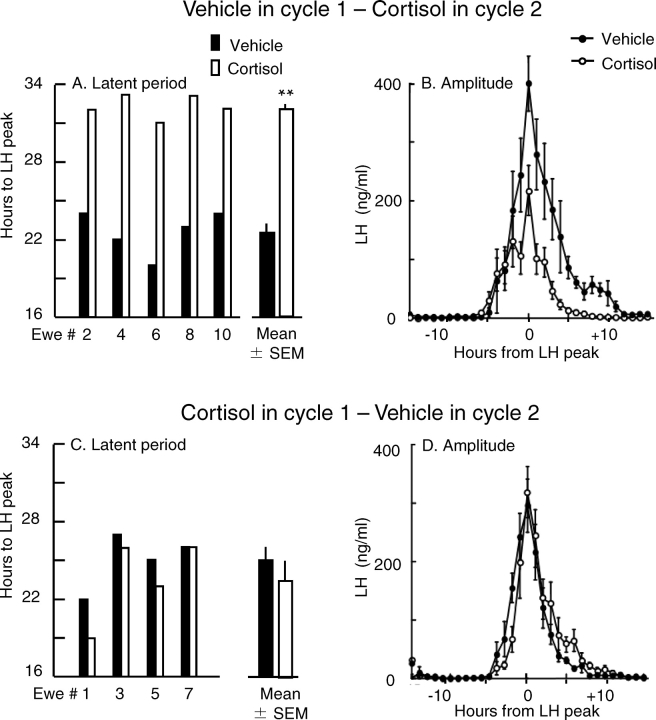
There was no effect of cortisol on incidence of the LH surge. Regardless of whether treated with vehicle or cortisol, all ewes responded with an unambiguous LH surge in both the first and second cycles. Cortisol, however, increased the latent period and reduced the amplitude of the LH surge, but both effects depended on treatment sequence. When vehicle was infused in cycle 1 and cortisol in cycle 2, the LH surge was delayed in each of the five ewes; the latent period increased by an average of 10 h (22.6 and 32.2 h in cycles 1 and 2, respectively; P < 0.001, paired t-test) (Fig. 1A). Furthermore, the LH surge amplitude was reduced by 53% compared with that in cycle 1 when vehicle was infused (mean ± SEM amplitude, 164 ± 31 vs. 352 ± 48 ng/ml; P < 0.05) (Fig. 1B). In contrast, when the sequence was reversed and cortisol was given in cycle 1, there was no effect on the latent period (Fig. 1C) or the amplitude (Fig. 1D). In addition, there was no difference in the duration of the LH surge in animals treated with cortisol in cycle 1 vs. vehicle in cycle 2 (mean ± SEM duration, 10.5 ± 1.2 vs. 9.0 ± 0.7 h; P > 0.05) or when treatments were reversed (10.8 ± 0.9 vs. 14.8 ± 4.4 h, vehicle in cycle 1 vs. cortisol in cycle 2; P > 0.05).
FIG. 1.
Influence of cortisol on the estradiol-induced LH surge in experiment 1. A and C show the latent period from estradiol stimulus to LH peak in individual ewes (thin bars) and the mean ± SEM for all ewes (thick bars) in each cycle. B and D show the mean ± SEM plasma LH concentrations normalized to the peak of the LH surge. In A and B, vehicle was infused in cycle 1, and cortisol was infused in cycle 2. In C and D, cortisol was infused in cycle 1, and vehicle was infused in cycle 2. Solid and open bars and symbols indicate vehicle- and cortisol-infused ewes, respectively. The mean ± SEM values represent five ewes in A and B and four ewes in C and D. **Group difference in the latent period, P < 0.001. In B, the LH surge amplitude was less in cortisol- than vehicle-treated ewes (P < 0.05).
