Abstract
Agonistic Abs to select costimulatory members of CD28 and TNFR family have shown efficacy in various preclinical cancer immunotherapeutic settings. However, the use of agonistic Abs is often associated with severe toxicity due to nonspecific activation of lymphocytes. We hypothesized that natural costimulatory ligands may serve as more potent and safer alternative to agonistic Abs for immunotherapy. In this communication, we focused on 4-1BBL as the molecule of choice because of the pleiotropic effects of 4-1BB signaling in the immune system and the demonstrated therapeutic efficacy of 4-1BB agonistic Abs in preclinical cancer and infection models. We report that a novel form of soluble ligand, SA-4-1BBL, delivered more potent and qualitatively different signals to T cells than an agonistic Ab. Importantly, while treatment of naïve mice with the agonistic Ab resulted in severe toxicity, as assessed by enlarged spleen and peripheral LNs, non-specific T cell proliferation, hepatitis, and systemic inflammatory cytokine production, treatment with SA-4-1BBL lacked these immune anomalies. Agonistic Ab treatment produced full toxicity in FcγR−/− or complement C1q−/− or C3−/− knockout mice, suggesting lack of involvement of stimulatory FcγRs or complement system in the observed toxicity. Naïve and memory T cells served as direct targets of anti-4-1BB Ab mediated toxicity. Potent immunostimulatory activity combined with lack of toxicity rationalizes further development of soluble SA-4-1BBL as an immunomodulatory component of therapeutic vaccines against cancer and chronic infections.
Keywords: Costimulation, 4-1BB, SA-4-1BBL, Vaccines, Toxicity, Adjuvant
1. Introduction
Costimulatory molecules of the CD28 and TNFR superfamilies play critical roles in modulating innate, adaptive, and regulatory immune responses [1]. As such, agonistic ligands to these receptors have the potential to serve as effective immunomodulatory components of vaccines. Consistent with this notion are a series of studies demonstrating that agonistic Abs to the CD28 and TNFR superfamilies were effective in generating potent immune responses with therapeutic potential in settings of chronic infections and cancer [2–5]. However, the use of agonistic Abs can be associated with severe toxicity arising from nonspecific, systemic activation of lymphocytes [6–12]. For example, a single intravenous dose of a superagonist anti-CD28 mAb in 6 healthy volunteers enrolled in a Phase I clinical trial resulted in a systemic inflammatory response and life threatening toxicity [8]. Therefore, the potential use of costimulatory agonists for therapeutic vaccines in humans requires the generation of agonists that transduce the appropriate stimulatory signals without toxic side effects in vivo.
Costimulatory receptors that are inducibly expressed or upregulated on activated T cells may serve as preferred targets for immunomodulation due to their potential to selectively target antigen-experienced T cells for expansion, survival, and establishment of long-term immune memory. Using the appropriate form of agonists would ideally lead to the generation of a highly specific immune response, and importantly, potentially avoid toxic side effects often associated with systemic immune activation. 4-1BB, a costimulatory receptor belonging to the TNF receptor family, is inducibly expressed to high levels on activated CD4+ and CD8+ T cells, and as such represents a promising target for immunomodulation [13,14]. Engagement of 4-1BB with its ligand, 4-1BBL, or agonistic Abs results in T cell activation, clonal expansion, survival, and the establishment and maintenance of long-term immune memory [1,15–18]. Agonistic Abs to 4-1BB have demonstrated impressive therapeutic efficacy in various preclinical cancer and viral models [5,16,18,19]. As a result, a humanized agonistic mAb was developed and tested in phase I and II clinical trials with metastatic or locally advanced solid tumors (National Institutes of Health Clinical trials database NCT00309023). However, we have recently reported that multiple injections of anti-4-1BB mAbs at therapeutic doses in naïve mice resulted in transient toxicity and the development of a series of immunological anomalies [7]. We hypothesized that natural 4-1BBL may have better efficacy and safety as compared with agonistic Abs by delivering signals quantitatively and/or qualitatively different from those transduced by Abs. Inasmuch as 4-1BBL functions as a membranous protein and has no costimulatory activity in soluble form [20,21], we recently generated a novel chimeric form of 4-1BBL, SA-4-1BBL, by fusing the extracellular domains of 4-1BBL to the C-terminus of a modified form of core streptavidin (SA). The SA-4-1BBL molecule exists as tetramers/oligomers, owing to the structural features of SA, and has the ability to cross-link 4-1BB receptors for potent costimulatory activity on T cells [22]. In addition, we have recently demonstrated that SA-4-1BBL served as a potent immunomodulatory component of a peptide-based vaccine and had better efficacy than an agonistic 4-1BB Ab (3H3) as well as TLR agonists LPS, MPL, and CpG in eradicating established tumors in an animal model of cervical cancer [23].
In this study, we directly compared the immunomodulatory activity and toxicity of SA-4-1BBL to the 3H3 agonistic Ab, which has shown therapeutic efficacy in various experimental settings [16,24,25]. SA-4-1BBL demonstrated more potent and qualitatively different immunostimulatory activity as compared with the agonistic mAb. Importantly, the better efficacy of SA-4-1BBL was achieved in the absence of Ab-associated severe systemic toxicity that did not involve FcγR or complement activation. Both naïve and memory CD4+ and CD8+ T cells served as direct targets for Ab-associated toxicity. Potent immunostimulatory activity and lack of toxicity support our perception that SA-4-1BBL may serve an effective immunomodulatory component of prophylactic and therapeutic vaccine formulations.
2. Materials and Methods
2.1 Mice
C57BL/6.SJL, C57BL/6, C57BL/6 Fcer1g−/−, C57BL/6 OT-Irag−/−, and C57BL/6 OT-IIrag−/− mice were purchased from The Jackson Laboratory or bred in our barrier animal facility at the University of Louisville. C57BL/6 4-1BB−/− mice were kindly provided by Dr. A.T. Vella of University of Connecticut, Farmington, CT, with permission from Dr. B.S. Kwon of University of Ulsan, Korea. C57BL/6 C3−/− and C57BL/6 C1q−/− mice were generously provided by Dr. M. Ratajczak of University of Louisville. All animals were cared for in accordance with institutional and National Institutes of Health guidelines.
2.2 Reagents
Construction, expression, purification, and characterization of SA-4-1BBL and core streptavidin (SA) were recently described [22] and endotoxin levels were nill. Anti-41BB agonistic Ab, 3H3, was previously described [26]. The preparation of Ab contained 0.6 EU/mg or 0.06 EU/100 µg endotoxin per injection. Rat IgG2a was purchased from Sigma-Aldrich. Fluorochrome-conjugated Abs to various immune cell surface markers or cytokines and isotype controls were purchased from BD Bioscience (San Jose, CA), eBioscience (San Diego, CA), and BioLegend (San Diego, CA). Chicken ovalbumin (OVA) was purchased from Pierce, and OVA SIINFEKL peptide (aa 257–264) was purchased from CPC Scientific Inc, San Jose, CA.
2.3 Flow cytometry
For phenotyping and sorting, spleens and LNs were processed into single-cell suspensions, treated with ACK solution to lyse RBC, Fc blocked (BD Pharmingen), and labeled with saturating concentrations of fluorochrome-conjugated Abs. Isotype matched Abs with the same fluorochrome were used as controls. For T cell sorting, lymphocytes were stained with CD4-FITC, CD25-PE, and CD8-APC Abs. CD4+CD25− (CD4+ Teff) and CD8+ (CD8+ Teff) T cells were sorted to > 95% purity using a FACSVantage cell sorter (BD Bioscience). Intracellular FoxP3 staining was performed according to the manufacturer’s protocol (eBiosciences). Intracellular cytokine staining on PMA (5 ng/ml, Sigma) and ionomycin (500 ng/ml, Sigma) stimulated cells was performed as previously described [23]. Serum cytokine levels were determined using the inflammatory CBA kit (BD Pharmingen) according to the manufacturer’s protocol. FACS Caliber or LSR-II (BD Bioscience) was used for FACS acquisition. Data was analyzed using CellQuest (BD Biosciences) and FlowJo (Tree Star) software.
