Abstract
Metabolic change in cancer cells by preferential production of energy through glycolysis is a well-documented characteristic of cancer. However, whether inhibition of glycolysis will enhance the efficacy of radiation therapy is a matter of debate. In this study which uses lung cancer as the model, we demonstrate that the improvement of radiotherapy by 2-deoxy-D-glucose (2DG) is p53-dependent. Based on clonogenic survival data, we show that p53-deficient lung cancer cells (H358) are more sensitive to 2DG treatment when compared to p53 wild-type lung cancer cells (A549). The effective doses of 2DG at 0.5-surviving fraction of A549 and H358 are 17.25 and 4.61 mM, respectively. Importantly, 2DG exhibits a significant radiosensitization effect in A549 cells but not in H358 cells. Treatment with 2DG increases radiation-induced p53 protein levels in A549 cells. siRNA inhibition of p53 in A549 cells reduces the radiosensitization effect of 2DG. Furthermore, ectopic expression of wild-type p53 in H358 cells significantly enhance the radiosensitization effect of 2DG as determined by colony formation assay. In nude mice injected with A549 cells, treatment of 2DG enhances the efficacy of radiation therapy. Together, these results suggest that inhibition of glycolysis may only be beneficial for radiation therapy of cancer expressing wild-type p53.
Keywords: 2-deoxy-D-glucose, p53, radiation, lung cancer, clonogenic survival
Introduction
Lung cancer is the most common cause of cancer deaths both in the United States and worldwide with an approximately 16% 5-year combined survival rate (1,2). Lung cancer can be categorized into two broadly defined groups: small cell lung carcinoma (SCLC) and non-small cell lung cancer (NSCLC) (3). NSCLC accounts for 86% of all lung cancer diagnosed. Radiation and chemotherapy are the primary treatments for non-resectable, locally advanced NSCLC. However, it has been observed that NSCLC often resists both chemo- and radio-therapy and that overexpression of glucose transporter 1 and 3 is associated with poor survival of non-small cell lung cancer (4,5).
It is well established that many tumor cells exhibit altered metabolism compared with normal cells (6). Tumor cells often rely on glycolysis rather than oxidative phosphorylation, even in the presence of oxygen (Warburg effect) (6,7). Accumulating data have demonstrated the selective growth advantage and mechanisms of the glycolytic phenotype of cancer (8,9). Cells from several types of cancer including lung have decreased expression of mitochondrial H+-ATP synthase (β-F1-ATPase), a protein complex required for oxidative phosphorylation (OXPHOS), and a high level of glycolytic enzymes such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and pyruvate kinase (PK) (10,11).
It has been shown that 2-deoxy-D-glucose (2DG), a glycolytic inhibitor, inhibits growth and induces apoptosis in a number of cancer cell lines (12,13). 2DG exhibits a cytotoxic effect in cancer cells, but the same dose spares normal cells (14–17). In addition, the cytotoxic effect of 2DG is greater in cancer cells that exhibit mitochondrial respiratory defects or are hypoxic (18–20). Many mechanisms are postulated to contribute to the antitumor effect of 2DG, including inhibition of glucose transport (21) and hexokinase II activity, depletion of cellular ATP, blockage of cell cycle progression, induction of apoptosis (20), induction of endoplasmic reticulum stress (22,23), and/or induction of oxidative stress (24–26). Combining glycolytic inhibition with conventional therapeutic modalities such as radiation and chemotherapy is a new approach being considered to selectively kill cancer cells. 2DG significantly sensitizes human osteosarcoma and non-small cell lung cancer to adriamycin and paclitaxel in mouse models (27). 2DG sensitizes cancer cells to radiation through mechanisms such as inhibiting DNA repair processes and recovery from potentially lethal damage (28,29), and inducing oxidative stress (24,25). However, in spite of several past attempts to test the role of 2DG in radiation therapy, only one clinical trial study on human cerebral gliomas has demonstrated that 2DG improves the efficacy of radiotherapy (30). Therefore, whether inhibition of glycolysis will enhance the efficacy of radiation therapy is still a matter of debate.
Recently, the tumor suppressor protein p53 has been identified as a key regulator of cell metabolism by its modulation of the balance between the glycolytic and mitochondrial respiratory pathways (31). It has been noted that nutrient deprivation can activate p53 expression through the AMP-activated protein kinase (AMPK) pathway (32). p53 transcriptionally regulates synthesis of cytochrome c oxidase 2 (SCO2), a regulator of the cytochrome c oxidase complex that is essential for mitochondrial respiration (33). Matoba et al (33) showed that p53−/− mice exhibit a decrease in mitochondrial respiration. p53 also downregulates phosphoglycerate mutase (PGM), an enzyme in the glycolytic pathway responsible for the conversion of 3-phosphoglycerate to 2-phosphoglycerate (34). In addition, p53 targets TP53-induced glycolysis and the apoptosis regulator (TIGAR), which functions to direct glucose to the pentose phosphate pathway (PPP), resulting in the generation of NADPH that increases the concentration of reduced glutathione, an antioxidant that scavenges reactive oxygen species (ROS) (35). Because p53 level is increased by radiation in p53 expressing cells, it is possible that the presence of p53 may be the determinant for the radiosensitizing effect of 2DG.