Experiment 2
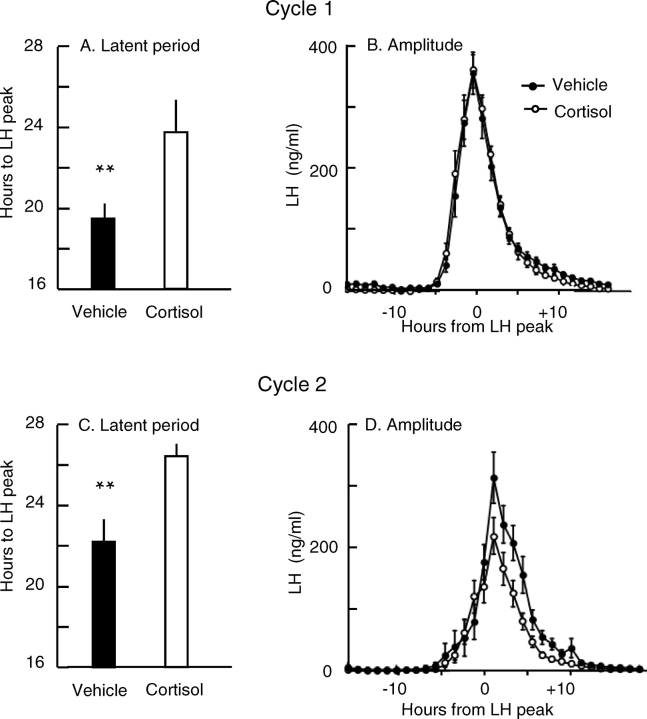
As in experiment 1, all ewes expressed an LH surge in both cycles regardless of treatment. The latent period in control ewes (i.e., saline infused in both cycles) was greater in cycle 2 than in cycle 1, thus indicating a cycle effect (21.9 ± 1.4 h vs. 18.7 ± 1.6 h; P < 0.02, paired t-test). Because of this and the cycle effect in experiment 1, LH surge parameters between cortisol- and vehicle-treated ewes were compared within each cycle by Student t-test rather than within ewes across cycles by paired t-test (11–12 ewes per group for each cycle). This analysis revealed that cortisol delayed the LH surge by an average of 4.3 h in cycle 1 (P < 0.01) and 4.2 h in cycle 2 (P < 0.01) (Fig. 2, A and C). Cortisol tended to reduce the LH surge amplitude in cycle 2 but not in cycle 1 (Fig. 2, B and D). The mean ± SEM amplitude in cycle 2 was 30% lower in ewes receiving cortisol than in ewes receiving vehicle (176 ± 25 vs. 252 ± 33 ng/ml; P = 0.086). Cortisol did not affect the mean ± SEM surge duration in cycle 1 (12.4 ± 0.8 vs. 11.6 ± 0.5 h, vehicle vs. cortisol; P > 0.05) or cycle 2 (11.4 ± 0.9 vs. 10.9 ± 0.6 h, vehicle vs. cortisol; P > 0.05).
FIG. 2.
Influence of cortisol on the estradiol-induced LH surge in cycle 1 (top) and cycle 2 (bottom) of experiment 2. A and C show the mean ± SEM for the latent period from estradiol stimulus to LH peak. B and D show the mean ± SEM plasma LH concentration normalized to the peak of the LH surge. Solid and open bars and symbols indicate values for vehicle- and cortisol-treated ewes, respectively. **Group difference, P < 0.01 (11–12 ewes per group).
DISCUSSION
Our findings indicate that cortisol can interfere with the estradiol-induced LH surge in ovariectomized ewes under conditions that simulate the follicular phase of the estrous cycle. Cortisol did not block the LH surge, but it had other disruptive effects. In experiment 1, cortisol reduced the LH surge amplitude by ∼50% and delayed the surge by 10 h, which represents almost half of the latent period for LH surge induction in this model. The detrimental effects of cortisol, however, were evident only in the second cycle. This suggested that exposure to ovarian steroids during the previous cycle sensitized the LH surge mechanism to cortisol in the second cycle. Therefore, the design for experiment 2 was modified to strengthen testing for cortisol effects within each cycle, as well as between consecutive cycles. In experiment 2, cortisol delayed the LH surge by 4 h in both cycles and tended to reduce the amplitude in cycle 2. Collectively, the two experiments indicate that cortisol can interfere with the positive feedback action of estradiol.
The lack of an effect of cortisol on the latent period in cycle 1 of experiment 1 is difficult to explain. It cannot be explained by the lack of exposure to ovarian steroids during a previous cycle because cortisol delayed the LH surge in the first cycle of experiment 2, when there was no such prior exposure to steroids. Furthermore, it cannot be explained by known experimental conditions (time of year, nutrition, etc.), as these were similar in experiments 1 and 2. More likely, the lack of an effect of cortisol to delay the surge in cycle 1 of experiment 1 (compared with vehicle in cycle 2) is related to the cycle effect whereby the latent period increases in the second cycle independent of cortisol action, as revealed in experiment 2. This cycle effect could also explain why the delay of the LH surge was so great (10 h) in experiment 1 when ewes were treated with vehicle in cycle 1 and cortisol in cycle 2 because the delaying effect of cortisol would be superimposed on the cycle effect. Of importance, the strengthened design of experiment 2 eliminated the influence of a cycle effect, and the action of cortisol to delay the estradiol-induced LH surge was unambiguous.