2.4 In vitro T cell proliferation
Sorted CD8+ and CD4+ T cells were cultured (2.5 × 104/well) with 0.25 µg/ml of soluble anti-CD3 Ab and irradiated (2000 cGy) syngeneic splenocytes (1 × 105/well) in the presence of varying concentrations of soluble SA-4-1BBL, equimolar quantity of control SA protein, or agonistic anti-4-1BB Ab (3H3) for 3 days in complete MLR medium. Cultures were pulsed with [3H]-thymidine during the last 16 hrs of the culture, and harvested on a Tomtec Harvester 96 (Tomtec Inc., Hamden, CT) for quantification of incorporated radioactivity. Results expressed as mean ±SD cpm of triplicate wells.
2.5 In vivo OT-I and OT-II proliferation
Flow cytometry-sorted OT-I CD8+ T cells and OT-II CD4+ T cells (CD45.2+) were labeled with 2.5 µM CFSE and 1 × 106 OT-I and OT-II T cells were transferred by i.v. injection into naïve C57BL/6 mice (SJL, CD45.1+). After 24 hrs, mice were immunized s.c. with OVA (10 µg) in conjunction with various doses of SA-4-1BBL, anti-4-1BB Ab, or equimolar concentration of SA control protein. After 3 days, peripheral LNs and spleens were harvested and OT-I and OT-II proliferation was assessed by analyzing the CFSE dilution of CD45.2+CD8+ and CD45.2+CD4+ cells using flow cytometry.
2.6 In vivo cytotoxicity
Naïve C57BL/6 mice were immunized i.v. with OVA (50 µg) in conjunction with various doses of SA-4-1BBL, anti-4-1BB Ab, or equimolar concentration of SA control protein. One week later, mice received CFSE labeled target cells pulsed with SIINFEKL as previously described [23].
2.7 Toxicity studies
Naïve mice were injected s.c. with 100 µg of 3H3 mAb, rat isotype IgG2a, SA-4-1BBL, or equimolar SA control protein either once or once weekly for 3 wks. One week following the last treatment, mice were euthanized and subjected to various analyses to determine toxicity.
2.8 Adoptive transfer model
C57BL/6 4-1BB−/− mice, which do not develop toxicity from agonistic Ab treatment [7], were adoptively transferred with various cell populations, including total splenocytes, DCs, and T cells, of WT mice. One day after adoptive transfer, mice were treated with 100 µg of the 3H3 Ab and analyzed for signs of toxicity one week later. C57BL/6 4-1BB−/− mice adoptively transferred with highly purified cell populations and treated with agonistic Ab were compared to C57BL/6 4-1BB−/− mice that underwent the same agonistic Ab treatment but were not adoptively transferred with WT cells.
2.9 Statistics
Data were compared using the Student’s t test or ANOVA and a p < 0.05 was considered significant.
3. Results
3.1 SA-4-1BBL has better immunostimulatory activity than an agonistic anti-4-1BB Ab
We compared the immunostimulatory activity of SA-4-1BBL on T cells to that of an agonistic 4-1BB Ab (3H3) with demonstrated therapeutic efficacy in various experimental settings [16,24,25]. SA-4-1BBL was more effective than the 3H3 Ab for driving the proliferation of CD8+ T cells in a CD3 Ab-based in vitro stimulation assay (Fig. 1A, left). Interestingly, while SA-4-1BBL induced proliferation of CD4+ T cells in a dose dependent manner, the 3H3 Ab showed little to no effect on the proliferation of CD4+ T cells in vitro (Fig. 1A, right). The better efficacy of SA-4-1BBL in driving T cell proliferation was not dependent on the dose of soluble CD3 Ab as SA-4-1BBL induced higher levels of proliferation of both CD8+ and CD4+ T cells compared to the agonistic anti-4-1BB Ab at all CD3 Ab concentrations tested (Supporting Fig. 1). Importantly, SA-4-1BBL showed no effect on the proliferation of both CD8+ and CD4+ T cells from C57BL/6 4-1BB−/− mice (Supporting Fig. 2), demonstrating that SA-4-1BBL acts directly through 4-1BB.
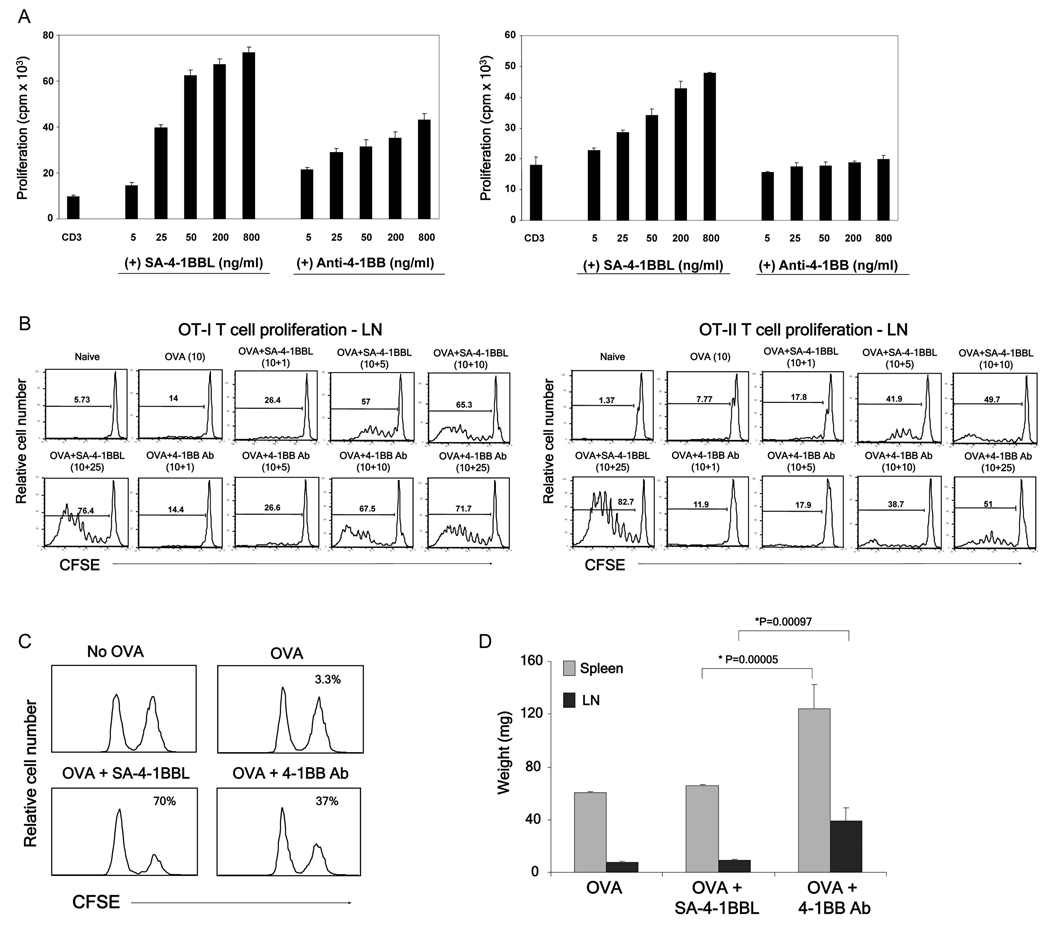
Fig 1.
