Abstract
Background
D-cycloserine (DCS), a partial glutamate NMDA receptor agonist, enhances extinction of conditioned fear responding in rodents and facilitates exposure-based learning in humans with anxiety disorders.
Objectives
This preliminary study investigates DCS pretreatment on response to cocaine cues in cocaine-dependent subjects.
Methods
Ten cocaine-dependent subjects were randomly assigned to receive either 50 mg DCS or matching placebo two hours before each of two 1-hour cocaine cue exposure sessions one day apart. Heart rate and craving ratings were obtained before and during cue exposure sessions.
Results
There was a trend towards increased craving to cocaine cues in cocaine-dependent individuals after administration of DCS.
Conclusions
The administration of DCS prior to cue exposure sessions may facilitate response activation.
Scientific Significance
While facilitation of extinction-based learning by DCS may have therapeutic potential for cocaine dependence, this drug may exhibit a different profile in cocaine-dependent individuals as compared to those with anxiety disorders.
Keywords: cocaine, craving, cue reactivity, extinction, D-cycloserine
INTRODUCTION
There is considerable evidence suggesting that the glutamate system plays an important role in associative learning and memory. Acute treatment with D-cycloserine (DCS), a partial glutamate NMDA receptor agonist, enhances learning processes underlying extinction of conditioned fear responding in animal studies [1]. Several recent clinical studies have built on this finding by demonstrating that DCS administered before exposure sessions enhances extinction of response to fear-eliciting cues in individuals with anxiety disorders [2–4]. If DCS enhances associative learning in general, it may also enhance the learning that occurs during extinction training for appetitively-conditioned cues. Preliminary support for this comes from recent investigations in which accelerated extinction of cocaine conditioned place preference (CPP) in rodents was seen when DCS was administered immediately after each extinction trial [5, 6] and a pilot study in humans in which DCS plus exposure therapy significantly attenuated response to smoking cues in nicotine-dependent individuals [7]. Thus, despite the opposite motivational valence of fear and drug cue conditioning and extinction, glutamate may play a critical role in both types of associative learning.
In an attempt to follow-up on the promising findings concerning DCS in anxiety disorders in humans and extend the recent preclinical cocaine-related findings, this pilot study was conducted to explore to the impact of DCS on response to repeated presentation of cocaine cues (i.e., extinction training sessions) in cocaine-dependent individuals.
METHODS AND MATERIALS
Subjects
Subjects were recruited primarily via media advertisements over a three-month period. Written informed consent was obtained prior to study assessment. All study procedures and consent forms were approved by Medical University of South Carolina’s (MUSC) Institutional Review Board. Men and women 18 years of age and older were eligible for participation. Subjects were required to meet DSM-IV criteria for cocaine dependence, agree to remain abstinent from all drugs of abuse (except nicotine) for 24 hours immediately prior to the laboratory sessions, and consent to random assignment to DCS or placebo. Exclusion criteria included (1) medical conditions that might interfere with the safe conduct of the study or collection of physiological data; (2) psychotic, affective or primary anxiety disorder; (3) pregnancy, nursing, or ineffective means of birth control; (4) current treatment with a medication that could interact with DCS; (5) hypersensitivity to DCS; and (6) DSM-IV criteria for substance dependence except cocaine and nicotine within the past 60 days. Participants were non-treatment seekers.
Procedures
Patients meeting preliminary screening criteria were evaluated for study eligibility using the SCID-IV [8]. A history and physical examination and serum chemistries were obtained. Substance use was assessed using the Timeline Follow-Back [9]. After inclusion/exclusion criteria were satisfied, subjects were scheduled for laboratory sessions in the MUSC General Clinical Research Center (GCRC). Subjects were admitted to the GCRC at 1200h on test days. Cigarette smokers were provided with a nicotine patch. After pregnancy and urine drug screen testing, subjects were administered either a 50 mg capsule of DCS or matching placebo (PBO) via random assignment using a permuted block random numbers table. Each subject received the same medication (DCS or PBO) two hours prior to each of two cue exposure sessions. The 50 mg dose and pre-session timing was chosen based on previous randomized, double-blind, PBO-controlled trials demonstrating these procedures to be effective in enhancing the attenuation of fear/anxiety responses during exposure therapy in persons with acrophobia [4] and social anxiety disorder [2]. Two baseline assessments were collected (1340h and 1355h) using the Craving/Distress/Mood Scale designed to rapidly assess craving and other mood feeling states during testing [10] via 100-mm visual analog scales anchored with adjectival modifiers (“not at all” to “extremely”). Baseline measures of heart rate (HR) were also assessed at these times. At 1400h, exposure to cocaine cues consisting of a small bag of the subject’s preferred style of simulated cocaine (powder or crack), crack pipe/lighter or razor blade/mirror, and money ($20 bill) began. The subject was asked to inspect and handle the cues for a five-minute period, then view a ten-minute film depicting cocaine use in a variety of settings. This sequence was repeated four times over a one-hour period. Subjective and HR measures were obtained at 15, 30, 45 and 60 minutes following the onset of cue exposure. Subjects remained in the hospital for an overnight stay and participated in a second laboratory session the next day, in which procedures were identical. Subjects returned for a follow-up visit one week later, in which cocaine craving was assessed without prior administration of DCS or PBO and cocaine use in the previous week was assessed using the TLFB.
