Abstract
The nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) is a key transcriptional regulator of both lipid metabolism and inflammation. The importance of PPARγ is accentuated by the widespread use of synthetic PPARγ agonists, thiazolidinediones (such as rosiglitazone), as drugs for insulin resistance and type II diabetes. Fractalkine (FKN) and FKN receptor (FR) play an important role in the immune responses by regulating leukocyte migration and adhesion to inflamed peripheral tissues. In this study, we have identified a novel link between PPARγ activation and FKN signaling. On one hand, the activation of PPARγ by rosiglitazone in macrophages not only represses the transcription of the FR gene, but also prevents the plasma membrane translocation of the FR protein. On the other hand, the activation of PPARγ by rosiglitazone in endothelial cells also impedes the nuclear export of FKN. Together, these data suggest that PPARγ activation represses FKN signaling. These findings indicate a previously unrecognized mechanism that may contribute to the anti-inflammatory effect of PPARγ.
Introduction
The migration of leukocytes into inflamed peripheral tissues and lymphoid organs involves a cascade of molecular events finely regulated by chemokines and cell adhesion molecules. Fractalkine (FKN), also known as CX3CL1, is a structurally unique chemokine that can act either as a soluble chemotactic factor or as a membrane-anchored adhesion molecule for circulating leukocytes. It is expressed on endothelial cells, smooth muscle cells, and neurons that are activated by pro-inflammatory cytokines. The FKN receptor (FR), also known as CX3CR1, is expressed on monocytes and mature macrophages, natural killer cells, cytotoxic effector T cells, and mucosal dendritic cells, all of which play important roles in the inflammatory and immune responses.
Accumulating evidence in both clinical studies and animal disease models has shown that FKN signaling is also involved in the pathogenesis of various chronic inflammatory diseases, such as atherosclerosis (Lesnik et al. 2003), age-related macular degeneration (AMD; Combadiere et al. 2007), and rheumatoid arthritis (Nanki et al. 2004). Abrogation of FKN signaling by FR deletion in mice results in reduced accumulation of tissue-specific macrophages, such as foam cells at atherogenic lesions and microglial cells at sites of retinal degeneration. In addition, polymorphisms in human FR, which reduce its binding activity to FKN, have been reported to increase the risk of AIDS and to reduce the risk of coronary artery disease (Faure et al. 2000, Moatti et al. 2001). Therefore, the FKN signaling represents a new target for the treatment of an array of inflammatory and immune disorders.
Peroxisome proliferator-activated receptor γ (PPARγ) is a member of the nuclear hormone receptor superfamily of ligand-responsive transcription factors (Evans et al. 2004). It forms a functional heterodimer with the retinoid receptor (RXR)α. Certain lipophilic compounds have been identified as PPARγ ligands that can bind to the receptor complex and stimulate its transcriptional activity. Naturally occurring PPARγ ligands include native and oxidized polyunsaturated fatty acids and arachidonic acid derivatives such as prostaglandins and eicosanoids. Synthetic PPARγ ligands include thiazolidinediones (TZDs) such as rosiglitazone (BRL; Willson & Wahli 1997). PPARγ regulates a diverse array of physiological processes including adipogenesis, lipid metabolism, and insulin sensitivity, as well as diseases such as obesity, diabetes, and atherosclerosis. The importance of this receptor is accentuated by the widespread use of TZDs as drugs for insulin resistance and type II diabetes.
Numerous studies using mouse genetic models or synthetic PPARγ agonists have suggested that PPARγ also regulates both native and acquired immune responses (Bensinger & Tontonoz 2008). For example, we have recently reported an unexpected yet important role of PPARγ in suppressing the production of inflammatory milk lipids in the lactating mammary glands, using a mouse model in which PPARγ is specifically deleted in the hematopoietic and endothelial cells. The ingestion of this ‘toxic’ milk by nursing neonates results in growth retardation and inflammatory alopecia (Wan et al. 2007). Furthermore, conditional deletion of PPARγ in macrophages and intestinal epithelial cells demonstrated that it is important in the regulation of inflammatory bowel disease (Adachi et al. 2006, Shah et al. 2007). Surprisingly, macrophage-specific deletion of PPARγ was shown to regulate diet-induced obesity and insulin sensitivity, which are the key components of type II diabetes and metabolic syndrome (Odegaard et al. 2007).