SA-4-1BBL demonstrates better immune activity than an agonistic anti-4-1BB Ab without Ab-associated toxicity. (A) In vitro CD8+ T cell (left) and CD4+ T cell (right) proliferation. Flow sorted CD8+ and CD4+ T cells were stimulated for 3 days with a suboptimal dose of an agonistic anti-CD3 Ab in conjugation with various doses of SA-4-1BBL or 3H3 Ab in the presence of irradiated syngeneic splenocytes. Cells were pulsed with [3H]-thymidine for the last 16 hrs of culture. (B) In vivo OT-I and OT-II T cell proliferation. One million flow sorted OT-I T cells and OT-II (CD45.2+) were labeled with CFSE and injected i.v. into naïve congenic C57BL/6.SJL (CD45.1+) mice. Mice were vaccinated i.v. with OVA (10 µg) in combination with various doses (µg) of SA-4-1BBL or 3H3 Ab as indicated. Proliferation was assessed using multiparameter flow cytometry 3 days after vaccination. (C) In vivo killing response. C57BL/6 mice were immunized i.v. with OVA (50 µg) in combination with 3H3 Ab (25 µg) or SA-41BBL (25 µg). Seven days post vaccination, mice received SIINFEKL-pulsed syngeneic splenocytes and peptide-specific killing was assessed 2 days later and expressed as percent lysis for each histogram. (D) In vivo toxicity as assessed by the weight of spleen and lymph nodes harvested from mice vaccinated in panel C. p values determined by Student’s t test. Data are representative of a minimum of three independent experiments for Panels A–C.
The immunostimulatory activity of SA-4-1BBL was also compared to 3H3 Ab in an in vivo OT-I and OT-II adoptive transfer model. Vaccination with ovalbumin (OVA) and SA-4-1BBL resulted in greater proliferation of OT-I CD8+ T cells in both the peripheral LNs (Fig. 1B, left) and spleen (Supporting Fig. 3) at lower doses (1 and 5 µg) and similar proliferation at higher doses (10 and 25 µg) as compared with the 3H3 Ab. Similar to the in vitro proliferation assays, vaccination with SA-4-1BBL resulted in higher levels of proliferation of OT-II CD4+ T cells in both the peripheral LNs (Fig. 1B right) and spleen (Supporting Fig. 3) at all concentrations tested as compared to vaccination with the agonistic 4-1BB Ab. The enhanced efficacy of the SA-4-1BBL over the Ab was even more pronounced in an in vivo killing assay using OVA as a cognate antigen for vaccination (Fig. 1C). Importantly, animals treated with the agonistic anti-4-1BB Ab, but not SA-4-1BBL, had enlarged spleen and lymph nodes (Fig. 1D), suggesting possible toxicity.
3.2 Treatment of naïve mice with agonistic anti-4-1BB Ab, but not SA-4-1BBL, results in nonspecific naïve and memory T cell proliferation, altered lymphocyte trafficking, and enlarged lymphoid organs
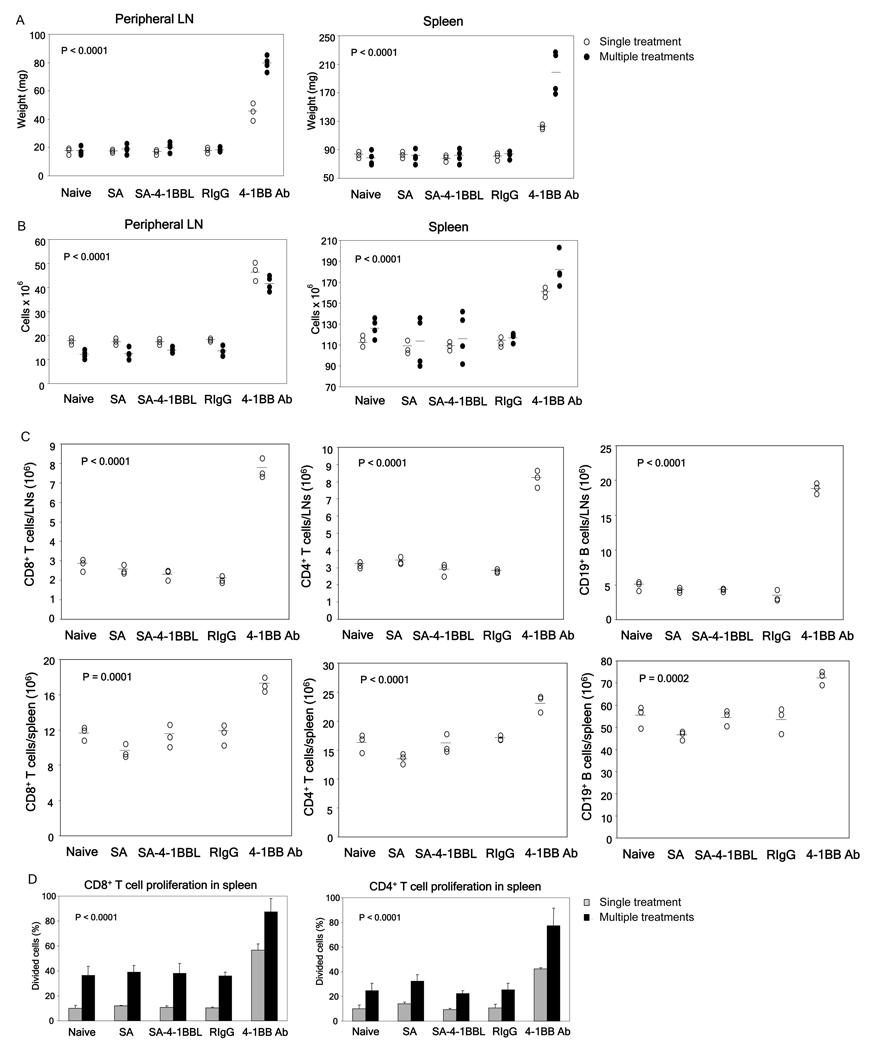
Our observation that treatment with one dose of agonistic anti-4-1BB Ab resulted in enlarged spleen and peripheral LNs is consistent with our recent study reporting severe toxicity from multiple high-dose therapeutic injections (200 µg) of various agonistic anti-4-1BB Abs, including 3H3 Ab.[7] We therefore compared the toxicity profile of SA-4-1BBL directly with the 3H3 Ab under similar conditions. Three weekly treatments of naïve mice with 100 µg of 3H3 Ab, a therapeutic dose used in various settings [27,28], resulted in gross enlargement of spleen and LNs as demonstrated by weight (Fig. 2A) and total cell numbers (Fig. 2B). In contrast, the same treatment with SA-4-1BBL did not result in lymphadenopathy, splenomegaly, and increased cell numbers (Fig. 2A,B). Treatment with 3H3 Ab resulted in > 2-fold increase in CD4+ T cells and > 3-fold increase in CD8+ T cells in the peripheral LNs, as well as significant increases in both T cell subsets in the spleen. However, the same treatment resulted in > 3-fold increase in CD19+ B cells in the peripheral LNs, but a dramatic decrease in CD19+ B cells in the spleen (Table I). We found similar toxicity results using only one injection of anti-4-1BB Ab (Fig. 2A–C) with the exception of an increase, rather than decrease, in the numbers of CD19+ B cells in the spleen.
Fig 2.
SA-4-1BBL treatment lacks Ab associated toxicity. Naïve C57BL/6 mice were injected s.c. once (○) or once weekly over a 3-wk period (●) with 100 µg of SA-4-1BBL, equivalent doses of anti-4-1BB Ab, or other appropriate controls. Animals were euthanized one week after the final treatment and assessed for various indicators of toxicity, including peripheral LN and spleen weights (A) and total peripheral LN and spleen cell numbers (B). (C) Anti-4-1BB Ab demonstrates super-agonistic activity in vivo. In vivo proliferation of spleen CD8+ and CD4+ T cells was determined by adoptively transferring 2 × 106 CFSE-labeled CD45.1+ congenic splenocytes into naïve C56BL/6 (CD45.2+) mice one day prior to the indicated treatments. Percentage of proliferating cells was determined by CFSE dilution using FACS analysis. p values determined by ANOVA, anti-4-1BB vs. all other groups within same treatment schedule. Data were obtained from a minimum of 3 mice per group.
Table 1.
