Abstract
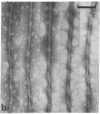
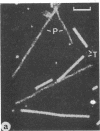
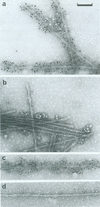
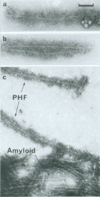
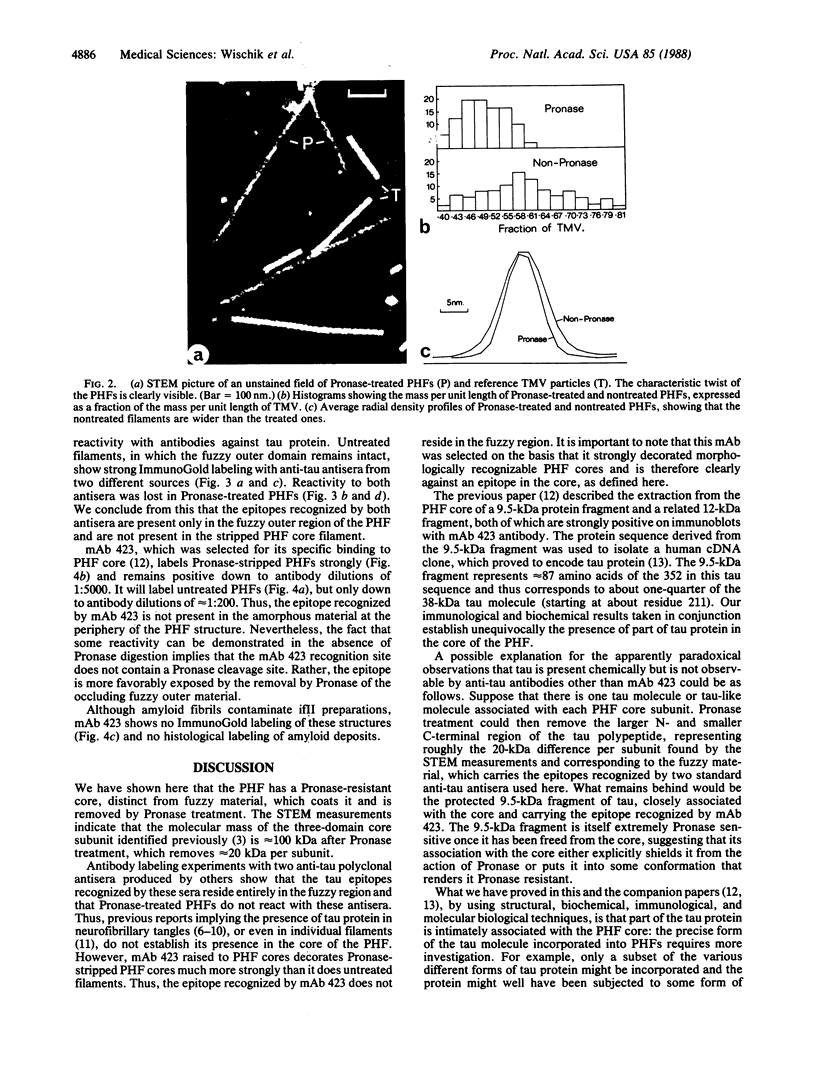
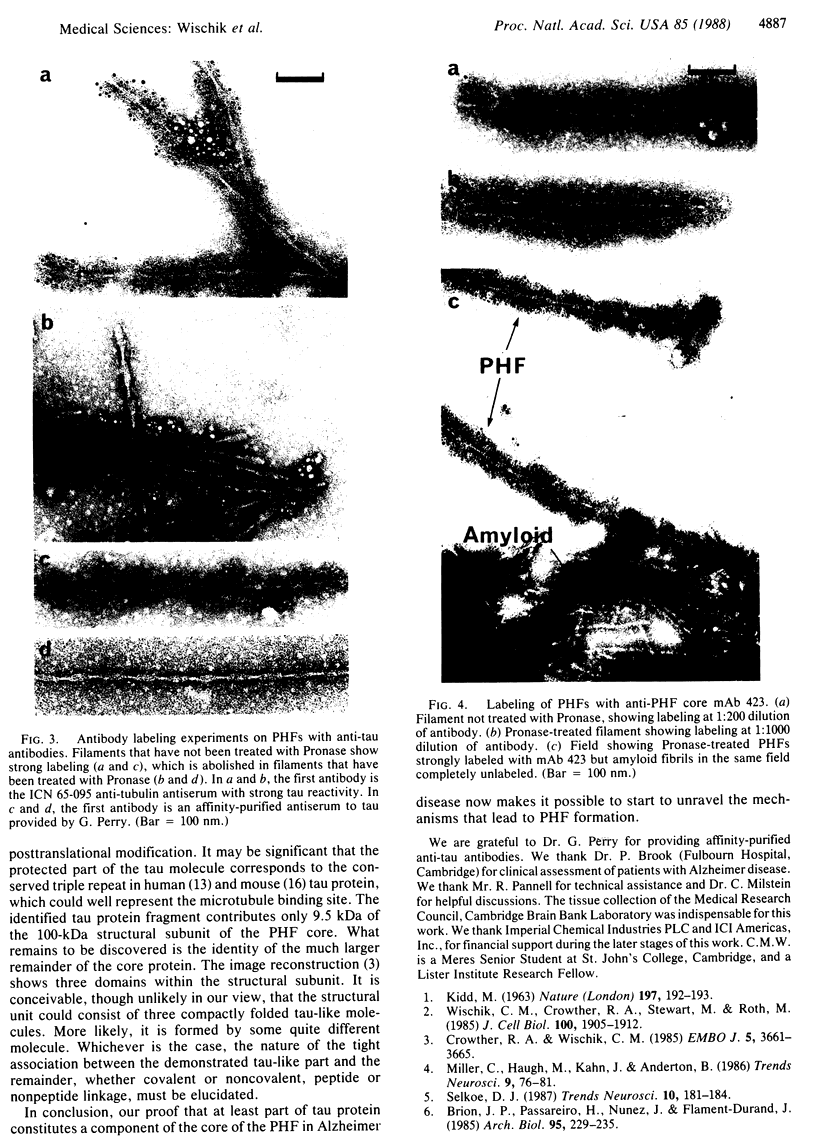
The paired helical filament, the principal constituent of the neurofibrillary tangles characteristic of Alzheimer disease, is shown to consist of two structurally distinct parts. An external fuzzy region can be removed by Pronase treatment to leave a Pronase-resistant morphologically recognizable core. Scanning transmission electron microscopy gives an estimate for the mass per unit length as 79 kDa.nm-1 before Pronase treatment and 65 kDa.nm-1 after treatment. The fuzzy region carries all the epitopes recognized by two different antisera against microtubule-associated protein tau. By contrast, a monoclonal antibody (mAb) we have raised to paired helical filament cores (mAb 423) decorates Pronase-treated filaments much more strongly than it does untreated ones. We have shown in previous papers that the epitope recognized by mAb 423 is carried by a central 9.5-kDa fragment of tau protein, which therefore forms part of the Pronase-resistant core structure. The remainder of the tau protein incorporated into the filaments must contribute part, if not all, of the fuzzy region. The mass per unit length measurements imply that the three-domain structural subunit of the core that we visualized previously by image reconstruction has a molecular mass of approximately equal to 100 kDa.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freeman R., Leonard K. R. Comparative mass measurement of biological macromolecules by scanning transmission electron microscopy. J Microsc. 1981 Jun;122(Pt 3):275–286. doi: 10.1111/j.1365-2818.1981.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Goedert M., Wischik C. M., Crowther R. A., Walker J. E., Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Nukina N., Ihara Y. One of the antigenic determinants of paired helical filaments is related to tau protein. J Biochem. 1986 May;99(5):1541–1544. doi: 10.1093/oxfordjournals.jbchem.a135625. [DOI] [PubMed] [Google Scholar]
- Perry G., Mulvihill P., Manetto V., Autilio-Gambetti L., Gambetti P. Immunocytochemical properties of Alzheimer straight filaments. J Neurosci. 1987 Nov;7(11):3736–3738. doi: 10.1523/JNEUROSCI.07-11-03736.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]