To further understand how PPARγ regulates the immune system, we have found that PPARγ activation by rosiglitazone suppresses the FKN signaling via multiple mechanisms. In macrophages, rosiglitazone suppresses both the expression and the membrane translocation of FR. In endothelial cells, rosiglitazone prevents the nuclear export of FKN. Taken together, this evidence provides a previously unrecognized mechanism that may contribute to the anti-inflammatory effect of PPARγ.
Materials and methods
Macrophage cell culture
For bone marrow-derived macrophages (BMDMs), mouse bone marrow was flushed from the femur and tibia with serum-free DMEM (Invitrogen). After passing through a 40 μm cell strainer, the cells were cultured in macrophage differentiation medium (DMEM containing 20% fetal bovine serum (FBS) and 30% L929 cell-conditioned medium) for 8 days. Differentiated macrophages were replated in RPMI medium containing 10% FBS for various experiments. Peritoneal macrophages were isolated 3 days after thioglycollate challenge and were cultured in RPMI medium containing 10% FBS. The human monocyte cell line THP-1 and the mouse macrophage cell line RAW264.7 were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were treated with the indicated stimulant in the presence or absence of a ligand before harvesting. BRL was purchased from Cayman Chemical Ann Arbor, Michigan, USA; GW501516 and LG268 were synthesized; TPA (12-0-tetradecanoylphorbol 13-acetate) was obtained from Sigma; and tumor necrosis factor-α (TNFα) was obtained from BD Biosciences (San Jose, CA, USA). Concentrations used were BRL, 1 μM; GW501516, 0·1 μM; LG268, 0·1 μM; TPA, 40 ng/ml; and TNFα, 10 ng/ml.
Embryonic stem cell macrophage differentiation
Embryonic stem (ES) cells were trypsinized and depleted of feeder cells by culturing in IMDM (Invitrogen) supplemented with 5% FCS for 3–4 h. Nonadherent ES cells were seeded in 0·9% methylcellulose in IMDM supplemented with 15% FCS, transferrin (300 μg/ml, Roche), PFHM-II (5%, Invitrogen), ascorbic acid (50 μg/ml), and monothioglycerol (4×0−4 M). Embryoid bodies were collected on day 6, trypsinized, and replated in IMDM supplemented with 15% L929 cell-conditioned medium and mIL-3 (1 ng/ml, BD Biosciences Pharmingen). Two days later, macrophage precursors that developed as nonadherent cells were collected and cultured in IMDM supplemented with 15% L929 cell-conditioned medium. Adherent macrophages developed over the next 4 to 5 days, and were used for the experiments starting on day 5 (Keller et al. 1993, Chawla et al. 2001a).
Transient transfection and reporter analyses
A luciferase reporter was co-transfected into the murine macrophage cell line RAW264.7 cells with expression plasmids for β-gal, PPARγ1, and RXRα using Lipofectamine 2000 (Invitrogen). Next day, the cells were treated with 1 μM BRL or DMSO vehicle control for 20–24 h. Luciferase activity was normalized by β-gal. Aox–PPRE–luc was a positive control. The hFR promoter–luciferase constructs P1, P2, and P3 were generously provided by Dr Combadiere's Laboratory (Garin et al. 2002). The P2-3 kb–luciferase plasmid was constructed by PCR cloning of the upstream genomic DNA sequence into the pGL3-Basic vector (Promega).