Repeated injections with anti-4-1BB Ab, but not the SA-4-1BBL, results in significant alterations in absolute number of peripheral lymphocytes
| LN | Spleen | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | CD8+ T cellsa | CD4+ T cells | CD19+ B cells | CD4+Foxp3+ T cells | CD8+ T cells | CD4+ T cells | CD19+ B cells | CD4+Foxp3+ T cells |
| None | 1.89 ± 0.6 | 2.09 ± 0.7 | 2.45 ± 0.6 | 3.80 ± 0.8 | 10.39 ± 2.1 | 14.21 ± 1.4 | 56.99 ± 14.0 | 2.48 ± 0.5 |
| SA | 1.99 ± 0.3 | 2.25 ± 0.2 | 3.20 ± 0.5 | 3.67 ± 0.6 | 10.79 ± 2.6 | 15.89 ± 3.8 | 60.84 ± 13.8 | 2.53 ± 0.6 |
| SA-4-1BBL | 1.83 ± 0.7 | 2.21 ± 0.8 | 3.32 ± 1.2 | 3.38 ± 1.3 | 12.37 ± 1.9 | 17.38 ± 2.8 | 61.80 ± 9.4 | 2.75 ± 0.7 |
| R-IgG | 2.21 ± 0.5 | 2.66 ± 0.5 | 4.23 ± 0.6 | 4.01 ± 0.7 | 10.78 ± 0.5 | 17.36 ± 0.5 | 64.14 ± 7.4 | 2.45 ± 0.1 |
| 4-1BB Ab | 8.20 ± 1.8* | 6.59 ± 1.4* | 13.14± 1.84* | 14.86 ± 3.7* | 23.43 ± 5.5* | 23.84 ± 3.4* | 35.49 ± 6.5* | 5.05 ± 1.7* |
All numbers, except Treg cells in LN × 105, are shown as × 106 ± SD.
p < 0.05, ANOVA, anti-4-1BB Ab vs. all other groups.
Anti-4-1BB Ab treatment also led to a dramatic increase in CD8+ T cells and decrease in CD19+ B cells in the bone marrow (data not shown). Unlike other lymphocytes, treatment with 3H3 Ab did not significantly alter the absolute number of NK cells (NK1.1+CD3ε−) in both the peripheral LNs and spleen. In addition, anti-4-1BB Ab treatment resulted in increased percentage as well as absolute number (2-fold) of CD4+FoxP3+ regulatory T cells (Table I). Importantly, these immune abnormalities were absent in SA-4-1BBL treated animals (Supporting Fig. 4 and Table I).
The enlargement in the spleen and LNs of Ab-treated mice was primarily due to non specific proliferation of T cells as demonstrated by adoptive transfer of CFSE-labeled congenic T cells one day before Ab treatment. While SA-4-1BBL showed no effect on the in vivo proliferation of cells compared to control animals, anti-4-1BB Ab acted as a superagonist in vivo and resulted in striking non-specific proliferation of the majority of CD4+CD45.1+ and CD8+CD45.1+ congenic T cells in both the spleen (Fig. 2D) and peripheral LNs of treated mice (Supporting Fig. 5). More than 85% and 55% of transferred T cells proliferated in response to multiple and single injections of anti-4-1BB Ab, respectively, as compared with < 40% and 15% for treatment groups receiving SA-4-1BBL or control reagents. Antibody treatment caused the proliferation of both newly activated naive (CD43highCD44high) and memory (CD43low/−CD44high) CD8+ T cells [29–31] (Supporting Fig. 6). Thus, although T cells could still be trafficking to the spleen and peripheral LNs, nonspecific proliferation of both memory and naïve T cells plays a major role in the increased absolute number of T cells. On the other hand, anti-4-1BB Ab treatment had no effect on the proliferation of CD19+ B cells (data not shown), suggesting that the increase or decrease in CD19+ B cell numbers in lymphoid organs and BM of these mice was due to cell trafficking.
3.3 Agonistic anti-4-1BB Ab, but not SA-4-1BBL, results in systemic cytokine response
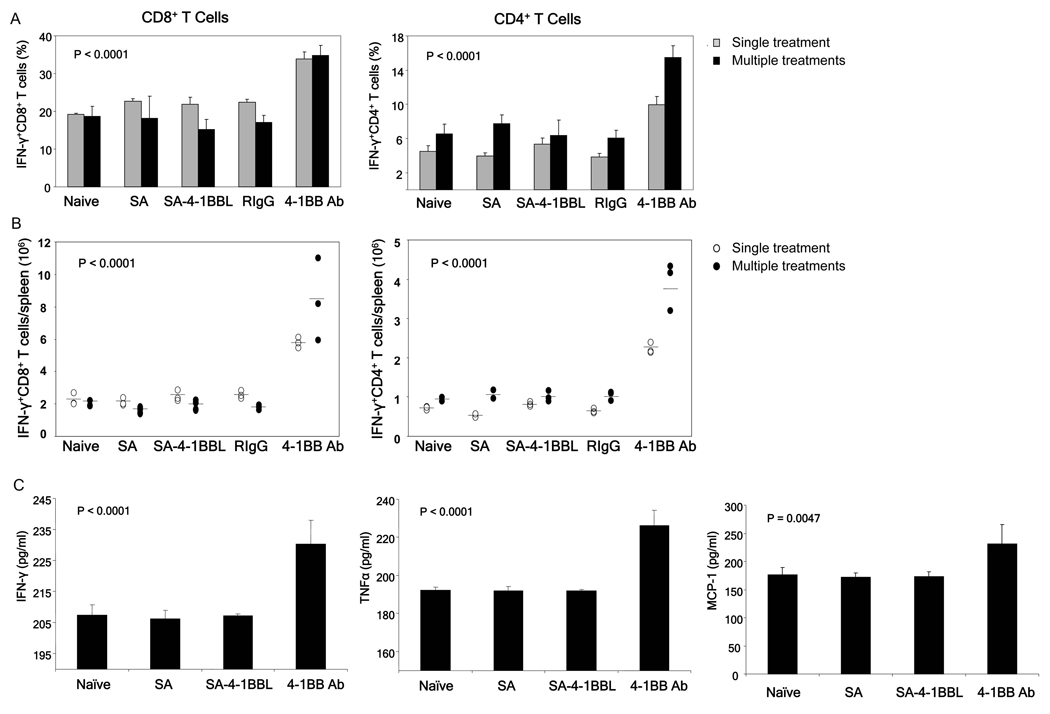
Toxic effects of agonistic Abs to CD28 and CD40 are associated with a systemic inflammatory cytokine response [6,8–12,32]. Therefore, we compared the effects of treatment with SA-4-1BBL and anti-4-1BB Ab on the production of inflammatory cytokines. Whereas treatment of naïve mice with SA-4-1BBL had no effect on the production of inflammatory cytokines, anti-4-1BB Ab resulted in a significant increase in the percentage (Fig. 3A) and absolute number (Fig. 3B) of total CD8+ T cells (left) and CD4+ T cells (right) producing IFN-γ and CD8+ T cells producing IL-2 and TNF-α (data not shown). Mice treated with multiple injections of anti-4-1BB Ab demonstrated approximately a 2-fold increase in percentage and a 4-fold increase in absolute number of splenic CD8+IFN-γ+ and CD4+IFN-γ+ T cells compared to all other groups. This effect was also observed with a single Ab treatment, but it was not as drastic as that obtained using multiple treatments. Importantly, repeated anti-4-1BB Ab treatment resulted in significant increases in the serum cytokine levels for IFN-γ, TNF-α, and MCP-1 (Fig. 3C). Of note, treatment with either SA-4-1BBL or anti-4-1BB Ab demonstrated no effect on the production of IL-4, IL-6 or IL-10 cytokines as compared to control groups (data not shown).
Fig 3.
Treatment with anti-4-1BB Ab, but not SA-4-1BBL, results in a drastic increase in the production of inflammatory cytokines. Lymphocytes and sera harvested from mice that underwent treatment as described in Figure 2 were analyzed for percentage (A) and absolute numbers (B) of splenic CD8+ and CD4+ T cells expressing IFN-γ. Spleen cells were stimulated with PMA and ionomycin for 6 hours and then surface stained with anti-CD8 and anti-CD4 Abs followed by intracellular staining for IFN-γ. (C) Cytokine levels in the serum of various treatment group, as determined using a CBA kit and flow cytometry. p values determined by ANOVA, anti-4-1BB vs. all other groups within same treatment schedule. Data were obtained from a minimum of 3 mice per group.
