Abstract
Although the Jak2-V617F mutation has generated strong awareness because of its causative role in myeloproliferative disorders, reports of Jak2 gene aberrations linked to hematologic malignancies have preceded those of V617F by nearly a decade. These malignant mutations include Jak2 amino acid substitutions, deletions, insertions, and chromosomal translocations. As a consequence, researchers are increasingly focused on identifying Jak2 inhibitors that suppress aberrant Jak2 kinase activity. Some of these inhibitors may one day become therapeutically beneficial for individuals with Jak2-related hematologic malignancies. This review summarizes various Jak2 mutations associated with hematologic malignancies and assesses some of the Jak2 inhibitors in the preclinical phase or in clinical trials. By reviewing these specific areas, we hope to have a better understanding of Jak2’s role in hematologic malignancies and to shed light on the utility of Jak2 inhibitors.
Introduction
Since its discovery in 1992 [1], Jak2 tyrosine kinase has emerged as an important molecule in mammalian development, physiology, and disease. Jak2 is a nonreceptor tyrosine kinase that is widely expressed, as it is found in virtually every cell type. It is essential for signaling through a variety of cytokine receptors, such as those that bind growth hormone, prolactin, erythropoietin, and thrombopoietin. In addition, it is important for the family of cytokines that signal through the interleukin-3 and gp130 receptors. Although intensive studies in the past decade have led to a general understanding of how most cytokine receptors activate the Jak/STAT signaling pathway, the exact molecular mechanisms of Jak2 activation are not fully understood and continue to be an active area of research. Jak2 is thought to be activated by a conformational change in the receptor that allows trans- and/or autophosphorylation of the two bound Jak2 molecules. This ligand-dependent tyrosine phosphorylation occurs principally on Tyr 1007 [2]. Activated Jak2 then phosphorylates specific tyrosine residues on the cytoplasmic tails of the receptors, creating docking sites for the SH2 domain–containing STAT proteins. Once bound to the receptors, STATs are themselves phosphorylated by Jak2 on tyrosine residues. Subsequently, phosphorylated STATs form dimers and translocate into the nucleus, where they regulate gene transcription. Thus, Jak2 is responsible for transducing a signal from the cell surface to the nucleus through a tyrosine phosphorylation signaling mechanism.
Although appropriate Jak2 expression levels need to be maintained for animal survival, too much Jak2 tyrosine kinase activity may have deleterious effects. For instance, mutations in the Jak2 allele leading to the proliferation of a neoplastic clone were identified recently in myeloproliferative disorders. The discovery of the Jak2-V617F mutation in nearly all polycythemia vera (PV) and a large subset of essential thrombocythemia and primary myelofibrosis patients has prompted researchers to closely study the Jak2 gene and its role in hematologic disorders. In addition, constitutive activation of Jak2 kinase activity by chromosomal translocations has been reported in various types of leukemia [3,4]. Currently, however, no US Food and Drug Administration–approved Jak2 inhibitor therapies are available for use in the clinic, although a few are being examined for their efficacy and safety in phase 1/2 clinical trials. Thus, the continual identification of novel activating Jak2 mutations, and their correlation with hematologic malignancies, highlights the requirement for the development of potent and therapeutically effective Jak2 inhibitors.
The Role of Jak2 in Myeloproliferative Disorders
In 2005, five independent studies reported the identification of a Jak2 somatic mutation (Val 617 to Phe) in several myeloproliferative disorders at a high frequency [5–9]. Studies employing sensitive detection methodologies indicated that the Jak2-V617F mutation on exon 14 can be detected in almost all PV patients and in approximately 50% of essential thrombocythemia and primary myelofibrosis patients [10]. These myeloproliferative disorders are characterized by the clonal overproduction of normally differentiated hematopoietic lineages. The V617F substitution leads to constitutive activation of Jak2 and downstream effector signaling pathways including the STAT transcription pathway and phosphoinositide 3-kinase and extracellular signal–regulated kinase (ERK) signaling networks, which in turn induce inappropriate cytokine-independent proliferation of cells [7,11]. The nature of this gain-of-function mutation is that Val 617 lies in the JH2/pseudokinase autoinhibitory domain of Jak2. Current molecular models of the pseudokinase domain suggest that it interacts with the activation loop of the kinase domain [12]. Moreover, structure/function studies have shown that amino acids located between positions 619 and 970 are critical for maintaining the inhibitory property of the pseudokinase domain [13]. Therefore, it is hypothesized that the V617F mutation impedes the pseudokinase domain from acting as an internal inhibitory regulator of the adjacent kinase domain, resulting in aberrant Jak2 tyrosine kinase activity.
Although the Jak2-V617F mutation is associated predominantly with myeloproliferative disorders, it is evident that other activating alleles of Jak2 also are involved in these disorders. For example, Scott et al. [14] identified a set of novel somatic Jak2 mutations on exon 12 in patients with Jak2-V617F–negative PV or idiopathic erythrocytosis. Specifically, these mutations mapped to amino acid residues 537 to 543, which is a region that links the SH2 and JH2 domains of Jak2. Patients harboring these mutations displayed isolated erythrocytosis, reduced serum erythropoietin, and factor-independent erythrocyte colony formation.