In this study, two non-small cell lung cancer cell lines with p53 wild-type cells (A549) and p53 deficiency (H358) were used to test the hypothesis in vitro that p53 is a critical factor for the radiosensitization effect of 2DG and determine the effect of 2DG in vivo.
Materials and methods
Cell and culture conditions
Human lung cancer cell lines A549 and H358 were obtained from American Type Culture Collection (ATCC). Cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin mixture (Gibco), and 2 mmol/l of L-glutamine (Gibco). Cells were grown in 5% CO2 and air in a humidified 37°C incubator.
Drug and radiation treatment
2-Deoxy-D-glucose (2DG) was purchased from Sigma-Aldrich, Inc. (St. Louis, MO). Stock solutions of 1 M 2DG were dissolved in PBS and diluted in culture medium to the indicated final concentration for cell treatment. A 130 kv X-ray machine (Faxitron X-ray Corporation) was used to radiate cells with a dose rate of 80.0 cGy/min.
Clonogenic survival assay
Cells were trypsinized and plated in triplicate into 6-well plates at 100–500 cells/well. Cells were treated with the indicated concentration of 2DG for 24 h prior to radiation exposure, Twenty-four hours later, the medium containing 2DG was removed and cells were maintained in culture medium. After 8–21 days of incubation, cells were washed and stained with crystal violet, and the colonies containing more than 50 cells were counted. Plating efficiency was calculated by dividing the average number of cell colonies per well by the amount of cells plated. Surviving fractions were calculated by normalization to the plating efficiency of appropriate control groups.
siRNA transfection
Control siRNA and p53 siRNA were purchased from Dharmacon. Cells were trypsinized and plated in triplicate into 12-well plates at 0.5×105 cells/well and transiently transfected with 25 pmol of p53 siRNA or control siRNA by using oligofectamine transfection reagent (Invitrogen) according to the manufacturer's instructions. Twenty-four hours after transfection, cells were treated with 20 mM 2DG for 4 h prior to radiation exposure. After radiation exposure, cells were immediately trypsinized and plated for clonogenic cell survival assay. For Western blot analysis, cells were collected and processed 48 h after radiation treatment.
Adenoviral transduction
Ad5CMVwtp53/eGFP (Adp53) and Ad5CMV/eGFP (AdGFP) were generated as previously described (36). H358 cells were seeded in 100-mm tissue culture dishes at 2×106 cells. Approximately 20 h later, cells were treated with 5 mM 2DG. After 4 h of exposure to 2DG, cells were irradiated with 4 Gy of X-radiation, then immediately trypsinized and plated for clonogenic cell survival and Western blot assay. Twenty-four hours after plating, 50 multiplicities of infection (MOIs) of AdGFP or Adp53 were added into cultured cells for 4 h and then removed. For clonogenic survival assay, 2DG were removed at 48 h after radiation exposure and cells were incubated at 37°C in a humidified atmosphere under 5% CO2 for 21 day. For Western blot analysis, cells were collected and processed at 24 h after incubation with virus particles.
Western blot analysis
Whole cell lysates were separated on 10% SDS-PAGE gel and transferred onto a nitrocellulose membrane. The membrane was incubated with primary antibody, followed by the corresponding secondary antibody. Signals were detected by the enhanced chemiluminescence system (Amersham) and quantified by Quantity One (Bio-Rad). Anti-p53 antibody was purchased from Santa Cruz Biotechnology, anti β-actin antibody from Sigma.
In vivo study
Six week-old athymic female nude mice were obtained from Harlan Sprague-Dawley (Indianapolis, IN, USA). A549 human lung cancer cells (4×106), suspended in 75 µl of FBS free RPMI medium, were subcutaneously injected into the right hind-leg region of mice. Tumors were allowed to grow to 250 mm3, then mice were randomly divided into 4 experimental groups of 10 mice/group consisting of: control, 2DG 3 mg/g × 15 days; radiation, 3 Gy/fraction × 10 days; and combination, 2DG + radiation. In radiation treatment groups, mice were exposed to X-radiation 3 Gy/fraction for 10 days. In the combination group, 2DG was intraperitoneally injected 1 day prior to and 30–45 min prior to radiation. Mice were weighed and tumors were measured weekly. Mice were humanelysacrificed when tumors reached the size of 2000 mm3 in accordance with protocol approved by the University of Kentucky Institutional Animal Care and Use Committee (IACUC). Radiation control groups and groups treated with both radiation and 2DG were terminated even though tumors never reached an average of 2000 mm3.
Statistical analysis
Statistical analysis was performed using one-way ANOVA (for comparison of multiple groups). For in vivo experiment, we used Student's t-test (for comparison of two groups).