Treatment with the agonistic anti-4-1BB Ab resulted in massive infiltration of CD8+ T cells into the liver as determined by confocal microscopy (Supporting Fig. 7) and H&E staining (data not shown), while SA-4-1BBL treated mice showed little evidence of any hepatitis. Taken together, these data demonstrate that in spite of its more potent antigen-specific immunostimulatory activity, SA-4-1BBL lacks toxicity caused by the agonistic anti-4-1BB Ab .
3.4 Toxic effects of anti-4-1BB Ab are FcγR and complement independent
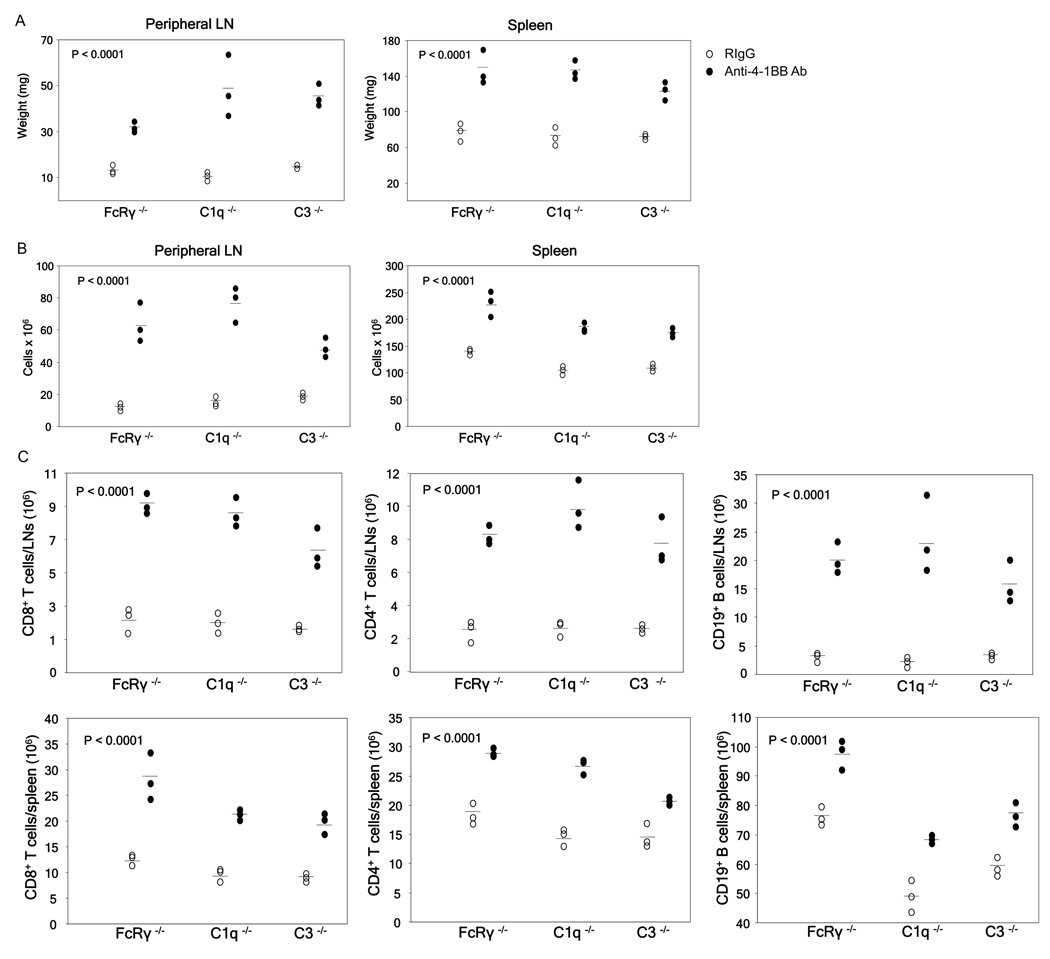
We next tested if the systemic inflammatory responses and the associated toxicity seen in this model is manifested by 3H3 Ab interaction with FcγRs expressed on various immune cell types, resulting in cellular activation and the production of inflammatory cytokines [33]. C57BL/6 mice lacking the expression of FcR γ-chain (Fcer1g−/−), and as a result surface expression and function of stimulatory Fc-γRI, Fc-γRIII, and Fc-γRIV [33,34], were injected with 100 µg of anti-4-1BB Ab or isotype control and analyzed for toxicity. Similar to wild type C57BL/6 mice, a single treatment with 100 µg of anti-4-1BB Ab resulted in severe toxicity in Fcer1g−/− mice as assessed by enlarged spleen and peripheral LNs (Fig. 4A), increased total cell numbers (Fig. 4B), and increased CD4+ and CD8+ T cells and CD19+ B cell populations (Fig. 4C) in these organs. We further eliminated the role of complement in 3H3 Ab associated toxicity by treating C57BL/6 mice deficient in C1q (C1q−/−) or complement factor C3 (C3−/−). A single treatment with 100 µg anti-4-1BB Ab resulted in full toxicity in C1q−/− and C3−/− mice, as demonstrated by enlarged spleen and peripheral LNs (Fig. 4A) and increased total leukocyte (Fig. 4B) and CD4+ and CD8+ T cell and CD19+ B cell populations (Fig. 4C). Taken together, these data strongly suggest that activating FcγRs and complement mediated mechanisms do not play a role in the anti-4-1BB Ab associated toxicity.
Fig 4.
Anti-4-1BB Ab associated toxicity is FcR and complement independent. Naïve C57BL/6 Fcer1g−/−, C57BL/6 C3−/−, and C57BL/6 C1q−/− male mice were injected once s.c. with 100 µg of anti-4-1BB Ab or control rat IgG, sacrificed one week after treatment, and assessed for various indicators of toxicity. Peripheral LN and spleen weights of treated mice (A), total peripheral LN and spleen cell numbers (B), as well as total T cell and B cell numbers as determined by FACS analysis (C). p values for anti-4-1BB vs. rat IgG for each type of KO mice was determined by Student’s t test. Data were obtained from a minimum of 3 mice per group.