Data Analysis
Group differences of demographic variables were evaluated with the non-parametric Mann-Whitney rank-sum test. The primary dependent measures were subjective craving rating and HR, which were also compared with the Mann-Whitney two-sample rank-sum test. An average of the two baseline assessments (20 and 5 minutes before presentation of the cues) was computed for each measure and compared between medication conditions, as were data from each subsequent time point. Findings are considered significant if p-values were less than 0.05 and marginally significant (denoted as a ‘trend’) if less than 0.10.
RESULTS
The demographic characteristics of the 10 subjects (5 DCS; 5 PBO) who completed the study are shown in Table 1. There were no significant between-group differences in any demographic variables, although a trend for higher age in the DCS-treated group was noted (p=0.09). There were no significant differences in cocaine-use variables including cocaine use in the month prior to testing.
Table 1.
Demographic Characteristics of Participants
| Treatment Group | |||
|---|---|---|---|
| Demographic Characteristic | DCS (N=5) | Placebo (N=5) | p-value |
| Age | |||
| Mean (SD) | 42.4 (6.1) | 28.4 (10.5) | 0.09 |
| Gender (male) | |||
| N (%) | 4 (80) | 4 (80) | 0.78 |
| Race (African American) | |||
| N (%) | 4 (80) | 4 (80) | 0.78 |
| Married (not married) | |||
| N (%) | 5 (100) | 5 (100) | 1.00 |
| Employed | |||
| N (%) | 1(25) | 4 (80) | 0.17 |
| Education N (%) | |||
| 7th–12th grade | 2 (40) | 3 (60) | 0.89 |
| High school graduate or above | 3 (60) | 2 (40) | |
| Years Since First Cocaine Use | |||
| Mean (SD) | 13.5 (5.07) | 7.5 (10.41) | 0.31 |
| # Days Cocaine Use: 30 Days Prior | |||
| Mean (SD) | 17 (9) | 11 (3) | 0.48 |
| # Days Cocaine Use: 7 Days Post | |||
| Mean (SD) | 3 (2.2) | 2 (1.4) | 0.74 |
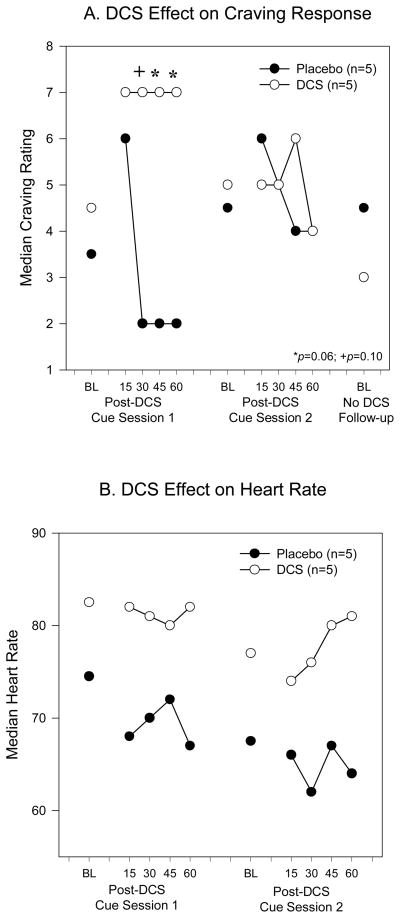
There was no significant difference between the DCS- and PBO-treated groups in the craving rating two hours after DCS/PBO administration, but before the cue presentation [DCS: 4.5 (4), PBO: 3.5 (4), Median (Interquartile Range), p=0.19; Figure 1A]. There was a trend towards higher craving ratings in the DCS group compared to the PBO group at 30 [DCS: 7 (4), PBO: 2 (5); p=0.10], 45 [DCS: 2 (3), PBO: 7 (5); p=0.06] and 60 [DCS: 7 (4), PBO: 2 (5); p=0.06] minutes after onset of cue presentation during the first session, but there was no difference between the two groups at the 15 minute time point [DCS: 7 (5), PBO: 6 (3)]. The differences in craving ratings between the groups in the second session were not statistically significant[DCS: 5 (5.5), 5 (6), 5 (5), 6 (5), 5 (6); PBO: 4.5 (4.5), 6 (3), 5 (5), 4 (6), 4 (5) at BL, 15, 30, 45, 60 minutes respectively]. There was no significant between-group difference in the craving rating obtained during the follow-up visit [DCS: 3 (2.8), PBO: 4.5 (5.5)].