Early work addressing the influence of corticosteroids on the LH surge revealed that synthetic glucocorticoids block the natural preovulatory LH surge and the estradiol-induced LH surge in ovariectomized rats [31, 32]. Early experiments in sheep, however, suggested that neither cortisol nor its synthetic analog, dexamethasone, could block the estradiol-induced LH surge, but the number of animals (e.g., two controls and three cortisol-treated ewes) was insufficient to test for effects on the latent period or the surge amplitude [33]. In a companion study using a different follicular-phase model in which estradiol was delivered as a single bolus rather than constant-release implants, cortisol was found to delay, attenuate, and even block the LH surge [34] or delay the expression of estrous behavior [35], and the effect on the LH surge was more profound in the breeding season compared with the nonbreeding season. Those findings reinforce the present observations by determining that cortisol can perturb the positive feedback action of estradiol and that the response includes an increased latent period and a decreased amplitude of the LH surge.
Several further questions arise from the present work. First, how do our findings fit with the effect of cortisol in the natural follicular phase, and through what mechanisms does cortisol act? Recent studies [14–16] indicate that a cortisol treatment similar to the one used in experiments 1 and 2 reduces the frequency of GnRH and LH pulses in the natural follicular phase, attenuates the preovulatory estradiol rise, and delays or blocks the LH surge. Although the reduction in circulating estradiol would be expected to compromise the LH surge, the present work suggests that the deleterious effect on the preovulatory LH surge also results from impaired neuroendocrine responsiveness to the positive feedback action of estradiol. Delay of the positive feedback response most likely reflects an effect on GnRH secretion because estradiol induces a large surge of GnRH in the artificial follicular-phase model used here [23, 25, 26] and because this is necessary for initiating the LH surge [36–38]. Inhibition of GnRH secretion is probably indirect because GnRH neurons in the ewe lack the type II glucocorticoid receptor [39], which mediates neuroendocrine responses to stress-like elevations in glucocorticoids [40]. Glucocorticoid receptors, however, are colocalized with progesterone receptor and estrogen receptor α in neurons of the preoptic area and arcuate nucleus of the ewe, providing a possible locus of interaction in regulating GnRH secretion [41]. Another possibility is that cortisol might cross-react with progesterone receptor, as progesterone also delays the LH surge in sheep [42], but cross-reaction is unlikely with physiological levels of cortisol because of low affinity of progesterone receptor for glucocorticoids [43]. The reduced amplitude of the LH surge in the present study likely includes an effect on the pituitary, as cortisol acts on the pituitary to inhibit response to GnRH in the ewe [44–46]. Supporting this possibility, glucocorticoids disrupt estradiol-induced LH release in rats by suppressing the response to exogenous GnRH [32], and transport stress has a similar effect in the ewe [5].
Another question arising from our findings is how might a delay and an attenuation of the preovulatory LH surge influence overall reproductive success? Although it is not known if attenuation of the LH surge would compromise fertility, there is reason to suspect that a delay of the LH surge could have deleterious effects. Because increased estradiol secretion constitutes the ovarian signal to the neuroendocrine axis that a follicle is nearing readiness to ovulate, a delay in the LH surge could desynchronize follicular and/or oocyte maturation and the ovulatory stimulus. There is evidence to suggest that uncoupling of ovarian and neuroendocrine events lowers fertility and produces epigenetic effects that compromise the offspring. For example, an 11-h delay of the LH surge in cattle, while increasing the number of ovulations, impairs early embryonic development [47]. Lengthening of the estrous cycle of the rat to 6 days leads to decreased fertilization and implantation rates and increased chromosomal aberrations, fetal abnormalities, and embryonic death [48, 49]. Further work is needed to determine if the magnitude of the detrimental effects of cortisol on the LH surge is sufficient to compromise fertilization, embryonic development, and production of viable offspring.