Results
2DG induces radiosensitization of p53 wild-type non-small cell lung cancer cells but not of p53-deficient cells
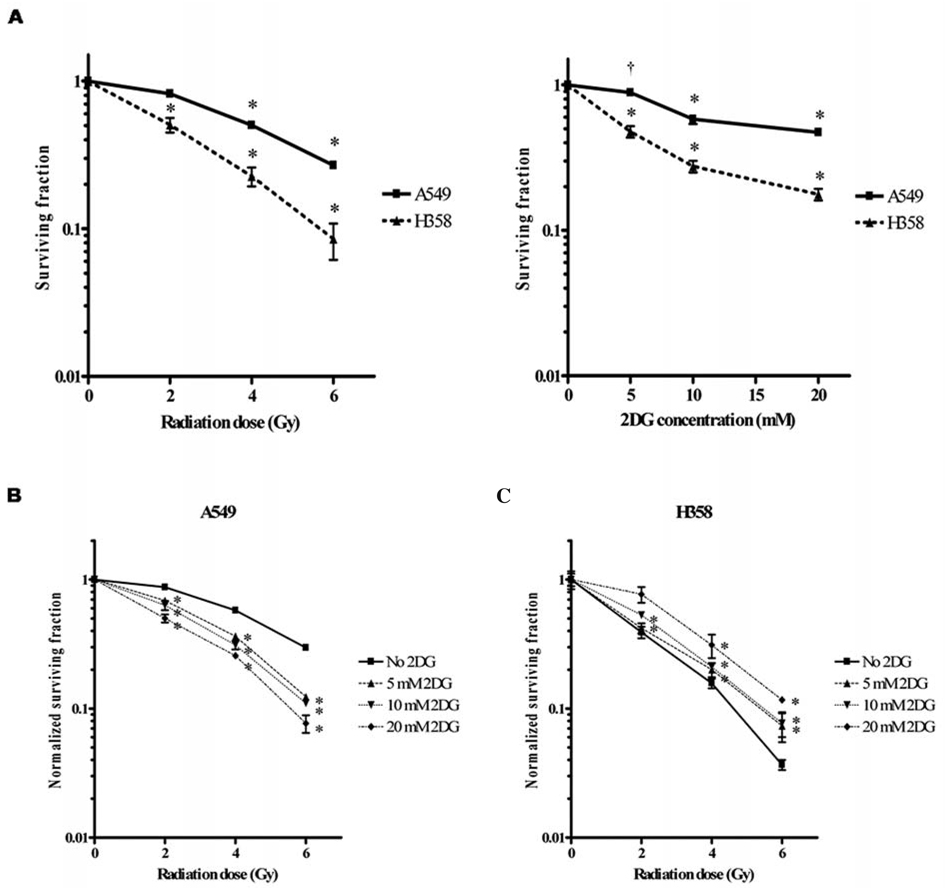
To evaluate the toxicity of 2DG combined with radiation treatment, A549 and H358 were cultured and plated in the same RPMI-1640 medium for clonogenic cell survival assay. Cells were exposed to graded doses of X-radiation (0–6 Gy) or increasing dosesof 2DG (0–20 mM) and allowed to form colonies. Fig. 1A shows the surviving fraction of cells treated with radiation alone or 2DG alone. Both cell lines showed decreased cell viability with radiation or 2DG treatment in a dose-dependent manner. Compared to p53 wild-type cells (A549) at 0.5-surviving fraction, p53-deficient lung cancer cells (H358) were 2 times more sensitive to radiation and 4 times more sensitive to 2DG treatment (Fig. 1A). The effective doses of 2DG at 0.5-surviving fraction of A549 and H358 are 17.25 and 4.61 mM, respectively. Thus, H358 cells were more sensitive to 2DG than to radiation compared to A549 cells. When the radiation sensitivity of A549 and H358 cells was evaluated in the absence or presence of increasing concentrations of 2DG, 2DG significantly increased radiosensitivity of A549 in a dose-dependent manner (p<0.001) (Fig. 1B). In contrast, treatment that combined 2DG and radiation conferred an antagonistic effect in H358 cells (Fig. 1C). These results suggest that the radiation affect of 2DG on lung cancer cells is not enhanced when p53 is absent.
Figure 1.
2DG induces radiosensitization of p53 wild-type cells (A549) but not of p53-deficient cells (H358). A, The cytotoxic effect of radiation and/or 2DG on survival of A549 and H358 cells was determined by clonogenic survival assay. B and C, Combined effect of radiation and 2DG on survival of A549 cells (B) and H358 cells (C). 2DG was applied 24 h prior to radiation treatment and replaced with fresh media 24 h following radiation. Colonies were counted 8–21 days following treatment. Values shown are the mean ± SE. *p<0.001; †p<0.05 compared with no 2DG control group.