RNA extraction, real-time quantitative PCR and northern blot
RNA was extracted using TRIZOL (Invitrogen) according to the manufacturer's protocol. For real-time quantitative PCR (RT-QPCR) analysis, RNA was first treated with RNase-free DNaseI using the DNA-free kit (Ambion, TX, USA) to remove all genomic DNA. Then the RNA was reverse transcribed into the first-strand cDNA using the Superscript First-Strand Synthesis System (Invitrogen). A negative control experiment without reverse transcriptase was performed for all samples to exclude genomic DNA contamination. RT-QPCR (SYBR Green) analyses of the cDNA were performed using an Applied Biosystems 7700 Sequence Detection System. Primer sequences for mouse FR were 5′-ATCAGCATCGACCGGTACCT-3′ (forward) and 5′-GTGACACCGTGCTGCACTGT-3′ (reverse). Each reaction was performed in triplicate or quadruplicate in a 384-well format. The expression of each gene was normalized to L19 expression. For the northern blot analysis, total RNA (10 μg) was separated on formaldehyde agarose gel and transferred to Hybond N+ nylon membrane (Amersham Pharmacia Biotech). cDNA probes were isolated from IMAGE clones and were labeled with a High Prime kit (Roche) and hybridized with the membrane at 42 °C overnight. After washing, the blot was exposed to a film and quantified by PhosphorImager.
Immunofluorescence staining
The cells were fixed in 2% paraformaldehyde at 4 °C for 30 min, washed, and stained with rabbit anti-hFR or rabbit anti-hFKN primary antibodies (Torrey Pines Biolabs, East Orange, NJ, USA) at 1:200 for 1 h (Hosokawa et al. 2005, Jamieson et al. 2008). Following two washes, the cells were stained with FITC-conjugated goat anti-rabbit IgG secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania, USA) at 1:1000 for 1 h. After two washes, cover slips were mounted with the Vectashield medium containing DAPI (Vector Laboratories Burlingame, CA, USA). Negative control experiments using a nonimmune IgG primary antibody, or a secondary antibody alone without a primary antibody, were performed under the same staining condition to exclude any background signal.
Flow cytometry
Macrophages were cultured in petri dishes and were then detached for fluorescence-activated cell sorting (FACS) analysis with ice-cold PBS containing 5 mM EDTA. All samples were blocked with mouse IgG for 15 min and were then incubated for 15 min at 4 °C with FITC-conjugated antibodies F4/80 (Serotec, Raleigh, NC, USA) or CD11b (Mac-1) (BD Pharmingen, San Diego, CA, USA). Propidium iodide was used for live cell gating. Samples were analyzed on the Becton–Dickinson FACScan using CellQuest software. A negative control experiment using a FITC-conjugated nonimmune IgG was performed under the same condition to determine the baseline.
Results
Rosiglitazone activation of PPARγ down-regulates FR in macrophages
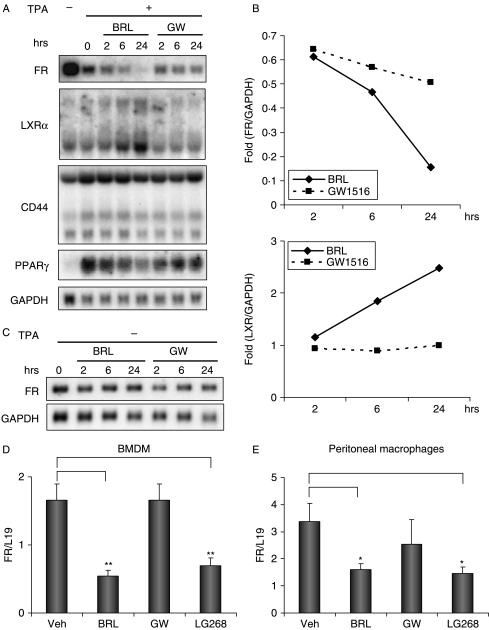
We investigated the effect of PPARγ activation by rosiglitazone on cytokine expression in the THP-1 human monocyte cell line and the TPA-differentiated THP-1 macrophages. The RNA expression of FR in TPA-differentiated THP-1 macrophages was inhibited by BRL in a time-dependent manner (Fig. 1A and B). After 24-h treatment, BRL reduced the FR expression by 84%. As a positive control, BRL induced the expression of LXRs by 2·2-fold as reported previously (Chawla et al. 2001b). In contrast, the PPARδ agonist GW501516 did not induce LXRs expression, but suppressed FR expression (49%). In addition, the expression of CD44, another cell adhesion receptor for hyaluronic acid, was not significantly affected by BRL or GW501516 treatment. Furthermore, the TPA-induced PPARγ expression was also suppressed by 29% after 24-h treatment of BRL, but not by GW501516 treatment. Interestingly, the suppression of FR expression by BRL occurred only in the THP-1 macrophages but not in the THP-1 monocyte precursors in the absence of TPA-induced macrophage differentiation (Fig. 1C).