Yet another question pertains to physiological relevance. Our treatment elevated plasma cortisol for the entire period between onset of the estradiol stimulus and generation of the LH surge. This treatment was based on observations that infusion of endotoxin, which disrupted the LH surge in the artificial follicular-phase model, elevated plasma cortisol for the entire latent period to the LH surge [10]. Thus, cortisol likely contributes to disruption of the positive feedback action of estradiol in response to severe immune/inflammatory stress. This is an attractive possibility because detrimental effects of endotoxin on the estradiol-induced LH surge are not mediated by prostaglandins, which relay effects of immune/inflammatory stress on pulsatile GnRH and LH secretion [8, 18]. However, caution must be exercised in extending our interpretation to less severe stressors such as transport that evoke smaller and briefer elevations in cortisol secretion. Nonetheless, the magnitude and duration of the cortisol increment needed to interfere with the positive feedback response are not known, and timing of the cortisol rise relative to the estradiol stimulus could be important. Clearly, further work is needed to assess the applicability of our findings to stress-induced disruption of reproductive neuroendocrine function.
Finally, although previous studies indicate that glucocorticoids can block or attenuate the estradiol-induced LH surge [32], this is the first study (to our knowledge) to reveal that a stress-like elevation in plasma cortisol can shift the timing of the positive feedback response to a fixed estradiol signal, as is seen in response to certain stressors. Interference with the response to estradiol constitutes one mechanism by which cortisol compromises the follicular phase of the estrous cycle, and it likely contributes to the means by which severe stressors such as endotoxin disrupt ovarian cyclicity. This could have the adaptive value of reducing the likelihood of pregnancy in animals with infectious or immune/inflammatory disease or with adrenal disorders that chronically elevate cortisol (e.g., Cushing disease), thus allowing metabolic energy to be partitioned in a manner that optimizes survival.
Acknowledgments
We thank Douglas Doop and Gary McCalla for care and maintenance of the experimental animals, Lisa Case-Doro and Andrew Pytiak for technical assistance with animal experimentation, Dr. Morton Brown for consultation on the data analysis, and Drs. Gordon D. Niswender and Leo E. Reichert, Jr. for supplying radioimmunoassay reagents.
Footnotes
1Supported by NIH- HD30773, T32-07048, and T32-08322.
REFERENCES
- Williams NI, Berga SL, Cameron JL.Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab 2007; 293: E270–E276. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Barth A, Zhu J, Ferin M.Stress and the menstrual cycle: relevance of cycle quality in the short- and long-term response to a 5-day endotoxin challenge during the follicular phase in the rhesus monkey. J Clin Endocrinol Metab 1998; 83: 2454–2460. [DOI] [PubMed] [Google Scholar]
- Battaglia DF, Krasa HB, Padmanabhan V, Viguié C, Karsch FJ.Endocrine alterations that underlie endotoxin-induced disruption of the follicular phase in ewes. Biol Reprod 2000; 62: 45–53. [DOI] [PubMed] [Google Scholar]
- Dobson H, Tebble JE, Phogat JB, Smith RF.Effect of transport on pulsatile and surge secretion of LH in ewes in the breeding season. J Reprod Fertil 1999; 116: 1–8. [DOI] [PubMed] [Google Scholar]
- Phogat JB, Smith RF, Dobson H.Effect of transport on pituitary responsiveness to exogenous pulsatile GnRH and oestradiol-induced LH release in intact ewes. J Reprod Fertil 1999; 116: 9–18. [DOI] [PubMed] [Google Scholar]
- Roozendaal MM, Swarts JJ, van Maanen JC, Wiegant VM, Mattheij JA.Inhibition of the LH surge in cyclic rats by stress is not mediated by opioids. Life Sci 1997; 60: 735–742. [DOI] [PubMed] [Google Scholar]
- Battaglia DF, Bowen JM, Krasa HB, Thrun LA, Viguié C, Karsch FJ.Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophyseal portal and peripheral blood. Endocrinology 1997; 138: 4273–4281. [DOI] [PubMed] [Google Scholar]
- Harris TG, Battaglia DF, Brown ME, Brown MB, Carlson NE, Viguié C, Williams CY, Karsch FJ.Prostaglandins mediate the endotoxin-induced suppression of pulsatile gonadotropin-releasing hormone and luteinizing hormone secretion in the ewe. Endocrinology 2000; 141: 1050–1058. [DOI] [PubMed] [Google Scholar]