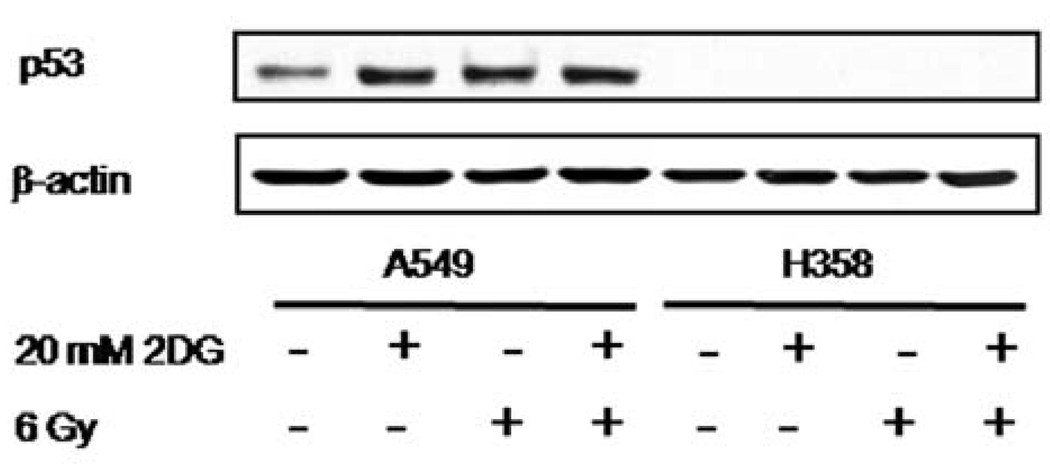
2DG increases p53 expression in A549 cells
To investigate the effect of 2DG and radiation on p53 expression, the p53 protein level was determined by Western blotting (Fig. 2). p53 expression was undetectable in H358. Treatment with 20 mM of 2DG or 6 Gy radiation alone or in combination caused an increase of p53 level in A549 cells.
Figure 2.
2DG increases p53 expression in A549 cells. A549 and H358 cells were treated with or without 20 mM of 2DG for 24 h before radiation. Cells were then collected and the level of p53 was determined by Western blot analysis.
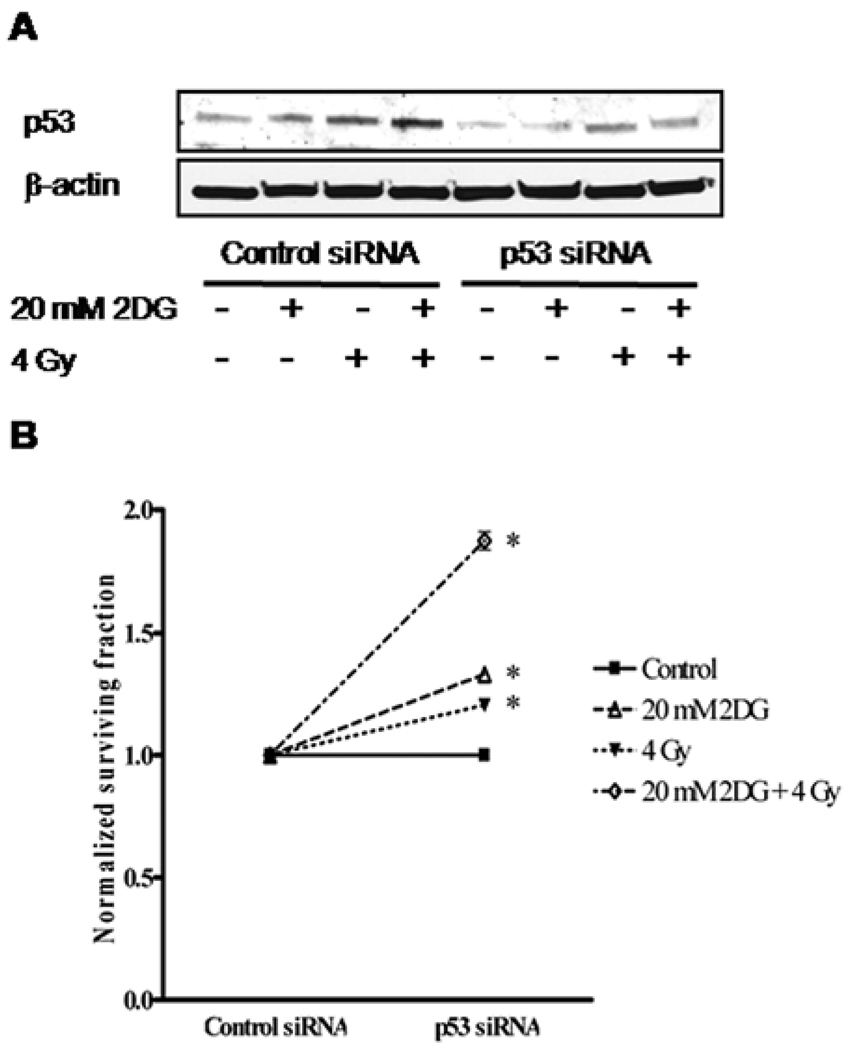
siRNA inhibition of p53 in A549 cells abolishes the radiosensitization effect of 2DG
To determine the role of p53 in 2DG-mediated radiosensitization of human lung cancer cells, p53 siRNA was transfected into p53 wild-type A549 cells, and cell viability after radiation and 2DG treatments was determined by clonogenic survival assay. The effect of p53 siRNA was confirmed by Western blots (Fig. 3A). Inhibition of p53 expression by p53 siRNA significantly increased cell survival in all treatment groups (p<0.001) (Fig. 3B). Compared with the cells transfected with control siRNA, the cells in which p53 expression was suppressed showed a normalized cell survival increase of 33% when treated with 20 mM of 2DG alone, 20% when treated with 4 Gy of radiation alone, and 87% when treated with 2DG combined with radiation. These results demonstrate that suppression of p53 level in A549 cells reduced the radiosensitization effect of 2DG in the combination treatment of 2DG and radiation.