The Role of Jak2 in Hematologic Malignancies
The first study indicating that a mutant Jak kinase could result in a hematologic malignancy was in 1995, when Luo et al. [15] demonstrated that a glycine to glutamic acid substitution at position 341 in the Drosophila hopscotch gene caused a leukemia-like hematopoietic defect. Two years later, studies linked Jak2 chromosomal translocations to human neoplastic growth. Specifically, a translocation event between the kinase domain of Jak2 and the helix-loop-helix domain of the ETS family transcription factor TEL was identified in a child with early B-precursor acute lymphoid leukemia and in an adult with atypical chronic myeloid leukemia [3,16]. The basis for the diverse phenotype detected in these two patients is the result of two distinct translocation events within the Jak2 and TEL genes that consequently give rise to distinct chimeras. Nevertheless, these TEL-Jak2 fusion proteins cause increased oligomerization of the Jak2 proteins that lead to growth factor–independent Jak2 activation and subsequent nuclear factor (NF)-κB signaling [17]. Moreover, creation of TEL-Jak2 transgenic mice revealed a causal relationship between the TEL-Jak2 gene product and leukemogenesis, as overexpression of this fusion protein resulted in the development of T-cell leukemia in these animals [18].
Apart from TEL-Jak2, studies have implicated Jak2 in other chromosomal translocations observed in various hematologic malignancies. Miyamoto et al. [19] showed that the Jak2 inhibitor AG490 reduced the growth of human B-precursor leukemic cells. Specifically, they found that AG490 significantly downregulated Jak2 phosphorylation in these cells at a concentration that had little effect on normal hematopoiesis. Consequently, this study correlated an 11q23 translocation or Philadelphia chromosome with constitutive Jak2 activation in human lymphoid leukemic cells. In addition, Joos et al. [20] analyzed four Hodgkin’s lymphoma cell lines and identified chromosomal rearrangements of the short arm of chromosome 2 involving REL, a transcription factor belonging to the NF-κ B family. This resulted in a copy number increase of Jak2 (9p24) in three of the four cell lines. These results suggested that REL and Jak2 may play an important role in the pathogenesis of Hodgkin’s lymphoma. Recent studies have demonstrated that human autoantigen pericentriolar material (PCM1) is a Jak2 translocation partner associated with chronic and acute leukemias, including chronic eosinophilic leukemia, acute myeloid leukemia, and acute lymphoblastic leukemia [4,21]. In all cases, the PCM1-Jak2 fusion involved a t(8;9)(p22;p24) translocation event. The chimeric gene product was predicted to encode a protein that maintains several of the coiled-coil domains of PCM1 and the kinase domain of Jak2. The PCM1 coiled motifs possibly serve as a dimerization motif to bring about constitutive activation of Jak2. Lastly, BCR-Jak2 fusions have been identified in patients with typical and atypical chronic myeloid leukemia [22,23••]. In each case, in situ hybridization revealed a t(9;22)(p24;q11.2) translocation in these patients as opposed to the typical t(9;22)(q34;q11.2) translocation. Although the breakpoints were variable in each patient, the rearrangement resulted in a BCR-Jak2 chimera rather than the classic BCR-ABL fusion protein. A common finding in these patients was that they exhibited relatively early blast crisis. All together, BCR-Jak2 represents a novel fusion protein detected in chronic myeloid leukemia.
Activating Jak2 somatic mutations such as amino acid substitution mutations and deletions also have been identified in hematologic malignancies. Mercher et al. [24••] identified a novel Jak2-T875N mutation in an acute megakaryoblastic leukemic cell line using a combination of mass spectrometry and growth inhibition assays via the use of a selective tyrosine kinase inhibitor. The authors demonstrated that the Jak2-T875N was constitutively active in vitro and induced a myeloproliferative disease with characteristics of megakaryoblastic leukemia in a murine bone marrow transplantation assay. Other novel mutations have been reported in the JH2 domain of Jak2 that confer constitutive activation of the Jak-STAT signaling pathway. These include the Jak2-K607N [25••] and Jak2-L611S [26] mutations found in acute myeloid leukemia and acute lymphoblastic leukemia, respectively. Finally, a deletion of amino acids 682 to 686 (Jak2-Δ682IREED) has been observed in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia [27•].
Collectively, the aforementioned studies indicate that the Jak2 locus is susceptible to chromosomal rearrangement, point mutations, and deletions, all of which are associated with hematologic malignancies. These Jak2 gene aberrations are summarized in Table 1. Jak2 translocation chimeras appear to increase Jak2 oligomerization and result in growth factor–independent Jak2 autoactivation, whereas Jak2 point mutations and deletions lead to hypersensitivity to growth factors through impaired Jak2 autoregulation. Nevertheless, the end result is that the aberrant Jak2 protein has constitutively active tyrosine kinase activity that results in a neoplastic phenotype.
Table 1.