- Williams CY, Harris TG, Battaglia DF, Viguié C, Karsch FJ.Endotoxin inhibits pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology 2001; 142: 1915–1922. [DOI] [PubMed] [Google Scholar]
- Battaglia DF, Beaver AB, Harris TG, Tanhehco E, Viguié C, Karsch FJ.Endotoxin disrupts the estradiol-induced luteinizing hormone surge: interference with estradiol signal reading, not surge release. Endocrinology 1999; 140: 2471–2479. [DOI] [PubMed] [Google Scholar]
- Breen KM, Billings HJ, Debus N, Karsch FJ.Endotoxin inhibits the surge secretion of gonadotropin-releasing hormone via a prostaglandin-independent pathway. Endocrinology 2004; 145: 221–227. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU.How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000; 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI, Clarke IJ.Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod 2000; 5: 105–113. [DOI] [PubMed] [Google Scholar]
- Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW, Karsch FJ.Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology 2005; 146: 2107–2115. [DOI] [PubMed] [Google Scholar]
- Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ.Cortisol reduces GnRH pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology 2008; 150: 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane MS, Breen KM, Sakurai H, Adams BM, Adams TE.Effect of duration of infusion of stress-like concentrations of cortisol on follicular development and the preovulatory surge of LH in sheep. Anim Reprod Sci 2000; 63: 167–175. [DOI] [PubMed] [Google Scholar]
- Smart D, Forhead AJ, Smith RF, Dobson H.Transport stress delays the oestradiol-induced LH surge by a non-opioidergic mechanism in the early postpartum ewe. J Endocrinol 1994; 142: 447–451. [DOI] [PubMed] [Google Scholar]
- Rivest S, Rivier C.Centrally injected interleukin-1 beta inhibits the hypothalamic LHRH secretion and circulating LH levels via prostaglandins in rats. J Neuroendocrinol 1993; 5: 445–450. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Horton RJ, Doughton BW.Investigation of the mechanism by which insulin-induced hypoglycemia decreases luteinizing hormone secretion in ovariectomized ewes. Endocrinology 1990; 127: 1470–1476. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M.Inhibitory effects of endotoxin on LH secretion in the ovariectomized monkey are prevented by naloxone but not by an interleukin-1 receptor antagonist. Neuroimmunomodulation 2000; 7: 6–15. [DOI] [PubMed] [Google Scholar]
- Breen KM, Karsch FJ.Does season alter responsiveness of the reproductive neuroendocrine axis to the suppressive actions of cortisol in ovariectomized ewes? Biol Reprod 2006; 74: 41–45. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ.Importance of variations in behavioural and feedback actions of oestradiol to the control of seasonal breeding in the ewe. J Endocrinol 1981; 89: 229–240. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Karsch FJ.The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 1990; 127: 1375–1384. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Karsch FJ, Foster DL.A new concept for control of the estrous cycle of the ewe based on the temporal relationships between luteinizing hormone, estradiol and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology 1977; 101: 807–817. [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Mauger DT, Padmanabhan V, Thrun LA, Karsch FJ.Does estradiol induce the preovulatory gonadotropin-releasing hormone (GnRH) surge in the ewe by inducing a progressive change in the mode of operation of the GnRH neurosecretory system? Endocrinology 1995; 136: 5511–5519. [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ.Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology 1997; 138: 5408–5414. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Midgley AR, Jr, Reichert LE., JrRadioimmunologic studies with murine, ovine and porcine luteinizing hormone. Rosenberg E.Gonadotropins Los Altos, CA:Geron-X;1968: 299–306. [Google Scholar]
- Niswender GD, Reichert LE, Jr, Midgley AR, Jr, Nalbandov AV.Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology 1969; 84: 1166–1173. [DOI] [PubMed] [Google Scholar]
- Breen KM, Oakley AE, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Karsch FJ.Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress? Endocrinology 2007; 148: 1882–1890. [DOI] [PubMed] [Google Scholar]
- Debus N, Breen KM, Barrell GK, Billings HJ, Brown M, Young EA, Karsch FJ.Does cortisol mediate endotoxin-induced inhibition of pulsatile luteinizing hormone and gonadotropin-releasing hormone secretion? Endocrinology 2002; 143: 3748–3758. [DOI] [PubMed] [Google Scholar]