Figure 3.
Reduction of the radiosensitization effect of 2DG by inhibition of p53 in A549 cells. Cells were transfected with p53 siRNA or control siRNA followed by pretreatment with 20 mM 2DG for 4 h prior to 4-Gy radiation exposure. Cell were then immediately trypsinized and plated for clonogenic survival and Western blot assay. A, The reduction of p53 by siRNA targeting was confirmed by Western blot analysis. B, Cell survival at 4 Gy (SF4) was quantified by clonogenic survival assay. Survival data were normalized to the corresponding control group. Data represent mean ± SE, *p<0.001 compared with control siRNA-transfected groups.
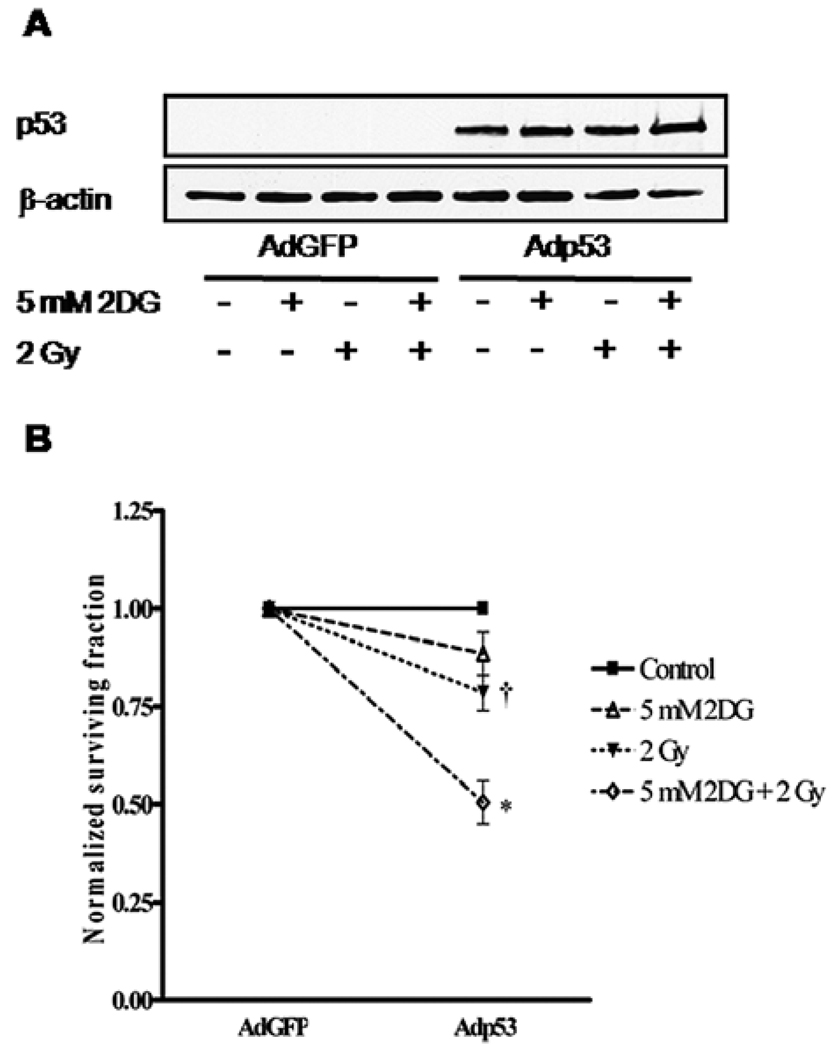
Overexpression of wild-type p53 in H358 cells increases the radiosensitization effect of 2DG
To verify the role of p53 in the radiosensitizing effect of 2DG, we transduced Ad5CMVwtp53/eGFP (Adp53) and Ad5CMV/eGFP (AdGFP) into H358 cells. The expression of p53 was verified by Western blots (Fig. 4A). Compared with the cells transduced with AdGFP alone, in the presence of wild-type p53 (Adp53) expression, the normalized cell survival was decreased by 11% when treated with 5 mM of 2DG alone, 22% when treated with 2 Gy of radiation alone, and 50% when treated with 2DG combined with radiation (Fig. 4B). Expression of Adp53 in H358 cells conferred the cells more susceptible to 2DG and radiation treatment (p<0.001), and also re-produced the radiosensitization effect of 2DG observed in p53 expressing cells.
Figure 4.
Ectopically expressing wild-type p53 in H358 cells. Cells were treated with 5 mM 2DG for 4 h prior to 2-Gy radiation, then cell were immediately trypsinized and plated for clonogenic survival and Western blot assay. Twenty-four hours after plating, cells were transduced with 50 MOI of Adp53 for 4 h. A, The expressed p53 in H358 cells was detected by Western blot analysis. B, Cell survival at 2 Gy (SF2) was determined and normalized to the corresponding control group. Data represent mean ± SE; *p<0.001; †p<0.05 compared with AdGFP-transduced groups.