Partial list of Jak2 gene aberrations reported in hematologic disorders*
| Study | Mutation type | Mutation | Phenotype | Year identified |
|---|---|---|---|---|
| Lacronique et al. [3], Peeters et al. [16], Carron et al. [18] | Translocation | TEL-Jak2 | ALL, aCML | 1997 |
| Joos et al. [20] | Translocation | REL-Jak2 | aCML, Hodgkin’s lymphoma | 2003 |
| Reiter et al. [4], Murati et al. [21] | Translocation | PCM1-Jak2 | aCML, AML, ALL | 2005 |
| Griesinger et al. [22] | Translocation | BCR-Jak2 | CML | 2005 |
| Lane et al. [23••] | Translocation | BCR-Jak2 | AML | 2008 |
| James et al. [5], Kralovics et al. [6], Levine et al. [7], Baxter et al. [8], Zhao et al. [9] | Substitution | Jak2-V617F | PV, ET, PMF | 2005 |
| Mercher et al. [24••] | Substitution | Jak2-T875N | Megakaryoblastic leukemia | 2006 |
| Lee et al. [25••] | Substitution | Jak2-K607N | AML | 2006 |
| Kratz et al. [26] | Substitution | Jak2-L611S | ALL | 2006 |
| Scott et al. [14] | Substitution | Jak2-K539L | PV, idiopathic erythrocytosis | 2007 |
| Malinge et al. [27•] | Deletion | Jak2-ΔIREED | ALL | 2007 |
These malignant mutations include Jak2 amino acid substitutions, deletions, and chromosomal translocations identified from 1997 to 2008. aCML—atypical chronic myeloid leukemia; ALL—acute lymphoblastic leukemia; AML—acute myeloid leukemia; CML—chronic myeloid leukemia; ET—essential thrombocythemia; PMF—primary myelofibrosis; PV—polycythemia vera.
Inhibitors of Jak2 Tyrosine Kinase
The causal relationship between constitutive Jak2 tyrosine kinase activity and neoplastic growth prompted researchers to identify potent and selective Jak2 small molecule inhibitors. In 1995, Meydan et al. [28] used a high-throughput screen of potential tyrosine kinase inhibitors and identified tyrphostin B42 (AG490) as the first Jak2 inhibitor. Their important finding was that AG490 blocked the growth of leukemic cells derived from patients who expressed constitutive Jak2 tyrosine kinase activity. The compound induced cellular apoptosis, without any deleterious effect on normal hematopoiesis. However, subsequent reports revealed that although AG490 is a potent inhibitor of Jak2, it suffers from a general lack of specificity [29].
To circumvent this problem, researchers have used different approaches to identify novel Jak2 selective inhibitors. In 2004, for example, Flowers et al. [30] developed a short peptide inhibitor of Jak2, termed Tkip, that mimics the actions of the Jak2 inhibitor protein SOCS1 (suppressor of cytokine signaling 1). They reported that the inhibitor peptide mimicked SOCS1 in that it specifically inhibited Jak2 tyrosine 1007 phosphorylation and suppressed IFN-γ signaling. In 2005, our group published a paper whereby we constructed a homology model of the Jak2 kinase domain and used a high-throughput program called DOCK to identify novel small molecule inhibitors of Jak2 tyrosine kinase [31]. Specifically, we tested 6451 compounds of known chemical structure in silico for their ability to interact with a pocket positioned adjacent to the activation loop of Jak2. The top seven scoring compounds were obtained from the National Cancer Institute and tested for their ability to inhibit Jak2 autophosphorylation in vitro. We found that one compound, C7, directly inhibited Jak2 tyrosine kinase activity. Characterization of C7 revealed that this compound suppressed Jak2 tyrosine autophosphorylation in a dose- and time-dependent manner. C7 significantly reduced growth hormone–dependent Jak2 autophosphorylation but had no effect on epidermal growth factor receptor tyrosine phosphorylation. Moreover, C7 was not cytotoxic to cells at doses as high as 100 μM, as measured by the ability of cells to exclude propidium iodide. All together, our results suggested that C7 may be a relatively specific Jak2 inhibitor, and we proposed that it may be useful for elucidating Jak2 signaling mechanisms.
The discovery of the Jak2-V617F mutation in 2005 and its identification in a high percentage of myeloproliferative disorders have further spurred interest in the development of small molecule inhibitors that selectively target Jak2. Moreover, the resolution of the crystal structures of portions of the kinase domains of Jak3 and Jak2 in 2005 and 2006, respectively, have provided a valuable tool for designing potent and specific Jak2 small molecule inhibitors [32,33]. Recently, a group developed several novel Jak2-selective small molecule compounds (TG101209 and TG101348) while considering the crystal structures of the kinase domains of both Jak2 and Jak3 [34•,35•]. They showed that TG101209 and TG101348 potently inhibit Jak2 tyrosine kinase, with considerably less activity against other tyrosine kinases, such as Jak3. These compounds suppress the proliferation of human erythroleukemia cells, which express the Jak2-V617F mutation. Furthermore, they demonstrated that both compounds effectively treat Jak2-V617F–induced hematopoietic disease in mice and reduce the growth of hemopoietic colonies from primary progenitor cells harboring Jak2-V617F mutations. Currently, the TG101348 compound has been assigned as a lead drug for clinical development for the potential treatment of Jak2-V617F–induced myeloproliferative disorders.
Another Jak2-selective inhibitor, INCB18424, is presently in phase 1/2 clinical trials in primary myelofibrosis patients at M.D. Anderson Cancer Center. Although it has reduced splenomegaly, unfortunately it has not diminished the marrow fibrosis [36].