- Baldwin DM, Sawyer CH.Effects of dexamethasone on LH release and ovulation in the cyclic rat. Endocrinology 1974; 94: 1397–1403. [DOI] [PubMed] [Google Scholar]
- Baldwin DM.The effect of glucocorticoids on estrogen-dependent luteinizing hormone release in the ovariectomized rat and on gonadotropin secretion in the intact female rat. Endocrinology 1979; 105: 120–128. [DOI] [PubMed] [Google Scholar]
- Moberg GP, Watson JG, Stoebel DP, Cook R.Effect of cortisol and dexamethasone on the oestrogen-induced release of luteinizing hormone in the anoestrous ewe. J Endocrinol 1981; 90: 221–225. [DOI] [PubMed] [Google Scholar]
- Pierce BN, Clarke IJ, Turner AI, Rivalland ET, Tilbrook AJ.Cortisol disrupts the ability of estradiol-17β to induce the LH surge in ovariectomized ewes. Domest Anim Endocrinol 2009; (in press). [DOI] [PubMed]
- Pierce BN, Hemsworth PH, Rivalland ET, Wagenmaker ER, Morrissey AD, Papargiris MM, Clarke IJ, Karsch FJ, Turner AI, Tilbrook AJ.Psychosocial stress suppresses attractivity, proceptivity and pulsatile LH secretion in the ewe. Horm Behav 2008; 54: 424–434. [DOI] [PubMed] [Google Scholar]
- Kaynard AH, Malpaux B, Robinson JE, Wayne NL, Karsch FJ.Importance of pituitary and neural actions of estradiol in induction of the luteinizing hormone surge in the ewe. Neuroendocrinology 1988; 48: 296–303. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT, Jenkin M, Phillips DJ.The oestrogen-induced surge of LH requires a ‘signal' pattern of gonadotrophin-releasing hormone input to the pituitary gland in the ewe. J Endocrinol 1989; 122: 127–134. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Dahl GE, Evans NP, Thrun LA, Wang Y, Brown MB, Karsch FJ.Importance of the gonadotropin-releasing hormone (GnRH) surge for induction of the preovulatory luteinizing hormone surge of the ewe: dose-response relationship and excess of GnRH. Endocrinology 1998; 139: 588–595. [DOI] [PubMed] [Google Scholar]
- Dufourny L, Skinner DC.Type II glucocorticoid receptors in the ovine hypothalamus: distribution, influence of estrogen and absence of co-localization with GnRH. Brain Res 2002; 946: 79–86. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M.Brain corticosteroid receptor balance in health and disease. Endocr Rev 1998; 19: 269–301. [DOI] [PubMed] [Google Scholar]
- Dufourny L, Skinner DC.Progesterone receptor, estrogen receptor α, and the type II glucocorticoid receptor are expressed in the same neurons of the ovine preoptic area and arcuate nucleus: a triple immunolabeling study. Biol Reprod 2002; 67: 1605–1612. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Harris TG, Evans NP.Duration and amplitude of the luteal phase progesterone increment times the estradiol-induced luteinizing hormone surge in ewes. Biol Reprod 2000; 63: 1135–1142. [DOI] [PubMed] [Google Scholar]
- Ojasoo T, Dore JC, Gibert J, Raynaud JP.Binding of steroids to the progestin and glucocorticoid receptors analyzed by correspondence analysis. J Med Chem 1988; 31: 1160–1169. [DOI] [PubMed] [Google Scholar]
- Breen KM, Karsch FJ.Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology 2004; 145: 692–698. [DOI] [PubMed] [Google Scholar]
- Pierce BN, Stackpole CA, Breen KM, Clarke IJ, Karsch FJ, Rivalland ET, Turner AI, Caddy DJ, Wagenmaker ER, Oakley AE, Tilbrook AJ.Estradiol enables cortisol to act directly upon the pituitary to suppress pituitary responsiveness to GnRH in sheep. Neuroendocrinology 2008; (in press). published online ahead of print 19 August 2008; DOI 10.1159/000151543. [DOI] [PubMed]
- Breen KM, Davis TL, Doro LC, Nett TM, Oakley AE, Padmanabhan V, Rispoli LA, Wagenmaker ER, Karsch FJ.Insight into the neuroendocrine site and cellular mechanism by which cortisol suppresses pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology 2008; 149: 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Leemput EE, Vos PL, Hyttel P, van den Hurk R, Bevers MM, van der Weijden GC, Dieleman SJ.Effects of brief postponement of the preovulatory LH surge on ovulation rates and embryo formation in eCG/prostaglandin-treated heifers. Theriogenology 2001; 55: 573–592. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Fugo NW.Overripeness and the mammalian ova, II: delayed ovulation and chromosome anomalies. Fertil Steril 1967; 18: 297–302. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Blue JD, Fugo NW.Overripeness and the mammalian ova, III: fetal development at midgestation and at term. Fertil Steril 1969; 20: 223–231. [DOI] [PubMed] [Google Scholar]