3.5 T cells are critical targets of anti-4-1BB Ab mediated toxicity
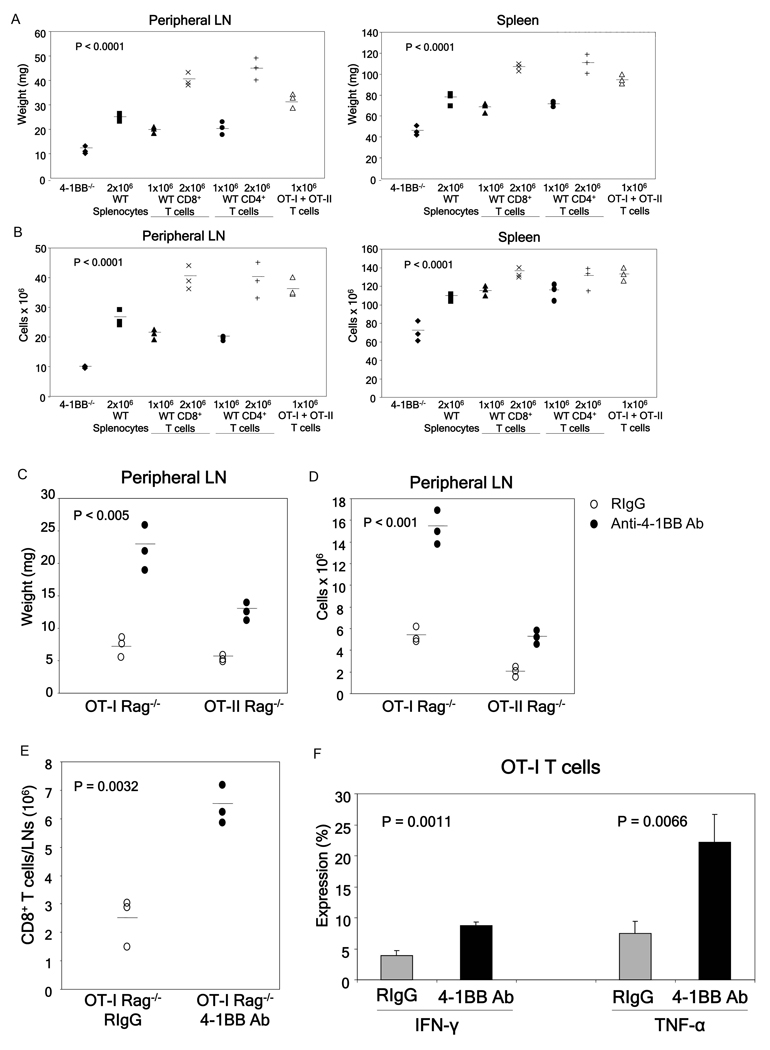
We next tested if T cells are the direct target of 3H3 Ab-induced toxicity. The cross-linking of 4-1BB receptor is essential for the induction of toxicity as all anti-4-1BB Ab associated immune anomalies are absent in C57BL/6 4-1BB−/− mice [7]. Therefore, we established an adoptive transfer model where highly purified lymphocyte populations from WT mice were transferred into naïve 4-1BB−/− mice that were subjected to Ab treatment. The adoptive transfer of 2 × 106 WT whole splenocytes into 4-1BB−/− mice followed by a single injection of 100 µg of anti-4-1BB Ab resulted in full toxicity as demonstrated by greatly enlarged spleen and peripheral LNs (Fig. 5A) and increased numbers of total leukocytes (Fig. 5B), CD4+ T cells, CD8+ T cells, and CD19+ B cells (Supporting Fig. 8).
Fig 5.
T cells are the direct target of anti-4-1BB Ab mediated toxicity. C57BL/6 4-1BB−/− mice were adoptively transferred with the indicated numbers and types of lymphocytes and treated one day later using 100 µg of anti-4-1BB Ab. Mice were sacrificed one week after treatment, and assessed for various indicators of toxicity. All the cells, except splenocytes, were sorted by flow cytometry to ensure purity before adoptive transfer. Peripheral LN and spleen weights (A) and total peripheral LN and spleen cell numbers (B) of the treated mice. p values determined by ANOVA, 4-1BB−/− without adoptive transfer vs. all other groups. (C–F) Anti-4-1BB Ab directly activates monoclonal T cells resulting in toxicity. A single treatment of OT-I Rag−/− and OT-II Rag−/− mice with 100 µg of anti-4-1BB Ab results in enlarged peripheral lymph nodes (C), total cell numbers (D), and CD8+ T cell numbers (E) as determined by flow cytometry. (F) As compared to controls, more CD8+ T cells from anti-4-1BB Ab treated OT-I Rag−/− mice produce IFN-γ and TNF-α as determined by intracellular cytokine staining. p values determined by Student’s t test. Data were obtained from a minimum of 3 mice per group.
To determine the cell population(s) that serves as the direct target for the agonistic anti-4-1BB Ab-mediated toxicity, we performed adoptive transfer experiments using various purified cell populations. Inasmuch as 4-1BB is constitutively expressed on a subpopulation of DCs [1;35], we first tested if these cells serve as initial targets for the anti-4-1BB Ab-mediated toxicity. The transfer of 2 × 106 highly purified bone marrow-derived WT DCs (purity >97%) into 4-1BB−/− mice followed by treatment with anti-4-1BB Ab did not result in detectable toxicity (data not shown). In marked contrast, adoptive transfer of flow cytometry sorted WT CD8+ or CD4+ T cells (both purities >95%) into 4-1BB−/− mice one day prior to anti-4-1BB Ab treatment resulted in full toxicity in a dose dependent manner as manifested by enlarged spleens and peripheral LNs (Fig. 5A), and increased total (Fig. 5B) and individual CD4+ T cells, CD8+ T cells, and CD19+ B cells in these organs (Supporting Fig. 8). This effect was not limited to polyclonal T cell populations as flow sorted monoclonal naïve OT-I Rag−/− CD8+ T cells and OT-II Rag−/−CD4+ T cells (1 × 106 of each cell type, both purities >95%) resulted in full blown toxicity when adoptively transferred into 4-1BB−/− mice treated with the anti-4-1BB Ab (Fig. 5A,B). In addition, over 95% of the transferred OT-I and OT-II T cells underwent proliferation as determined by CFSE dilution assay (data not shown). Furthermore, treatment of naïve OT-I Rag−/− or OT-II Rag−/− mice with one injection of anti-4-1BB Ab resulted in significant toxicity (Fig. 5C–F). Taken together, these data demonstrate that both CD8+ and CD4+ T cells serve as targets for the anti-4-1BB induced toxicity irrespective of their TCR antigen specificity or TCR repertoire diversity.
4. Discussion
The development of immunomodulators that invoke desired immune responses without adverse toxicity is crucial to the success of vaccines against cancer and infections. As costimulatory molecules of the CD28 and TNFR superfamilies play critical roles in the modulation of innate, adaptive, and regulatory immunity[1], they may serve effective immunomodulatory components of vaccines. Consistent with this notion are the demonstrated efficacy of agonistic Abs to costimulatory receptors in various virus and cancer immunotherapy settings [2–5]. However, systemic toxicity appears to be a common side effect of treatment with agonistic Abs to the members of CD28 and TNFR superfamilies [6–12,32]. The failed Phase I clinical trial resulting from a single intravenous dose of a superagonist anti-CD28 monoclonal Ab that led to multi-organ failure and a systemic inflammatory response [8] highlights the importance of developing costimulatory agonists that transduce the appropriate stimulatory signals without toxic side effects in vivo. We hypothesized that toxicity reported using agonistic mAbs to costimulatory receptors may be inherent to Abs, rather than being a generalized feature of the costimulatory system. Natural ligands may lack such toxicity and have better efficacy due to their potential ability to transduce signals having quantitative and/or qualitative differences with those elicited by agonistic Abs.
This hypothesis was tested using a novel form of 4-1BBL with potent immunomodulatory activity in soluble form [22,23] as a test case. The rationale for using 4-1BBL is several-fold. First, 4-1BB receptor is constitutively expressed by a subpopulation of DCs, which receive a stimulatory signal via 4-1BB resulting in up-regulation of costimulatory molecules and increased secretion of cytokines [35,36]. Second, 4-1BB is transiently expressed to high levels on activated CD4+ and CD8+ T cells, but not naïve T cells, thereby potentially allowing for selected modulation of antigen-specific T cells. Third, signaling via 4-1BB endows T effector cells refractory to suppression by CD4+CD25+FoxP3+ Treg cells [22,23,27] as well as reverses anergy in CD8+ T cells [3]. Therefore, 4-1BB/4-1BBL system has the potential to serve as an effective component of therapeutic vaccines against chronic infections and cancer due to its pleiotropic effects on innate, adaptive, and regulatory immunity. Consistent with this notion is our recent publication demonstrating that SA-4-1BBL as the immunomodulatory component of a peptide-based vaccine had better therapeutic efficacy in a mouse cervical cancer model than TLR agonists MPL and CpG [23].