Figure 1.
Rosiglitazone activation of PPARγ down-regulates FR in macrophages. (A) Northern blot analyses of RNA expression in TPA-differentiated THP-1 macrophages. THP-1 cells were differentiated with TPA (40 ng/ml) for 48 h before being treated with rosiglitazone (BRL, 1 μM) or GW501516 (GW, 0·1 μM) for the indicated amount of time. RNA was isolated and analyzed by northern blot using gene-specific probes. (B) Quantification of the northern blot analyses of FR and LXR in 1A with PhosphorImager. The expression of FR and LXR was normalized by the expression of GADPH. (C) Northern blot analyses of RNA expression in undifferentiated THP-1 monocytes. THP-1 cells were treated with rosiglitazone (BRL, 1 μM) or GW501516 (GW, 0·1 μM) for the indicated amount of time in the absence of TPA. RNA was isolated and analyzed by northern blot using gene-specific probes. (D and E) RNA expression analysis of FR in bone marrow-derived macrophages (BMDMs) (D) and thioglycollate-elicited peritoneal macrophages (E). Primary mouse macrophages were treated with rosiglitazone (BRL, 1 μM), GW501516 (GW, 0·1 μM), or LG100268 (LG268, 0·1 μM) for 24 h. RNA was isolated and analyzed by RT-QPCR. FR expression was normalized by the expression of the ribosomal protein gene L19. All experiments were repeated at least three times, and representative results are shown. *Indicates P<0·05; **indicates P<0·01.
We next examined the effect of PPARγ activation by BRL on FR expression in primary macrophages that were differentiated ex vivo. First, mouse bone marrow cells were differentiated into macrophages (BMDMs), and treated with BRL, GW501516, LG268 (an RXRα agonist), or vehicle control for 24 h. Both BRL and LG268, but not GW501516, suppressed FR expression (Fig. 1D). Secondly, mouse peritoneal macrophages were elicited with thioglycollate and were cultured in the presence of each ligand or vehicle control for 24 h. Consistently, FR expression was inhibited by both BRL and LG268, while GW501516 had much less suppressive effect (Fig. 1E). These results suggested that the activation of the PPARγ–RXRα receptor complex by their agonists, BRL and LG268, results in the down-regulation of the transcription of the gene encoding the FR in both human and murine macrophages.