In 2008, Verstovsek et al. [37•] demonstrated that a novel analogue of AG490, WP1066, potently suppressed proliferation and induced apoptosis in erythroid human cells harboring the Jak2-V617F mutation. In addition, WP1066 inhibited the expansion of peripheral blood hematopoietic progenitors of PV patients who were positive for the Jak2-V617F mutation. Interestingly, WP1066 was previously shown to inhibit phosphorylation of Jak2 in acute myelogenous leukemia cells, but unlike AG490, this compound also degraded the Jak2 protein [38]. Collectively, the data suggest that WP1066 is a potent Jak2 inhibitor in vitro and ex vivo and warrants further development for treating myeloproliferative disorders and other hematologic malignancies associated with constitutive Jak2 activity.
Our laboratory recently contributed to the continuing development of small molecule inhibitors that target aberrant Jak2 activity by using a rapid structure-based approach combining molecular docking with cell-based functional testing. Like others, we took into consideration the crystal structure for portions of the Jak3 kinase domain to generate an atomic model of the kinase domain of murine Jak2 and then used the DOCK program to predict the ability of 20,000 small molecules to interact with a structural pocket adjacent to the adenosine tri-phosphate–binding site. Consequently, we identified a Jak2-selective inhibitor termed Z3 [39]. We found that it bound to Jak2 with a favorable energy score and inhibited Jak2-V617F autophosphorylation in a dose-dependent manner but was not cytotoxic to cells at concentrations that inhibited kinase activity. Z3 selectively inhibited Jak2 as it had no effect on Tyk2 and c-Src kinase activity. Furthermore, Z3 significantly inhibited proliferation of the Jak2-V617F–expressing HEL cells, and this Z3-mediated reduction in cell growth correlated with reduced Jak2 and STAT3 tyrosine phosphorylation levels, as well as marked cell cycle arrest. Finally, Z3 inhibited the growth of hematopoietic progenitor cells isolated from the bone marrow of an essential thrombocythemia patient carrying the Jak2-V617F mutation and a PV patient harboring a Jak2-F537I mutation. Together, our results suggest that Z3 is a specific inhibitor of Jak2 tyrosine kinase.
In addition to the drugs that were targeted specifically for Jak2, there is a group of drugs that were developed for treating nonmyeloproliferative disorders but are now considered to have therapeutic potential in myeloproliferative disorders because of their significant off-target Jak2 inhibitory activity. Some of these drugs are even in phase 1/2 clinical trials. For example, MK-0457 (formerly VX-680), a potent inhibitor of Aurora kinases, effectively inhibits BCR-ABL, FLT3, and Jak2 [40]. A phase 1/2 clinical trial of MK-0457 was initiated in patients with chronic myelogenous leukemia or Philadelphia chromosome–positive acute lymphoblastic leukemia who carried the T315I BCR-ABL resistance mutation, as well as in patients with refractory Jak2-V617F–positive myeloproliferative disease. This compound showed encouraging antineoplastic growth activity and a good safety profile [41•]. Another off-target Jak2 inhibitor, CEP-701 (lestaurtinib), was originally developed to suppress tropomyosin receptor kinase A activity for possible use in prostate cancer but was later discovered to exhibit FLT3 inhibitory activity as well. CEP-701 has been shown to inhibit Jak2 tyrosine kinase activity and inhibit the proliferation of progenitor cells obtained from patients with myeloproliferative disorders [42]. Unfortunately, CEP-701 has shown little to no activity in treating primary myelofibrosis in phase 2 clinical studies. Finally, AT9283, another Aurora kinase as well as a potent Jak2 inhibitor, is in phase 1/2 clinical trials for the treatment of acute leukemias, chronic myelogenous leukemia, and primary myelofibrosis [43•].
Other non-Jak2 selective inhibitors are still in pre-clinical testing for the treatment of Jak2-associated hematologic disorders. For example, Gö6976, an inhibitor of the calcium-dependent isozymes of PKC and FLT3 tyrosine kinase, has been found to be a direct and potent inhibitor of Jak2 in vitro. This compound also suppresses signaling, survival, and proliferation of cells expressing the TEL-Jak2 fusion protein or the Jak2-V617F mutation [44]. These data suggest that Gö6976 may be useful for treating myeloproliferative disorders or other Jak2-associated hematologic malignancies. In addition, erlotinib (Tarceva; Genentech, South San Francisco, CA), which is used for treating patients with locally advanced or meta-static non–small cell lung cancer, inhibited the growth and expansion of Jak2-V617F–expressing PV hematopoietic progenitor cells and human erythroleukemia HEL cells while having little effect on normal cells [45].
Another compound with Jak2-inhibitory properties is Atiprimod (Callisto Pharmaceuticals, New York, NY), an orally bioavailable agent that has been investigated for its anti-inflammatory and anticancer properties. Faderl et al. [46] reported that Atiprimod inhibits Jak2/STAT phosphorylation and blocks clonogenic growth of acute myelogenous leukemia cell lines and patient-derived acute myelogenous leukemic marrow cells by inducing apoptosis. Their data suggest that the antiproliferative and proapoptotic activities of Atiprimod toward acute myelogenous leukemia cells might be attributed to the inhibition of the Jak-STAT signaling pathway. Interestingly, the inhibitory effect of this compound has not been evaluated on Jak2-V617F–dependent pathologic cell growth. Thus, Atiprimod may warrant further evaluation as a drug candidate for treating hematologic disorders associated with constitutive Jak2 activation.