We herein report that SA-4-1BBL had better costimulatory activity on both CD4+ and CD8+ T cells as compared with the agonistic 3H3 Ab at various doses tested in vitro and in vivo. Indeed, the agonistic Ab to 4-1BB had no detectable costimulatory effect on CD4+ T cells in vitro while such an effect in vivo was moderate. This observation is consistent with various studies demonstrating the preferential induction of CD8+ T cells by agonistic Abs to 4-1BB [3,17,26]. The potent costimulatory activity of SA-4-1BBL on CD8+ T cells and its differential effect on CD4+ T cells as compared with 3H3 Ab is consistent with the better efficacy of the ligand in generating in vivo killing responses and eradicating established tumors as the immunomodulatory component of a therapeutic cancer vaccine [23]. With potent costimulatory activity on CD4+ T cells, SA-4-1BBL may serve as a powerful adjuvant in subunit vaccines targeting CD4+ T cells, such as those against HIV and tuberculosis. The better efficacy of natural ligands as compared with agonistic Abs may be due to the kinetics of on/off rates with their respective receptors, the better ability of natural ligands to crosslink their respective receptors on the cell surface, and/or the quality/quantity of downstream signaling. Most importantly, the better immunostimulatory activity of SA-4-1BBL was achieved in the absence of severe toxicity associated with therapeutic doses of agonistic 3H3 Ab in naïve mice that caused enlarged spleen and LNs, non-specific T cell proliferation and expansion, systemic proinflammatory cytokine response, altered lymphocyte trafficking, and hepatitis. The toxicity associated with the use of 3H3 Ab in this study is consistent with our recently published study using various agonistic Abs to 4-1BB for immunomodulation [7]. However, lack of toxicity with SA-4-1BBL treatment is inconsistent with previous reports demonstrating toxicity in transgenic mice over-expressing 4-1BBL under the control of MHC class II promoter [37,38]. This may be due to 4-1BBL reverse signaling in the case of the transgenic mice [39–42]. Reverse signaling through 4-1BBL can lead to APC maturation, upregulation of T cell costimulatory molecules, production of proinflammatory TNF-α and IL-6, and inhibition of IL-10 production [39–42].
Our observation that 3H3 Ab causes nonspecific proliferation of T cells in naïve mice is consistent with a recent report by Zhu et al. using a different agonistic anti-4-1BB Ab (clone 2A) [38]. Unlike the studies of Zhu et al. that demonstrated the proliferative effect of the 2A Ab on memory, but not naïve, T cells, we observed the proliferative effect of 3H3 Ab on both naive and memory T cells. First, using Abs to the CD43 (mAb 1B11) and CD44 to distinguish between newly activated naïve CD8+ T cells (CD43highCD44high) and memory CD8+ T cells (CD43low/− CD44high) cells [29–31], we demonstrated that both T cell populations undergo proliferation in response to 3H3 Ab treatment. Second, treatment of 4-1BB−/− mice adoptively transferred with CFSE labeled sorted naïve OT-I Rag−/− or OT-II Rag−/− T cells (both purities >95%) resulted in excessive T cell proliferation and full toxicity. Third, direct treatment of naïve OT-I Rag−/− or OT-II Rag−/− mice with one injection of 3H3 Ab resulted in significant toxicity. Although the source of discrepancy between our data and those of Zhu et al. is not known, differences in experimental conditions and nature of agonistic Abs used may provide an explanation. Consistent with this notion are reports that certain CD28 superagonist Abs directly induce the proliferation of both naïve and T memory cells [43,44], while others act strictly on T memory cells [45].
However, unlike CD28 receptor that is constitutively expressed on T cells, 4-1BB is selectively expressed at high levels in activated, but not naive, T lymphocytes as assessed by both mRNA as well as protein expression [1;13;14]. Therefore, our observations that naïve T cells serve as a direct target of the agonistic anti-4-1BB Ab is surprising. A plausible explanation is that Ab-mediated toxicity could be initiated by a subpopulation of newly activated or memory T cells specific for environmental antigens with upregulated expression of 4-1BB on their surface. This contention, however, is inconsistent with Ab-mediated toxicity observed in OT-I Rag−/− and OT-II Rag−/− mice or 4-1BB−/− mice adoptively transferred with sorted naïve OT-I CD8+ and OT-II CD4+ T cells as these cells are not expected to react to environmental antigens due to their strict specificities for OVA, and as such represent naïve T cells. Alternatively, all naïve or a subpopulation of T cells could express undetectable levels of 4-1BB that upon supraphysiological agonistic 4-1BB Ab stimulation initiates immune responses resulting in full blown Ab-mediated toxicity.
The mechanisms of T-cell activation by agonistic mAbs to CD28 and 4-1BB Ab in the absence of antigen are unclear, but in both cases involve receptor cross linking. The signaling ability of CD28 superagonist Abs depends on a functional TCR [44;46]. Therefore, it is hypothesized that CD28 superagonist Abs activate T cells by amplifying low level tonic signals from unligated TCR. A similar mechanism may operate in anti-4-1BB Ab activation of antigen inexperienced T cells. Similar to CD28 superagonist Abs, treatment with anti-4-1BB Ab resulted in in vivo expansion of CD4+CD25+FoxP3+ Treg cells. In rodents, CD28 superagonist Ab administration resulted in almost immediate severe lymphopenia similar to that seen in humans, and induced strong naïve and effector T cell activation and increased mRNA levels of proinflammatory cytokines and chemokines. This initial wave of effector T cell activation was followed by a second wave of activation that resulted in the preferential expansion of Treg cells, and there was no detectable increase in secreted pro-inflammatory cytokines [47]. It was hypothesized that expanded Treg cells were able to suppress conventional T cell inflammatory cytokine secretion before it reached harmful levels. In support of this hypothesis, Treg depleted mice treated with a CD28 superagonist Ab resulted in the rapid, systemic release of high levels of inflammatory cytokines [48]. However, anti-4-1BB Ab administration resulted in increases in both serum and intracellular pro-inflammatory cytokines, irrespective of Treg expansion. This may be due to the ability of 4-1BB signaling to endow T effector cells refractory to the suppression function of Treg cells [22,27,49].
Mechanism(s) responsible for the differential effects of agonistic Abs and natural ligands vis-a-vis toxicity vs. efficacy are not known. Engagement of the activating FcγR receptors on immune cells by anti-4-1BB Ab or activation of the complement system do not play a role as treatment of mice lacking activating FcγRs or complement C1q and C3 with the agonistic anti-4-1BB Ab resulted in full blown toxicity. However, it is conceivable that the inhibitory FcγRIIB, which does not require FcR γ-chain expression for cell surface expression or function [34], could create cognates between T cells and monocytes or other cell types and could trigger toxicity. Such cognates could increase low level tonic signals to T cells leading to T cell activation.
In summary, we demonstrated that a soluble form of 4-1BBL chimeric with core streptavidin has better immunostimulatory activity and safety profile than an agonistic Ab to 4-1BB receptor. These observations support our notion that active forms of natural ligands, such as 4-1BBL, to costimulatory molecules have the potential to serve as more powerful and safer alternatives to agonistic mAbs to the receptors as immunomodulatory components of vaccine formulations. Generation of additional costimulatory proteins having potent immunomodulatory functions in soluble form and testing their efficacy in various prophylactic and therapeutic vaccine settings will be critical to the development of costimulatory ligands as a new class of adjuvants.
Supplementary Material
Acknowledgments
This work was funded in parts by grants from the NIH (R43 AI071618, R41 CA121665, R44 AI071618, and R43AI074176), Kentucky Lung Cancer Research Program, W.M. Keck Foundation, and the Commonwealth of Kentucky Research Challenge Trust Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The SA-4-1BBL described in this manuscript is licensed from UofL by ApoImmune, Inc., Louisville, KY, for which Haval Shirwan serves as CSO and Haval Shirwan and Esma S.Yolcu have significant equity interest in the Company. The other authors disclosed no potential conflict of interest.