2DG enhances radiation effect of non-small cell lung cancer in vivo
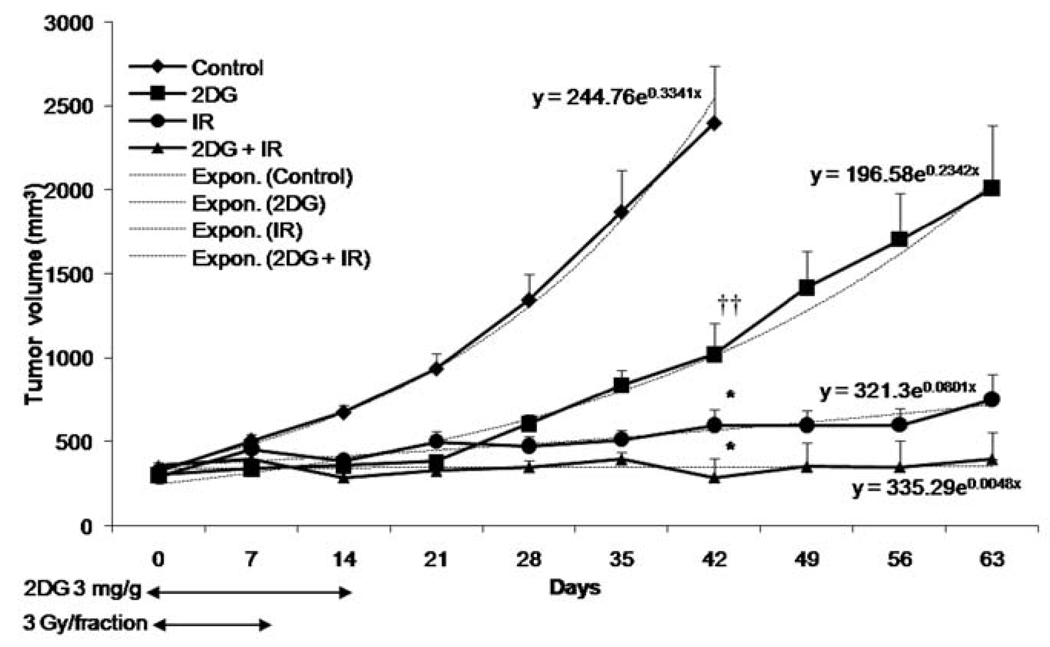
To verify the effect of 2DG on radiation therapy in vivo, A549 cells were injected into nude mice and tumors were allowed to grow until they reached an average size of 250 mm3. Mice were separated into 4 groups, each group having similar average tumor size prior to treatments: control, 2DG, radiation, 2DG + radiation. Forty-two days post tumor implantation, the control tumors reached the maximum growth allowed for tumor-bearing mice. Compared to the controls, at day 42 the tumor volume was significantly decreased in 2DG-treated mice (p<0.01) (Fig. 5). At the doses used, radiation alone almost completely controlled the growth of tumors (p<0.001). However, treatment that combined 2DG and radiation further decreased tumor volume (p<0.001) compared to the control group. To compare the treatment groups for tumor growth, exponential regression analysis was used. Tumor growth differed significantly between the groups. Both 2DG and radiation decreased tumor growth compared to the control. The tumor control remained stable when treated with a combination of 2DG and radiation. These results confirm that 2DG enhances efficacy of radiation therapy in vivo.
Figure 5.
2DG enhances radiosensitization of p53 expressing non-small cell lung cancer in vivo. Arrows indicate the duration of drug and/or radiation treatment. Tumor growth rates were determined by using exponential analysis. Y = Start*exp (k*x), begins at Y = Start and increases exponentially with constant k. Values shown are the mean ± SE. *p<0.001; ††p<0.01 compared with control group.
Discussion
In this report, we have demonstrated that p53 plays an important role in the radiosensitization effect of 2DG in lung cancer cells. Although both cell lines were cultured in the same medium, the effect of 2DG was different. H358 cells, which have no functional p53, showed higher sensitivity to 2DG compared to p53 wild-type cells, A549 (Fig. 1). These data support the concept that p53-deficient cells are more glycolytic-dependent than p53 wild-type cells are. Consistent with this possibility, the recommended growth medium for A549 contains 7 mM of D-glucose, whereas the recommended growth medium for H358 contains 25 mM of glucose. Thus, the difference in sensitivity of A549 and H358 cells to 2DG may be due, in part, to the intrinsic differences in glucose dependence of the two cell lines. These features are consistent with previous studies demonstrating that: i) p53 functions as a negative regulator of glycolysis by transcriptionally regulating SCO2, a regulator of the cytochrome c oxidase complex that is essential for mitochondrial respiration (33); ii) p53 serves as a positive regulator of TIGAR, an inhibitor of the glycolytic pathway and a negative regulator of glycolytic enzyme PGM expression, respectively (34,35); iii) p53 regulates glucose metabolism through the NF-κB target gene, glucose transporter 3 (Glut3) (37); and iv) loss of p53 leads to activation of NF-κB and causes an increase in the rate of glycolysis and upregulation of Glut3 (37).