Finally, CP-690,550, a selective Jak3 inhibitor, also exhibits Jak2-inhibitory properties. Manshouri et al. [47] demonstrated that it exerts potent antiproliferative activity against cells expressing the Jak2-V617F mutation. In fact, CP-690,550 suppressed Jak2-V617F–dependent cell growth in vitro (50% inhibitory concentration [IC50] = 0.2 μM) ten times more potently than wild-type (WT) Jak2 (IC50 = 2.1 μM). It induced a marked proapoptotic effect on cells harboring the Jak2-V617F mutation, whereas a smaller effect was observed for cells carrying WT Jak2. Furthermore, CP-690,550 selectively inhibited the growth of Jak2-V617F–positive cells in ex vivo expanded progenitors from PV patients, which correlated with a decrease in Jak2-V617F mutant allele frequency. Taken together, the data suggest that CP-690,550 is a putative inhibitor of Jak2-V617F in vitro and ex vivo.
Collectively, work by many groups, including our own, has identified various small molecule inhibitors that suppress Jak2 tyrosine kinase activity. Some of these small molecule compounds may be classified as Jak2 selective (class I) because they specifically target Jak2. Alternatively, many of these compounds may be categorized as non-Jak2 selective (class II) because they initially were developed for nonmyeloproliferative disorders but were subsequently shown to have considerable Jak2 inhibition. These inhibitors are summarized in Table 2.
Table 2.
Partial list of class I and II Jak2 inhibitors identified since 1996*
| Study | Inhibitor | Class | Current status | Year identified |
|---|---|---|---|---|
| Meydan et al. [28] | AG490 | I | Preclinical | 1996 |
| Flowers et al. [30] | Tkip | 1 | Preclinical | 2004 |
| Sandberg et al. [31] | Z3 | I | Preclinical | 2005 |
| Pardanani et al. [34•] | TG101209 | I | Preclinical | 2007 |
| Verstovsek et al. [37•] | WP1066 | I | Preclinical | 2008 |
| Sayyah et al. [39] | C7 | I | Preclinical | 2008 |
| Wernig et al. [35•] | TG101348 | I | Phase 1/2 | 2008 |
| Pardanani [40] | INCB018424 | I | Phase 1/2 | 2008 |
| Grandage et al. [44] | Gö6976 | II | Preclinical | 2006 |
| Li et al. [45] | Erlotinib | II | Preclinical | 2007 |
| Faderl et al. [46] | Atiprimod | II | Preclinical | 2007 |
| Manshouri et al. [47] | CP-690,550 | II | Preclinical | 2008 |
| Ravandi et al. [43•] | AT9283 | II | Phase 1/2 | 2006 |
| Hexner et al. [42] | CEP-701 | II | Phase 2 | 2007 |
| Wang and Serradell [41•] | MK-0457 | II | Phase 1/2 | 2007 |
The class I Jak2 inhibitors are considered to be Jak2-selective compounds, whereas the class II inhibitors are categorized as non–Jak2 selective. The Jak2 small molecule inhibitors listed are in preclinical or phase 1/2 clinical trials.
Conclusions
Although the Jak2-V617F mutation on exon 14 is the predominant disease-associated allele, a growing number of Jak2 somatic cell mutations and chromosomal translocations are linked to hyperkinetic Jak2 kinase activity and hematologic malignancies. As a consequence, it seems rational to develop highly sensitive and specific diagnostic tools for detecting Jak2 mutations. In this regard, testing for the Jak2-V617F mutation is becoming increasingly more common [48,49]. Additionally, very recent basic science studies have provided methodologies for detecting several Jak2 mutations on exon 12 [50]. Given the large number of Jak2 mutations now found in myeloproliferative disorders and hematologic malignancies, one wonders whether entire Jak2 gene screens will become a viable diagnostic tool in the future.
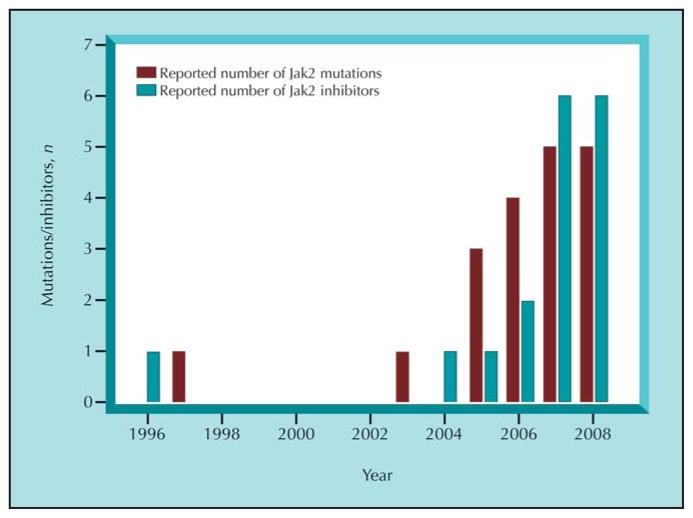
With the growing number of reported Jak2 mutations found in hematologic malignancies and myeloproliferative disorders, we also have seen a marked increase in the number of reported Jak2 inhibitors (Fig. 1). What is absolutely stunning about the development of the inhibitors is 1) their sheer number and 2) the speed at which they are entering clinical trials. In the case of the TG101209 compound, for example, clinical trials were initiated in Jak2-V617F–positive myeloproliferative individuals less than 3 years after the Jak2-V617F mutation was discovered. For comparison, it was nearly 40 years after the identification of the Philadelphia chromosome that imatinib (Gleevec; Novartis, East Hanover, NJ) was introduced into human medicine. One possible weakness facing the current state of Jak2 inhibitors, however, is that although these compounds suppress mutant Jak2 tyrosine kinase activity, they also inhibit WT Jak2 function. For example, Pardanani et al. [34•] demonstrated that a 500-nM dose of TG101209 completely inhibited WT Jak2 tyrosine kinase activity. Moreover, our laboratory showed that the Z3 compound inhibited Jak2-WT tyrosine autophosphorylation (IC50 = ~ 15 μM) more effectively relative to Jak2-V617F (IC50 = ~ 28 μM) [39]. Given that normal Jak2 function is critical for hematopoiesis and transmission of the growth hormone signaling cascade, one wonders about the possible deleterious effects of blocking WT Jak2 function.