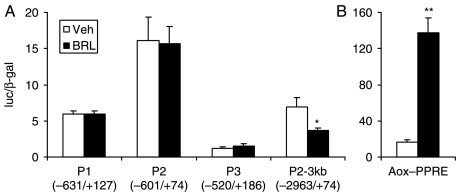
Rosiglitazone-activated PPARγ suppresses the upstream FR promoter
To test if PPARγ directly regulates the promoter for the FR gene, we performed transient transfection and reporter analysis in the murine macrophage cell line RAW264.7 cells. The promoter and upstream genomic DNA region for the human FR gene has been sequenced and characterized (Garin et al. 2002). Expression vectors for PPARγ and RXRα were co-transfected with a luciferase reporter driven by an FR promoter. First, we tested the three proximal promoter regions described by Garin et al. (P1, P2, and P3). As shown in Fig. 2A, these promoter fragments were not regulated by BRL treatment. To test the upstream promoter region, we cloned a 3 kb genomic DNA fragment into the luciferase reporter construct (P2-3 kb). Luciferase expression from this larger promoter was suppressed by BRL by 47%, suggesting that the PPARγ–RXRα receptor complex functions through the upstream FR promoter region to exert the inhibition of its transcription. As a positive control, luciferase expression from the PPRE identified in the acyl-CoA oxidase promoter (Aox–PPRE) was induced by BRL by 8-fold (Fig. 2B).
Figure 2.
Rosiglitazone-activated PPARγ suppresses the upstream FR promoter. (A) Transient transfection analysis of the human FR promoter-driven luciferase reporters in the RAW264.7 cells. Cells were transfected with plasmids for receptors and reporters for 20–24 h before being treated with BRL (1 μM) or vehicle control. Reporter activity assays were performed 20–24 h later. Luciferase activity was normalized to an internal β-galactosidase control. The position of the genomic DNA fragment relative to the transcriptional start site in each promoter construct is shown in parentheses. (B) Transient transfection analysis of the acyl-CoA oxidase (Aox)–PPRE-driven luciferase reporter as a positive control for BRL-mediated PPARγ activation. For all experiments, n=3; *indicates P<0·05; **indicates P<0·01.
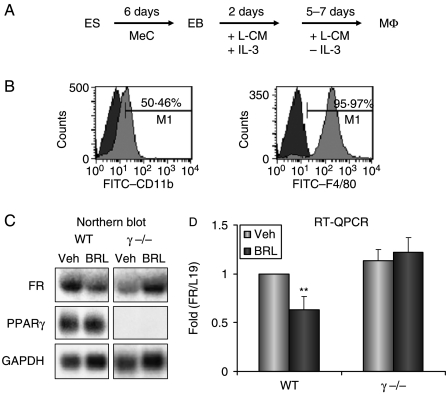
Rosiglitazone suppression of FR expression is PPARγ dependent
We next examined whether the inhibitory effect of BRL on FR expression was PPARγ dependent or PPARγ independent. Since the complete deletion of PPARγ in mice results in embryonic lethality (Barak et al. 1999), we differentiated macrophages from PPARγ−/− or wild-type ES cells as described previously (Fig. 3A; Chawla et al. 2001a). As shown in Fig. 3B, ES cells were efficiently differentiated into macrophages, evidenced by the expression of macrophage cell surface markers F4/80 and CD11b by FACS analysis. Both northern blot (Fig. 3C) and RT-QPCR analyses (Fig. 3D) demonstrated that BRL inhibited FR expression only in the wild-type macrophages but not in the PPARγ−/− macrophages. This indicates that the suppression of FR transcription by BRL is a PPARγ-dependent effect, rather than a receptor-independent effect.
Figure 3.
Rosiglitazone suppression of FR expression is PPARγ dependent. (A) A schematic diagram for the macrophage differentiation from embryonic stem cells. ES, embryonic stem cell; EB, embryoid body; MeC, methylcellulose; L-CM, L-conditioned medium; Mϕ, macrophage. (B) Flow cytometry analysis of the ES-derived macrophages with FITC-conjugated antibodies for macrophage cell surface markers (F4/80 or CD11b, light color histogram in the front layer) or FITC-conjugated nonimmune IgG isotype control (dark color histogram in the back layer). The histogram for CD11b (left) or F4/80 (right) is overlaid on the histogram for the nonimmune IgG isotype control. The percentage of the cells positive for each macrophage marker relative to the isotype control is illustrated. (C and D) Northern blot (C) and RT-QPCR (D) analyses of RNA expression in wild-type or PPARγ−/− macrophages treated with BRL or vehicle control for 24 h. In the northen blot analysis (C), BRL suppressed FR expression (normalized by GAPDH) by 81% in the wild-type macrophages, while increased FR expression (normalized by GAPDH) by 12% in the PPARγ−/− macrophages. For all the experiments, n=3; **indicates P<0·01.