In the present study, we show that p53-deficient cells are also more sensitive to radiation-induced cytotoxicity. This finding appears to contradict the well-documented role of p53 in apoptosis. However, it has been shown that p53 regulates cellular redox status (31,35,38,39). At low/constitutive stress levels, p53 plays a protective role by regulating expression of genes involved in cell-cycle arrest, antioxidants, and DNA repair, whereas it functions as apoptotic inducer when cells are exposed to high or acute stress level (39). Our results, which demonstrate that p53-deficient cells are more sensitive to ionizing radiation and that overexpression of p53 confers radiosensitivity, are consistent with these possibilities.
In contrast to p53 wild-type cells, our data show that 2DG exerts an antagonistic effect when combined with radiation in H358 cells (Fig. 1). These results suggest that p53 status might be a determinant of the radiosensitization effect of 2DG. This hypothesis is supported by two molecular approaches: a) inhibition of p53 expression in p53 wild-type lung cancer cells (A549), and b) overexpression of wild-type p53 in p53-deficient lung cancer cells (H358). Inhibition of p53 significantly increases survival of A549 cells treated with 2DG and/or radiation in comparison to the control siRNA-transfected group (Fig. 3). The 2DG-mediated radiosensitization effect was also significantly diminished in the p53 siRNA-transfected group. Ectopic expression of wild-type p53 in p53-deficient cells results in a radiosensitization effect of 2DG (Fig. 4). Together, these results suggest that the presence of p53 plays an important role in the radiosensitization effect of 2DG.
Our in vivo data confirm the radiosensitization effect of 2DG (Fig. 5). We show that treatment with either 2DG or with radiation significantly inhibits tumor growth compared to the control group. While high doses of radiation almost completely suppressed tumor growth in the radiation-treated group, addition of 2DG can further enhance the efficacy of radiation. Importantly, the radiation enhancement effect is stable long after the combined treatment of 2DG and radiation. Our result is consistent with previous studies showing that 2DG increases the effect of radiation in pancreatic cancer and head and neck cancer in mice (25,40). Thus, with p53-positive cancer, a lower dose of radiation could be effective when used in combination with 2DG.
In conclusion, we show that the radiosensitization effect of 2DG is determined by the presence of wild-type p53. Mutation of p53 function is one of the most common genetic alterations present in lung cancer; approximately 70% of SCLC and 50% of all NSCLC have mutations in one allele of p53, which is often accompanied by loss of the wild-type allele (41). Our findings suggest that p53 status could be used as a predictive marker of the sensitivity of lung cancer to radiation and glycolytic inhibitor treatment. A combination of 2DG with ionizing radiation may only be beneficial in p53-positive cancer, which might explain some of the contradictory results from clinical trials of 2DG and radiation.
Acknowledgements
This work was supported by NIH grants CA 115801, CA 049797, and the RGJ program, Thailand Research Fund, Thailand.
References
- 1.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Facts & Figures 2008. American Cancer Society; 2008. [Google Scholar]
- 3.Travis WD, Lubin J, Ries L, Devesa S. United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer. 1996;77:2464–2470. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2464::AID-CNCR8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 4.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–392. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 5.Younes M, Brown RW, Stephenson M, Gondo M, Cagle PT. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer. 1997;80:1046–1051. doi: 10.1002/(sici)1097-0142(19970915)80:6<1046::aid-cncr6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 7.Weber G. Enzymology of cancer cells (second of two parts) N Engl J Med. 1977;296:541–551. doi: 10.1056/NEJM197703102961005. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Cuezva JM, Chen G, Alonso AM, et al. The bioenergetic signature of lung adenocarcinomas is a molecular marker of cancer diagnosis and prognosis. Carcinogenesis. 2004;25:1157–1163. doi: 10.1093/carcin/bgh113. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Rios F, Sanchez-Arago M, Garcia-Garcia E, et al. Loss of the mitochondrial bioenergetic capacity underlies the glucose avidity of carcinomas. Cancer Res. 2007;67:9013–9017. doi: 10.1158/0008-5472.CAN-07-1678. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XD, Deslandes E, Villedieu M, et al. Effect of 2-deoxy-D-glucose on various malignant cell lines in vitro. Anticancer Res. 2006;26:3561–3566. [PubMed] [Google Scholar]
- 13.Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-Dglucose as a chemotherapeutic agent: mechanism of cell death. Br J Cancer. 2002;87:805–812. doi: 10.1038/sj.bjc.6600547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain VK, Kalia VK, Gopinath PM, Naqvi S, Kucheria K. Optimization of cancer therapy: Part III - effects of combining 2-deoxy-D-glucose treatment with gamma-irradiation on normal mice. Indian J Exp Biol. 1979;17:1320–1325. [PubMed] [Google Scholar]
