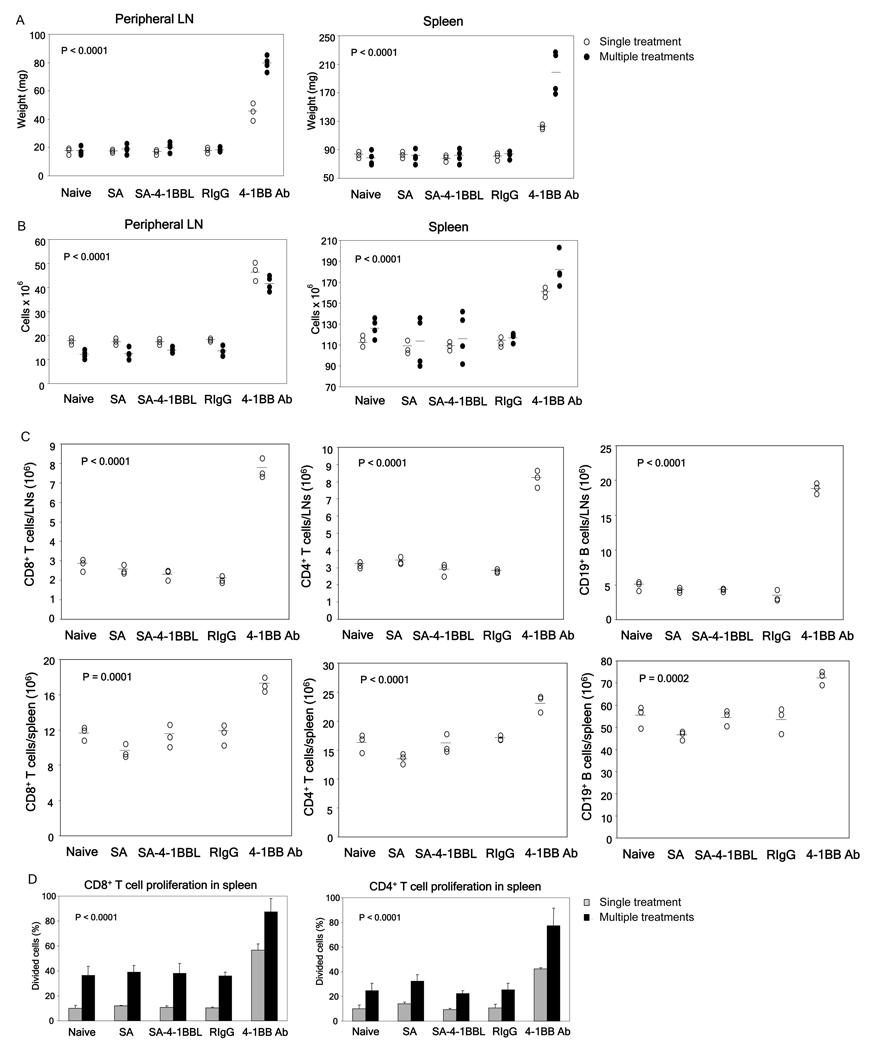
Fig 2.
SA-4-1BBL treatment lacks Ab associated toxicity. Naïve C57BL/6 mice were injected s.c. once (○) or once weekly over a 3-wk period (●) with 100 µg of SA-4-1BBL, equivalent doses of anti-4-1BB Ab, or other appropriate controls. Animals were euthanized one week after the final treatment and assessed for various indicators of toxicity, including peripheral LN and spleen weights (A) and total peripheral LN and spleen cell numbers (B). (C) Anti-4-1BB Ab demonstrates super-agonistic activity in vivo. In vivo proliferation of spleen CD8+ and CD4+ T cells was determined by adoptively transferring 2 × 106 CFSE-labeled CD45.1+ congenic splenocytes into naïve C56BL/6 (CD45.2+) mice one day prior to the indicated treatments. Percentage of proliferating cells was determined by CFSE dilution using FACS analysis. p values determined by ANOVA, anti-4-1BB vs. all other groups within same treatment schedule. Data were obtained from a minimum of 3 mice per group.