Figure 1.
Effect of DCS on Cocaine Cue Reactivity. DCS administered 2h prior to cocaine cue exposure sessions resulted in increased craving ratings (A), compared to placebo, during the first but not second cue exposure session. Craving ratings were not significantly different between the groups during baseline measures. Heart rate (B) was elevated after DCS, compared to placebo, although this difference did not reach statistical significance.
The heart rate data before and after each cue presentation are shown in Figure 1B. An elevation in median heart rate in the DCS-treated group can be observed as compared to the PBO-treated group, although these differences did not reach statistical significance for either session [Session 1-DCS: 82.5 (17), 82 (19), 81 (12), 80 (16), 82 (15); PBO: 74.5 (17), 68 (12), 70 (13), 72 (11), 67 (6) and Session 2-DCS: 77 (13.5), 74 (9), 76 (9), 80 (20), 81 (4); PBO: 67.5 (9), 66 (14), 62 (9), 67 (6), 64 (6).]
Since these data were preliminary in nature, a post-hoc power analysis was performed to assess the likelihood of finding significant differences where they do indeed exist. The power for the test performed is 48.9%; a sample size of 10 subjects per group is necessary to obtain 80% power.
DISCUSSION
The data from the present study are preliminary in nature but important to consider in the design of medication-facilitated exposure therapy for the treatment of cocaine dependence. These data suggest that contrary to expectation, DCS administration may acutely increase craving and reactivity to cocaine cues in cocaine-dependent individuals. In the present study, DCS was administered orally two hours before each extinction session. This methodology was modeled after that used in the successful clinical trials in anxiety disorders [2, 4] in which the dosing schedule was designed so that the exposure session and post-exposure memory consolidation period would occur during the time of peak DCS serum levels (two to four hours following oral administration). In the anxiety disorders studies, the acute administration of DCS did not alter physiologic or subjective responses during session one, and effects on anxiety measures were not seen until session two.
The difference in response to cocaine cues between the DCS and PBO groups suggests that DCS administration may acutely stimulate cocaine craving and enhance response to cocaine cues through stimulation of glutamatergic systems. Increasing evidence implicates the role of glutamate in cocaine dependence, and manipulation of glutamate function has been shown to affect motivation for cocaine in cocaine-dependent individuals (for review, see [11]). For example, restoring normal extra-synaptic glutamate levels with N-acetylcysteine decreases cocaine-seeking behavior in rodents [12, 13] and reduces the desire to use cocaine in humans [14]. The role of glutamate in cocaine dependence as compared to anxiety disorders may explain the fact that our study did not produce the hypothesized results and highlights an important consideration in adapting successful treatments across disorders.
In a recently published pilot investigation of DCS-facilitation of extinction of response to smoking cues in nicotine-dependent subjects [7], the timing of DCS administration was also similar to that used in the anxiety disorders studies. Consistent with the anxiety disorders literature, they found no acute increase in response to nicotine cues when DCS was administered one hour before the cue exposure session and a significant attenuation of response during session 2 in the DCS as compared to the placebo group. While the study by Santa Ana and colleagues suggests that DCS can facilitate extinction of appetitively-motivated behaviors, the present study highlights the importance of considering neurobiologic differences across disorders in study design.