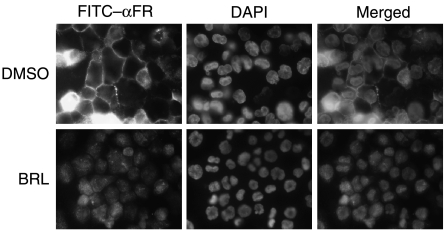
Rosiglitazone activation of PPARγ disrupts the membrane translocation of FR in macrophages
The function of the FR requires its plasma membrane translocation for ligand binding and signal transduction. We next examined whether BRL activation of PPARγ altered the subcellular localization of FR. Immunofluorescent staining using an antibody against the human FR demonstrated that BRL treatment reduced not only the receptor expression but also its membrane association in THP-1 macrophages (Fig. 4). In the vehicle-treated cells, the majority of the receptors were at the plasma membrane, demonstrated by the bright ring at the cell junctions. In contrast, in the BRL-treated cells, this bright ring was absent, and most of the receptors resided in the cytoplasm and the nucleus. This result suggests that BRL-activated PPARγ employs a second mechanism to inhibit FR function in macrophages by hampering its plasma membrane translocation.
Figure 4.
Rosiglitazone activation of PPARγ disrupts the membrane translocation of FR in macrophages. TPA-differentiated THP-1 macrophages were treated with BRL or DMSO control for 24 h and stained with a rabbit anti-hFR primary antibody followed by a FITC-conjugated goat anti-rabbit IgG secondary antibody. The nuclei were counterstained with DAPI. Negative control experiments were performed using a nonimmune IgG primary antibody, or a secondary antibody alone without a primary antibody, and no background signal was detected (blank images not shown). All experiments were repeated at least three times, and representative results are shown.
Rosiglitazone activation of PPARγ prevents FKN nuclear export in endothelial cells
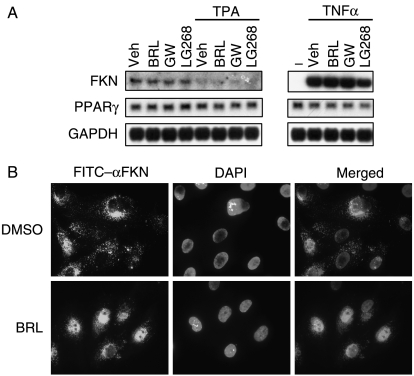
The migration and adhesion of macrophages that express FR also depend on the FKN ligand expressed on the cells in the inflamed tissues, such as endothelial cells. We next examined if BRL treatment regulates the expression and subcellular localization of FKN in the human endothelial cell line HUVEC. First, we measured the RNA expression of FKN using northern blot analysis. FKN was dramatically induced by TNFα by 48-fold, while it was down-regulated by TPA by 76% (Fig. 5A). LG268 treatment for 24 h suppressed the TNFα-induced FKN expression by 30%. In contrast, neither BRL nor GW501516 had an effect on the FKN expression (Fig. 5A).
Figure 5.
Rosiglitazone activation of PPARγ prevents FKN nuclear export in endothelial cells. (A) Northern blot analysis of RNA expression in HUVECs. The cells were treated with TPA (40 ng/ml) or TNFα (10 ng/ml) for 24 h in the presence of DMSO vehicle control, BRL (1 μM), GW501516 (0·1 μM), or LG100268 (0·1 μM). (B) Immunofluorescence analysis of the subcellular localization of FKN in HUVECs. The cells were treated with BRL or DMSO control for 24 h and stained with a rabbit anti-hFKN primary antibody followed by a FITC-conjugated goat anti-rabbit IgG secondary antibody. The nuclei were counterstained with DAPI. Negative control experiments were performed using a nonimmune IgG primary antibody, or a secondary antibody alone without a primary antibody, and no background signal was detected (blank images not shown). All experiments were repeated at least three times, and representative results are shown.