Figure 1.
An approximation of the number of reported Jak2 mutations and Jak2 inhibitors discovered by year. The reported Jak2 gene aberrations include Jak2 amino acid substitutions, deletions, insertions, and chromosomal translocations identified since 1997. The Jak2 inhibitors consist of Jak2 and non-Jak2 selective compounds in preclinical or clinical trials.
Currently, the lack of structural information regarding the Jak2 autoinhibitory domain may be an impediment to the design of inhibitors that selectively target pathologic Jak2 kinase activity. To overcome this obstacle, the crystal structure of full-length Jak2, or at least the autoinhibitory domain coupled to the kinase domain, may need to be resolved so we can have a better understanding of the structural differences between mutant and WT protein. Presumably, this would allow for the development of inhibitors that block only mutant Jak2 kinase activity. As our structural knowledge regarding the Jak2 protein increases, perhaps it is not unreasonable to think we may evolve toward Jak2 designer drugs based on specific mutations and/or particular hematologic malignancies.
In summary, activating Jak2 mutations are found in almost all individuals with PV and a substantial proportion of individuals with essential thrombocythemia and primary myelofibrosis. An increasing number of Jak2 aberrations, such as substitution mutations, deletions, insertions, and gene translocations, also are being found in several hematopoietic malignancies. The expanding compendium of Jak2 aberrations found in hematologic disorders justifies the need for quantitative Jak2 mutation testing in the clinic and validates their candidacy for targeted therapy. As such, the role of Jak2 inhibitors as therapeutic agents in hematologic malignancies seems more than rational.
Acknowledgments
This work was supported by a Biomedical Research Support Program for Medical Schools Award to the University of Florida College of Medicine by the Howard Hughes Medical Institute, a University of Florida Opportunity Fund Award, an American Heart Association Greater Southeast Affiliate Grant in Aid (#0855361E), and National Institutes of Health Awards R01-HL67277 and T32-HL83810.
Footnotes
Disclosures
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Harpur AG, Andres AC, Ziemiecki A, et al. JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene. 1992;7:1347–1353. [PubMed] [Google Scholar]
- 2.Feng J, Witthuhn BA, Matsuda T, et al. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol. 1997;17:2497–2501. doi: 10.1128/mcb.17.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacronique V, Boureux A, Valle VD, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 4.Reiter A, Walz C, Watmore A, et al. The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer Res. 2005;65:2662–2667. doi: 10.1158/0008-5472.CAN-04-4263. [DOI] [PubMed] [Google Scholar]
- 5.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 6.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 7.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine RL, Belisle C, Wadleigh M, et al. X-inactivation-based clonality analysis and quantitative JAK2V617F assessment reveal a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F-negative ET and MMM patients with clonal hematopoiesis. Blood. 2006;107:4139–4141. doi: 10.1182/blood-2005-09-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shannon K, Van Etten RA. JAKing up hematopoietic proliferation. Cancer Cell. 1995;7:291–293. doi: 10.1016/j.ccr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Giordanetto F, Kroemer RT. Prediction of the structure of human Janus kinase 2 (JAK2) comprising JAK homology domains 1 through 7. Protein Eng. 2002;15:727–737. doi: 10.1093/protein/15.9.727. [DOI] [PubMed] [Google Scholar]
- 13.Saharinen P, Vihinen M, Silvennoinen O. Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain. Mol Biol Cell. 2003;14:1448–1459. doi: 10.1091/mbc.E02-06-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott LM, Tong W, Levien RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo H, Hanratty WP, Dearolf CR. An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 1995;14:1412–1420. doi: 10.1002/j.1460-2075.1995.tb07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peeters P, Raynaud SD, Cools J, et al. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997;90:2535–2540. [PubMed] [Google Scholar]
- 17.Lacronique V, Boureux A, Monni R, et al. Transforming properties of chimeric TEL-JAK proteins in Ba/F3 cells. Blood. 2000;95:2076–2083. [PubMed] [Google Scholar]
- 18.Carron C, Cormier F, Janin A, et al. TEL-JAK2 transgenic mice develop T-cell leukemia. Blood. 2000;95:3891–3899. [PubMed] [Google Scholar]