In the present study, only one dose of DCS was tested and all subjects received the DCS at the same time relative to the extinction training session. The utility of a different dosage of DCS and a different schedule of DCS dosing relative to the extinction session cannot be ruled out. The timing of DCS dosing relative to the extinction session and amount of time between dosing has been demonstrated to be critical in animal studies of the facilitation of extinction learning in fear conditioning [15, 16] and cocaine CPP [5, 6]. In addition, a recent study of DCS-facilitated extinction learning in rat and monkey cocaine self-administration highlighted not only the importance of dose timing, but also the contribution of arousal to the consolidation of extinction learning [17]. It is possible that the participants in our study experienced intense arousal during presentation of the cues, which has been shown to be disruptive to memory consolidation [18]. Thus, if the dose of DCS could be timed so that it did not overly stimulate subjects, yet was active during memory consolidation, it might have a facilitatory effect on extinction learning in cocaine-dependent individuals. Therefore, while facilitation of extinction-based learning by glutamatergic agents may have therapeutic potential for cocaine dependence, the critical role of glutamate in the reinforcing properties of cocaine may give these agents a different profile in cocaine-dependent individuals and the parameters of DCS administration must be carefully explored.
There are a number of important study limitations. The sample size is small, making the findings preliminary in nature. In addition, no measures of craving were obtained prior to DCS administration. However, at the one-week follow-up visit, craving was assessed for all subjects without DCS or placebo administration. There were no between-group differences in this craving measurement which is the closest thing to a true baseline (without drug administration) measure of craving that we have in this study. It is likely that baseline between-group differences in craving would have been reflected in this rating. While there were no statistically significant differences between the DCS- and PBO-treated subjects in age, years since first cocaine use, or baseline craving, the DCS group was older and subsequently reported longer cocaine-use histories, which may have impacted craving ratings. However, there were no significant differences in cocaine use in the month before and week after study participation and no differences in post-study craving, so it is unlikely that these factors significantly impacted response. Despite these limitations, the findings are intriguing and further work exploring the impact of DCS on cocaine cue responding and extinction using larger samples and different dosing schedules could lead to important treatment developments.
Acknowledgments
NIH/NCRR: 5 M01 RR01070 NIH/NIDA: 5 K24 DA00435
Footnotes
Declaration of Interest: Authors Price, Saladin, Moran, DeSantis and Back have no relevant financial relationships with commercial interests to disclose. Kathleen Brady lists the following: Consultant: Pfizer, Eli Lilly, Abbott, GlaxoSmithKline, Forest, Ovation, Marinus, Novartis; Speakers’ Bureau: Pfizer, Eli Lilly, Abbott, GlaxoSmithKline, Forest; Research Support: Abbott, GlaxoSmithKline, Forest; Scientific Advisory Board: Embera NeuroTherapeutics; Independent Contractor: Medscape; Investigator: Titan Pharmaceuticals. Aimee McRae lists: Research Support: Bristol-Myers Squibb, Eli Lilly, Wyeth Ayerst, Shire, and GlaxoSmithKline.
References
- 1.Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by D-cycloserine: theoretical and clinical implications. Learn Mem. 2004;11(5):510–6. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann SG, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63(3):298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Kushner MG, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62(8):835–8. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Ressler KJ, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136–44.1. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 5.Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172(1):173–8. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202(1–3):403–9. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- 7.Santa Ana EJ, et al. D-Cycloserine attenuates reactivity to smoking cues in nicotine-dependent smokers: A pilot investigation. Drug Alc Dep. 2009;104:220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.First MB, et al. Structured clinical interview for Axis I DSM-IV disorders. 2004 Patient Edition. SCID-I/P, vs 20. [Google Scholar]
- 9.Sobell L, Sobell MB. Timeline follow-back: A technique for assessing self-reported ethanol assumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psychological and biological methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- 10.Childress AR, McLellan AT, O’Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict. 1986;81(5):655–60. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 12.Baker DA, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 13.Moran MM, et al. Cystine/glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine-seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaRowe SD, et al. Is Cocaine Desire Reduced by N-Acetylcysteine? Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 15.Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57(8):841–7. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Quartermain D, et al. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol. 1994;257(1–2):7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- 17.Nic Dhonnchadha BA, et al. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacol. 2009 doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGaugh JL, Roozendall B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]