However, immunofluorescent staining using an antibody against the human FKN demonstrated that BRL treatment did alter its subcellular localization in HUVECs (Fig. 5B). In the vehicle-treated cells, FKN was concentrated more in the cytoplasm than in the nucleus. In contrast, in the BRL-treated cells, FKN was mainly localized in the nucleus. This result suggests that BRL activation of PPARγ may dampen the FKN/FR signaling by impeding FKN nuclear export.
Discussion
In this study, we have identified a novel link between PPARγ and the FKN/FR signaling. On one hand, the activation of PPARγ by rosiglitazone in macrophages not only represses the transcription of the FR gene, but also prevents the plasma membrane translocation of FR protein. On the other hand, the activation of PPARγ by rosiglitazone in endothelial cells also impedes the nuclear export of FKN. Together, these findings indicate a previously unrecognized mechanism that may contribute to the anti-inflammatory effect of PPARγ.
We have found that BRL-activated PPARγ acts through an upstream promoter region to suppress the transcription of the FR gene in macrophages. A recent study proposed a model for the molecular events that underlie PPARγ-mediated transrepression of inflammatory genes in macrophages (Pascual et al. 2005). Activation of PPARγ inhibits inflammatory gene expression by preventing the inflammatory signal-specific removal of the co-repressor complex. In future study, it would be interesting to test whether PPARγ regulates the FR promoter via this transrepression mechanism.
Our data have demonstrated that BRL-activated PPARγ also prevents FR plasma membrane translocation, which is pivotal for its function as a chemoattractant and adhesion molecule. Human genetic studies identified five SNPs in the FR coding sequence, all of which were in the transmembrane domains of the receptor (Faure et al. 2000). Two of the mutations (V249I and T280M) were associated with increased susceptibility to HIV infection and accelerated progression to AIDS, as well as reduced risk of acute coronary events, potentially due to a significant decrease in the number of FKN-binding sites per cell (Moatti et al. 2001). T280M homozygosity was also found to be more frequent in AMD patients, which is likely due to impaired monocyte chemotaxis (Combadiere et al. 2007). Furthermore, the T280M mutation was independently associated with a lower risk of cardiovascular disease, possibly as the result of reduced cell–cell adhesion under physiological shear conditions (McDermott et al. 2003). Collectively, these studies demonstrated that the FR transmembrane domain is critical for the FKN-mediated chemotaxis and cell adhesion. Our finding that PPARγ activation can disrupt FR membrane association in macrophages suggests a secondary but important mechanism by which PPARγ inhibits FR function.
We have shown that although BRL-activated PPARγ does not affect FKN expression, it does alter FKN subcellular localization. BRL treatment prevents the nuclear export of FKN, resulting in its nuclear accumulation. This suggests that PPARγ activation can impede the FKN/FR signaling by an additional mechanism in the endothelial cells where it decreases the membrane association and/or secretion of FKN, thus reducing leukocyte migration and adhesion. In addition, the RXRα agonist LG100268, but not BRL or GW501516, down-regulated the TNFα-induced FKN expression in HUVECs by 30%, suggesting that it may dimerize and activate a nuclear receptor other than PPARγ and PPARδ to inhibit FKN transcription.