- 19.Miyamoto N, Sugita K, Goi K, et al. The JAK2 inhibitor AG490 predominantly abrogates the growth of human B-precursor leukemic cells with 11q23 translocation or Philadelphia chromosome. Leukemia. 2001;15:1758–1768. doi: 10.1038/sj.leu.2402260. [DOI] [PubMed] [Google Scholar]
- 20.Joos S, Granzow M, Holtgreve-Gez H, et al. Hodgkin’s lymphoma cell lines are characterized by frequent aberrations on chromosomes 2p and 9p including REL and JAK2. Int J Cancer. 2003;103:489–495. doi: 10.1002/ijc.10845. [DOI] [PubMed] [Google Scholar]
- 21.Murati A, Gelsi-Boyer V, Adelaide J, et al. PCM1-JAK2 fusion in myeloproliferative disorders and acute erythroid leukemia with t(8;9) translocation. Leukemia. 2005;19:1692–1696. doi: 10.1038/sj.leu.2403879. [DOI] [PubMed] [Google Scholar]
- 22.Griesinger F, Hennig H, Hillmer F, et al. A BCR-JAK2 fusion gene as the result of a t(9;22)(p24;q11.2) translocation in a patient with a clinically typical chronic myeloid leukemia. Genes Chromosomes Cancer. 2005;44:329–333. doi: 10.1002/gcc.20235. [DOI] [PubMed] [Google Scholar]
- 23••.Lane SW, Fairbairn DJ, McCarthy C, et al. Leukaemia cutis in atypical chronic myeloid leukaemia with a t(9;22) (p24;q11.2) leading to BCR-JAK2 fusion. Br J Haematol. 2008;142:503. doi: 10.1111/j.1365-2141.2008.07164.x. This study identified a BCR-Jak2 fusion protein as a result of a t(9;22) (p24;q11.2) translocation event in atypical chronic myeloid leukemia. [DOI] [PubMed] [Google Scholar]
- 24••.Mercher T, Wernig G, Moore SA, et al. JAK2T875N is a novel activating mutation that results in myeloproliferative disease with features of megakaryoblastic leukemia in a murine bone marrow transplantation model. Blood. 2006;108:2770–2779. doi: 10.1182/blood-2006-04-014712. This study identified a novel Jak2-T875N mutation in an acute megakaryoblastic leukemic cell line. This mutation was shown to be constitutively active in vitro and induced a myeloproliferative disease with characteristics of megakaryoblastic leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Lee JW, Kim YG, Soung YH, et al. The JAK2 V617F mutation in de novo acute myelogenous leukemias. Oncogene. 2006;25:1434–1436. doi: 10.1038/sj.onc.1209163. This study demonstrated the presence of the Jak2-V617F mutation as well as a novel Jak2-K607N mutation in de novo acute myelogenous leukemias. This is the 3 rst report confirming Jak2 gene mutations in acute myelogenous leukemia. [DOI] [PubMed] [Google Scholar]
- 26.Kratz CP, Boll S, Kontny U, et al. Mutational screen reveals a novel JAK2 mutation, L611S, in a child with acute lymphoblastic leukemia. Leukemia. 2006;20:381–383. doi: 10.1038/sj.leu.2404060. [DOI] [PubMed] [Google Scholar]
- 27•.Malinge S, Ben-Abdelali R, Settegrana C, et al. Novel activating JAK2 mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia. Blood. 2007;109:2202–2204. doi: 10.1182/blood-2006-09-045963. This study reported the identification of a novel Jak2-acquired mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia. The Jak2ΔIREED mutation involves a 3 ve–amino acid deletion within the pseudokinase domain of Jak2. [DOI] [PubMed] [Google Scholar]
- 28.Meydan N, Grunberger T, Dadi H, et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 29.Gu Y, Zuo Y, Aikawa R, et al. Growth hormone signaling and apoptosis in neonatal rat cardiomyocytes. Mol Cell Biochem. 2001;223:35–46. doi: 10.1023/a:1017941625858. [DOI] [PubMed] [Google Scholar]
- 30.Flowers LO, Johnson HM, Mujtaba MG, et al. Characterization of a peptide inhibitor of Janus kinase 2 that mimics suppressor of cytokine signaling 1 function. J Immunol. 2004;172:7510–7518. doi: 10.4049/jimmunol.172.12.7510. [DOI] [PubMed] [Google Scholar]
- 31.Sandberg EM, Ma X, He K, et al. Identification of 1,2,3,4,5,6-hexabromocyclohexane as a small molecule inhibitor of Jak2 tyrosine kinase autophosphorylation. J Med Chem. 2005;48:2526–2533. doi: 10.1021/jm049470k. [DOI] [PubMed] [Google Scholar]
- 32.Boggon TJ, Li Y, Manley PW, Eck MJ. Crystal structure of the Jak3 kinase domain in complex with a staurosporine analog. Blood. 2005;106:996–1002. doi: 10.1182/blood-2005-02-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucet IS, Fantino E, Styles M, et al. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood. 2006;107:176–183. doi: 10.1182/blood-2005-06-2413. [DOI] [PubMed] [Google Scholar]
- 34•.Pardanani A, Hood J, Lasho T, et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia. 2007;21:1658–1668. doi: 10.1038/sj.leu.2404750. First report demonstrating that a novel Jak2 inhibitor developed by TargeGen, Inc. (San Diego, CA) potently and selectively inhibits Jak2-dependent pathologic cell growth in vitro, ex vivo, and in vivo. [DOI] [PubMed] [Google Scholar]