- 15.Kalia VK, Jain VK, Otto FJ. Optimization of cancer therapy: Part IV - effects of 2-deoxy-D-glucose on radiation induced chromosomal damage in PHA-stimulated peripheral human leukocytes. Indian J Exp Biol. 1982;20:884–888. [PubMed] [Google Scholar]
- 16.Singh SP, Singh S, Jain V. Effects of 5-bromo-2-deoxy-uridine and 2-deoxy-D-glucose on radiation-induced micro-nuclei in mouse bone marrow. Int J Radiat Biol. 1990;58:791–797. doi: 10.1080/09553009014552171. [DOI] [PubMed] [Google Scholar]
- 17.Swamy RK, Manickam J, Adhikari JS, Dwarakanath BS. Glycolytic inhibitor, 2-deoxy-D-glucose, does not enhance radiation-induced apoptosis in mouse thymocytes and splenocytes in vitro. Indian J Exp Biol. 2005;43:686–692. [PubMed] [Google Scholar]
- 18.Liu H, Hu YP, Savaraj N, Priebe W, Lampidis TJ. Hyper-sensitization of tumor cells to glycolytic inhibitors. Biochemistry. 2001;40:5542–5547. doi: 10.1021/bi002426w. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Savaraj N, Priebe W, Lampidis TJ. Hypoxia increases tumor cell sensitivity to glycolytic inhibitors: a strategy for solid tumor therapy (Model C) Biochem Pharmacol. 2002;64:1745–1751. doi: 10.1016/s0006-2952(02)01456-9. [DOI] [PubMed] [Google Scholar]
- 20.Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004;53:116–122. doi: 10.1007/s00280-003-0724-7. [DOI] [PubMed] [Google Scholar]
- 21.Nelson CA, Wang JQ, Leav I, Crane PD. The interaction among glucose transport, hexokinase, and glucose-6-phosphatase with respect to 3H-2-deoxyglucose retention in murine tumor models. Nucl Med Biol. 1996;23:533–541. doi: 10.1016/0969-8051(96)00037-6. [DOI] [PubMed] [Google Scholar]
- 22.Heminger K, Jain V, Kadakia M, Dwarakanath B, Berberich SJ. Altered gene expression induced by ionizing radiation and glycolytic inhibitor 2-deoxy-glucose in a human glioma cell line: implications for radio sensitization. Cancer Biol Ther. 2006;5:815–823. doi: 10.4161/cbt.5.7.2812. [DOI] [PubMed] [Google Scholar]
- 23.Pahl HL, Baeuerle PA. Activation of NF-kappa B by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 1996;392:129–136. doi: 10.1016/0014-5793(96)00800-9. [DOI] [PubMed] [Google Scholar]
- 24.Lin X, Zhang F, Bradbury CM, et al. 2-Deoxy-D-glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism. Cancer Res. 2003;63:3413–3417. [PubMed] [Google Scholar]
- 25.Coleman MC, Asbury CR, Daniels D, et al. 2-deoxy-D-glucose causes cytotoxicity, oxidative stress, and radiosensitization in pancreatic cancer. Free Radic Biol Med. 2008;44:322–331. doi: 10.1016/j.freeradbiomed.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67:3364–3370. doi: 10.1158/0008-5472.CAN-06-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maschek G, Savaraj N, Priebe W, et al. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–34. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 28.Dwarkanath BS, Zolzer F, Chandana S, et al. Heterogeneity in 2-deoxy-D-glucose-induced modifications in energetics and radiation responses of human tumor cell lines. Int J Radiat Oncol Biol Phys. 2001;50:1051–1061. doi: 10.1016/s0360-3016(01)01534-6. [DOI] [PubMed] [Google Scholar]
- 29.Jha B, Pohlit W. Reversibility of inhibition of DNA double strand break repair by 2-deoxy-D-glucose in Ehrlich ascites tumour cells. Int J Radiat Biol. 1993;63:459–467. doi: 10.1080/09553009314550611. [DOI] [PubMed] [Google Scholar]
- 30.Mohanti BK, Rath GK, Anantha N, et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35:103–111. doi: 10.1016/s0360-3016(96)85017-6. [DOI] [PubMed] [Google Scholar]
- 31.Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol. 2007;17:286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Matoba S, Kang JG, Patino WD, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 34.Kondoh H, Lleonart ME, Gil J, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–185. [PubMed] [Google Scholar]
- 35.Bensaad K, Tsuruta A, Selak MA, et al. TIGAR, a p53- inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad IM, Abdalla MY, Aykin-Burns N, et al. 2-Deoxyglucose combined with wild-type p53 overexpression enhances cytotoxicity in human prostate cancer cells via oxidative stress. Free Radic Biol Med. 2008;44:826–834. doi: 10.1016/j.freeradbiomed.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawauchi K, Araki K, Tobiume K, Tanaka N. p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol. 2008;10:611–618. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
- 38.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 40.Simons AL, Fath MA, Mattson DM, et al. Enhanced response of human head and neck cancer xenograft tumors to cisplatin combined with 2-deoxy-D-glucose correlates with increased 18F-FDG uptake as determined by PET imaging. Int J Radiat Oncol Biol Phys. 2007;69:1222–1230. doi: 10.1016/j.ijrobp.2007.07.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brambilla C, Fievet F, Jeanmart M, et al. Early detection of lung cancer: role of biomarkers. Eur Respir J (Suppl) 2003;39:S36–S44. doi: 10.1183/09031936.02.00062002. [DOI] [PubMed] [Google Scholar]