Reference List
- 1.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu.Rev.Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 2.Uno T, Takeda K, Kojima Y, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat.Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J.Clin.Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat.Immunol. 2002;3:536–541. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 5.Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat.Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 6.Hixon JA, Blazar BR, Anver MR, Wiltrout RH, Murphy WJ. Antibodies to CD40 induce a lethal cytokine cascade after syngeneic bone marrow transplantation. Biol.Blood Marrow Transplant. 2001;7:136–143. doi: 10.1053/bbmt.2001.v7.pm11302547. [DOI] [PubMed] [Google Scholar]
- 7.Niu L, Strahotin S, Hewes B, et al. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J.Immunol. 2007;178:4194–4213. doi: 10.4049/jimmunol.178.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N.Engl.J.Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 9.Ferlin WG, von der WT, Cottrez F, et al. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 mAb. Eur.J.Immunol. 1998;28:525–531. doi: 10.1002/(SICI)1521-4141(199802)28:02<525::AID-IMMU525>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 10.Hixon JA, Anver MR, Blazar BR, et al. Administration of either anti-CD40 or interleukin-12 following lethal total body irradiation induces acute lethal toxicity affecting the gut. Biol.Blood Marrow Transplant. 2002;8:316–325. [PubMed] [Google Scholar]
- 11.van Mierlo GJ, den Boer AT, Medema JP, et al. CD40 stimulation leads to effective therapy of CD40(−) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc.Natl.Acad.Sci.U.S.A. 2002;99:5561–5566. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J.Clin.Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 13.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc.Natl.Acad.Sci.U.S.A. 1989;86:1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollok KE, Kim YJ, Zhou Z, et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J.Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- 15.Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc.Natl.Acad.Sci.U.S.A. 2004;101:1291–1296. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers L, Lee SW, Rossi RJ, et al. Combined CD137 (4-1BB) and adjuvant therapy generates a developing pool of peptide-specific CD8 memory T cells. Int.Immunol. 2006;18:325–333. doi: 10.1093/intimm/dxh371. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J.Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 18.Waller EC, McKinney N, Hicks R, et al. Differential costimulation through CD137 (4-1BB) restores proliferation of human virus-specific "effector memory" (CD28(−) CD45RA(HI)) CD8(+) T cells. Blood. 2007;110:4360–4366. doi: 10.1182/blood-2007-07-104604. [DOI] [PubMed] [Google Scholar]
- 19.Miller RE, Jones J, Le T, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J.Immunol. 2002;169:1792–1800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 20.Rabu C, Quemener A, Jacques Y, et al. Production of recombinant human trimeric CD137L (4-1BBL). Cross-linking is essential to its T cell co-stimulation activity. J.Biol.Chem. 2005;280:41472–41481. doi: 10.1074/jbc.M506881200. [DOI] [PubMed] [Google Scholar]
- 21.Saoulli K, Lee SY, Cannons JL, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J.Exp.Med. 1998;187:1849–1862. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elpek KG, Yolcu ES, Franke DD, et al. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J.Immunol. 2007;179:7295–7304. doi: 10.4049/jimmunol.179.11.7295. [DOI] [PubMed] [Google Scholar]
- 23.Sharma RK, Elpek KG, Yolcu ES, Schabowsky RH, Zhao H, Bandura-Morgan L, Shirwan H. Costimulation as a Platform for the Development of Vaccines: A Peptide-Based Vaccine Containing a Novel form of 4-1BB Ligand Eradicates Established Tumors. Cancer Res. 2009;69:4319–4326. doi: 10.1158/0008-5472.CAN-08-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukushima A, Yamaguchi T, Ishida W, et al. Engagement of 4-1BB inhibits the development of experimental allergic conjunctivitis in mice. J.Immunol. 2005;175:4897–4903. doi: 10.4049/jimmunol.175.8.4897. [DOI] [PubMed] [Google Scholar]
- 25.Irie J, Wu Y, Kachapati K, Mittler RS, Ridgway WM. Modulating protective and pathogenic CD4+ subsets via CD137 in type 1 diabetes. Diabetes. 2007;56:186–196. doi: 10.2337/db06-0793. [DOI] [PubMed] [Google Scholar]
- 26.Shuford WW, Klussman K, Tritchler DD, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J.Exp.Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson SJ, Messer RJ, Carmody AB, et al. CD137 costimulation of CD8+ T cells confers resistance to suppression by virus-induced regulatory T cells. J.Immunol. 2008;180:5267–5274. doi: 10.4049/jimmunol.180.8.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Guo Z, Dong Y, et al. Role of 4-1BB in allograft rejection mediated by CD8+ T cells. Am.J.Transplant. 2003;3:543–551. doi: 10.1034/j.1600-6143.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 29.Chang JT, Palanivel VR, Kinjyo I, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 30.Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J.Exp.Med. 2000;191:1241–1246. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manjunath N, Shankar P, Wan J, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J.Clin.Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stebbings R, Findlay L, Edwards C, et al. "Cytokine storm" in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J.Immunol. 2007;179:3325–3331. doi: 10.4049/jimmunol.179.5.3325. [DOI] [PubMed] [Google Scholar]
- 33.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 34.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Wilcox RA, Chapoval AI, Gorski KS, et al. Cutting edge: Expression of functional CD137 receptor by dendritic cells. J.Immunol. 2002;168:4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 36.Futagawa T, Akiba H, Kodama T, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int.Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 37.Zhu G, Flies DB, Tamada K, et al. Progressive depletion of peripheral B lymphocytes in 4-1BB (CD137) ligand/I-Ealpha)-transgenic mice. J.Immunol. 2001;167:2671–2676. doi: 10.4049/jimmunol.167.5.2671. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, Zhu G, Luo L, Flies AS, Chen L. CD137 stimulation delivers an antigen-independent growth signal for T lymphocytes with memory phenotype. Blood. 2007;109:4882–4889. doi: 10.1182/blood-2006-10-043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang D, Chen Y, Schwarz H. CD137 induces proliferation of murine hematopoietic progenitor cells and differentiation to macrophages. J.Immunol. 2008;181:3923–3932. doi: 10.4049/jimmunol.181.6.3923. [DOI] [PubMed] [Google Scholar]
- 40.Langstein J, Michel J, Fritsche J, et al. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J.Immunol. 1998;160:2488–2494. [PubMed] [Google Scholar]
- 41.Lippert U, Zachmann K, Ferrari DM, et al. CD137 ligand reverse signaling has multiple functions in human dendritic cells during an adaptive immune response. Eur.J.Immunol. 2008;38:1024–1032. doi: 10.1002/eji.200737800. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz H. Biological activities of reverse signal transduction through CD137 ligand. J.Leukoc.Biol. 2005;77:281–286. doi: 10.1189/jlb.0904558. [DOI] [PubMed] [Google Scholar]
- 43.Sester U, Wabnitz GH, Kirchgessner H, Samstag Y. Ras/PI3kinase/cofilin-independent activation of human CD45RA+ and CD45RO+ T cells by superagonistic CD28 stimulation. Eur.J.Immunol. 2007;37:2881–2891. doi: 10.1002/eji.200737206. [DOI] [PubMed] [Google Scholar]
- 44.Waibler Z, Sender LY, Merten C, et al. Signaling signatures and functional properties of anti-human CD28 superagonistic antibodies. PLoS.ONE. 2008;3:e1708. doi: 10.1371/journal.pone.0001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh M, Basu S, Camell C, et al. Selective expansion of memory CD4(+) T cells by mitogenic human CD28 generates inflammatory cytokines and regulatory T cells. Eur.J.Immunol. 2008;38:1522–1532. doi: 10.1002/eji.200737929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dennehy KM, Elias F, Na SY, et al. Mitogenic CD28 signals require the exchange factor Vav1 to enhance TCR signaling at the SLP-76-Vav-Itk signalosome. J.Immunol. 2007;178:1363–1371. doi: 10.4049/jimmunol.178.3.1363. [DOI] [PubMed] [Google Scholar]
- 47.Muller N, van den BJ, Odoardi F, et al. A CD28 superagonistic antibody elicits 2 functionally distinct waves of T cell activation in rats. J.Clin.Invest. 2008;118:1405–1416. doi: 10.1172/JCI32698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gogishvili T, Langenhorst D, Luhder F, et al. Rapid regulatory T-cell response prevents cytokine storm in CD28 superagonist treated mice. PLoS.ONE. 2009;4:e4643. doi: 10.1371/journal.pone.0004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi BK, Bae JS, Choi EM, et al. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J.Leukoc.Biol. 2004;75:785–791. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.