- 35•.Wernig G, Kharas MG, Okabe R, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13:311–320. doi: 10.1016/j.ccr.2008.02.009. This study reported that TG101348, a selective Jak2 inhibitor, exhibited therapeutic efficacy in a murine model of myeloproliferative disease induced by the Jak2-V617F mutation. Currently, this compound is designated as a lead drug for clinical development for the potential treatment of Jak2-V617F–induced myeloproliferative disorders. [DOI] [PubMed] [Google Scholar]
- 36.Verstovsek S, Kantarjian H, Pardanani A, et al. INCB18424, an oral, selective Jak2 inhibitor, shows significant clinical activity in a phase I/II study in patients with primary myelofibrosis and post polycythemia vera/essential thrombocythemia myelofibrosis [abstract 7004]. Presented at the 44th Annual Meeting of the American Society of Clinical Oncology.; Chicago, IL. May 30–June 3, 2008. [Google Scholar]
- 37•.Verstovsek S, Manshouri T, Quintas-Cardama, et al. WP1066, a novel JAK2 inhibitor, suppresses proliferation and induces apoptosis in erythroid human cells carrying the JAK2 V617F mutation. Clin Cancer Res. 2008;14:788–796. doi: 10.1158/1078-0432.CCR-07-0524. This report shows that a novel analogue of AG490, WP1066, potently suppressed proliferation and induced apoptosis in human cells carrying the Jak2-V617F mutation. This compound was previously shown to inhibit Jak2 phosphorylation in acute myelogenous leukemia cells. [DOI] [PubMed] [Google Scholar]
- 38.Ferrajoli A, Faderl S, Van Q, et al. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res. 2007;23:11291–11299. doi: 10.1158/0008-5472.CAN-07-0593. [DOI] [PubMed] [Google Scholar]
- 39.Sayyah J, Magis A, Ostrov DA, et al. Z3, a novel Jak2 tyrosine kinase small molecule inhibitor that suppresses Jak2-mediated pathologic cell growth. Mol Cancer Ther. 2008;7:2308–2318. doi: 10.1158/1535-7163.MCT-08-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardanani A. JAK2 inhibitor therapy in myeloproliferative disorders: rationale, preclinical studies and ongoing clinical trials. Leukemia. 2008;22:23–30. doi: 10.1038/sj.leu.2404948. [DOI] [PubMed] [Google Scholar]
- 41•.Wang Y, Serradell N. VX-680/MK-0457. Drugs Fut. 2007;32:144. MK-0457, an effective Jak2 inhibitor, showed encouraging antineoplastic growth activity and a good safety profile during phase 1/2 clinical trials involving patients with refractory Jak2-V617F–positive myeloproliferative disease. [Google Scholar]
- 42.Hexner EO, Serkikoff C, Jan M, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111:5663–5671. doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Ravandi F, Foran J, Verstovsek S, et al. A phase I trial of AT9283, a mutlitargeted kinase inhibitor, in patients with refractory hematological malignancies [abstract 904]. Presented at the 49th Annual Meeting of the American Society of Hematology; Atlanta, GA. December 8–11, 2007; AT9283, a potent Jak2 inhibitor, is currently in phase 1/2 clinical trials for the treatment of acute leukemias, chronic myelogenous leukemia, and primary myelofibrosis. [Google Scholar]
- 44.Grandage VL, Everington T, Linch DC, Khwaja A. Gö6976 is a potent inhibitor of the JAK2 and FLT3 tyrosine kinases with significant activity in primary acute myeloid leukaemia cells. Br J Haematol. 2006;135:303–316. doi: 10.1111/j.1365-2141.2006.06291.x. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Xu M, Xing S, et al. Erlotinib effectively inhibits JAK2V617F activity and polycythemia vera cell growth. J Biol Chem. 2007;282:3428–3432. doi: 10.1074/jbc.C600277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faderl S, Ferrajoli A, Harris D, et al. Atiprimod blocks phosphorylation of JAK-STAT and inhibits proliferation of acute myeloid leukemia (AML) cells. Leuk Res. 2007;31:91–95. doi: 10.1016/j.leukres.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 47.Manshouri T, Quintas-Cardama A, Nussenzveig RH, et al. The JAK kinase inhibitor CP-690,550 suppresses the growth of human polycythemia vera cells carrying the JAK2V617F mutation. Cancer Sci. 2008;99:1265–1273. doi: 10.1111/j.1349-7006.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith CA, Fan G. The saga of JAK2 mutations and translocations in hematological disorders: pathogenesis, diagnostic and therapeutic prospects, and revised World Health Organization diagnostic criteria for myeloproliferative neoplasms. Hum Pathol. 2008;39:795–810. doi: 10.1016/j.humpath.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Steensma DP. JAK2 V617F in myeloid disorders: molecular diagnostic techniques and their clinical utility: a paper from the 2005 William Beaumont Hospital Symposium on Molecular Pathology. J Mol Diagn. 2006;8:397–411. doi: 10.2353/jmoldx.2006.060007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones AV, Cross NC, White HE, et al. Rapid identification of JAK2 exon 12 mutations using high resolution melting analysis. Haematologica. 2008;93:1560–1564. doi: 10.3324/haematol.12883. [DOI] [PubMed] [Google Scholar]