Emerging evidence reveals that PPARγ is a key regulator of both lipid metabolism and inflammation. The ability to integrate metabolic signaling and inflammatory signaling makes PPARγ an attractive target for intervention in human metabolic diseases and immune disorders. Recent studies demonstrated that the FKN/FR signaling plays an important role in leukocyte migration and adhesion to inflammatory sites, thereby regulating disease processes such as atherosclerosis. Our study revealed a novel link between PPARγ activation and FKN/FR signaling, providing a potential mechanism underlying the anti-inflammatory role of PPARγ. These findings may enhance our understanding of the effect of the diabetic drug rosiglitazone on diseases including atherosclerosis, type II diabetes, AIDS, and AMD.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the funding from Howard Hughes Medical Institute, NIH grants (HD027183, DK057978, and HL56989), and a postdoctoral fellowship from American Cancer Society (PF-03-081-01-TBE).
Acknowledgements
We would like to thank Dr Combadiere (France) for providing the hFR–luciferase constructs; and Lita Ong, Sally Ganley, and Anittra Gray for administrative assistance. R M Evans is an investigator of the Howard Hughes Medical Institute at the Salk Institute and March of Dimes Chair in Molecular and Developmental Biology. Y Wan is a Virginia Murchison Linthicum Scholar in Medical Research at the University of Texas Southwestern Medical Center.
References
- Adachi M, Kurotani R, Morimura K, Shah Y, Sanford M, Madison BB, Gumucio DL, Marin HE, Peters JM, Young HA, et al. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut. 2006;55:1104–1113. doi: 10.1136/gut.2005.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR-gamma is required for placental, cardiac, and adipose tissue development. Molecular Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nature Medicine. 2001a;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Molecular Cell. 2001b;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. Journal of Clinical Investigation. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nature Medicine. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Faure S, Meyer L, Costagliola D, Vaneensberghe C, Genin E, Autran B, Delfraissy JF, McDermott DH, Murphy PM, Debre P, et al. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274–2277. doi: 10.1126/science.287.5461.2274. [DOI] [PubMed] [Google Scholar]
- Garin A, Pellet P, Deterre P, Debre P, Combadiere C. Cloning and functional characterization of the human fractalkine receptor promoter regions. Biochemical Journal. 2002;368:753–760. doi: 10.1042/BJ20020951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa Y, Nakanishi T, Yamaguchi D, Nakae H, Matsuo T. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, in periodontal diseased tissue. Clinical and Experimental Immunology. 2005;139:506–512. doi: 10.1111/j.1365-2249.2005.02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson WL, Shimizu S, D'Ambrosio JA, Meucci O, Fatatis A. CX3CR1 is expressed by prostate epithelial cells and androgens regulate the levels of CX3CL1/fractalkine in the bone marrow: potential role in prostate cancer bone tropism. Cancer Research. 2008;68:1715–1722. doi: 10.1158/0008-5472.CAN-07-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Molecular and Cellular Biology. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. Journal of Clinical Investigation. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, D'Agostino RB, O'Donnell CJ, Patel DD, et al. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. Journal of Clinical Investigation. 2003;111:1241–1250. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moatti D, Faure S, Fumeron F, Amara Mel W, Seknadji P, McDermott DH, Debre P, Aumont MC, Murphy PM, de Prost D, et al. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- Nanki T, Urasaki Y, Imai T, Nishimura M, Muramoto K, Kubota T, Miyasaka N. Inhibition of fractalkine ameliorates murine collagen-induced arthritis. Journal of Immunology. 2004;173:7010–7016. doi: 10.4049/jimmunol.173.11.7010. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah YM, Morimura K, Gonzalez FJ. Expression of peroxisome proliferator-activated receptor-gamma in macrophage suppresses experimentally induced colitis. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2007;292:G657–G666. doi: 10.1152/ajpgi.00381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Saghatelian A, Chong LW, Zhang CL, Cravatt BF, Evans RM. Maternal PPAR gamma protects nursing neonates by suppressing the production of inflammatory milk. Genes and Development. 2007;21:1895–1908. doi: 10.1101/gad.1567207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson TM, Wahli W. Peroxisome proliferator-activated receptor agonists. Current Opinion in Chemical Biology. 1997;1:235–241. doi: 10.1016/s1367-5931(97)80015-4. [DOI] [PubMed] [Google Scholar]