Abstract
Cardiac fibroblasts are emerging as key components of normal cardiac function as well as the response to stressors and injury. These most numerous cells of the heart interact with myocytes via paracrine mechanisms, alterations in extracellular matrix homeostasis, and direct cell-cell interactions. It is possible that they are a contributor to the inability of adult myocytes to proliferate, and may influence cardiac progenitor biology. Furthering our understanding of how cardiac fibroblast and myocytes interact may provide an avenue to novel treatments for heart failure prevention. This review discusses the most recent concepts in cardiac fibroblast-myocyte communication and areas of potential future research.
Keywords: Cardiac Failure, Fibroblasts, Cardiac Differentiation, Cytokines, Growth Factors
Introduction
Cardiac fibroblasts have received relatively little attention compared to their more famous neighbors, the cardiomyocytes. Cardiac fibroblasts are often regarded as the “spotters”, nonchalantly watching the cardiomyocytes do the real weight-lifting, and waiting for a catastrophe that requires their actions. However, emerging data now reveal the fibroblast as not only a critical player in the response to injury, but also as an active participant in normal cardiac function.
Interest in cardiac fibroblasts has grown with the recognition that cardiac fibrosis is a prominent contributor to diverse forms of myocardial disease.(1–5) In the early 1990’s, identification of angiotensin receptors on the surface of cardiac fibroblasts linked the renin-angiotensin-aldosterone system directly with pathologic myocardial and matrix extracellular remodeling.(6, 7) Fibroblasts were also revealed as a major source of not only extracellular matrix, but the proteases that regulate and organize matrix. New research has uncovered paracrine and well as direct cell-to-cell interactions between fibroblasts and their cardiomyocyte neighbors, and cardiac fibroblasts appear to be dynamic participants in ventricular physiology and pathophysiology.
This review will focus on several aspects of fibroblast-myocyte communication, including mechanisms of paracrine communication. Because it is now clear that fibroblasts can directly affect several important physiological properties of myocardium, this topic is particularly timely given the broad interest in cardiac regeneration, including cell therapy. Ongoing efforts at regeneration of cardiac tissue focus primarily on increasing the number of cardiomyocytes in damaged myocardium. Although getting cardiomyocytes into myocardium is an important goal, understanding intercellular paracrine communication between different cell types, including endothelial cells but also fibroblasts, may prove crucial to regenerating stable myocardium that responds to physiological conditions appropriately.
Fibroblast - cardiomyocyte interactions during development and repair
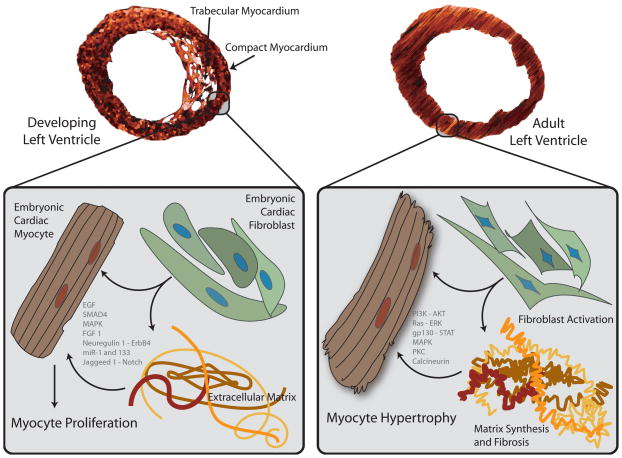
Cardiac fibroblasts may communicate with cardiomyocytes early in development, but surprisingly few investigations have addressed this question in vivo. This may be, in part, due to the lack of a single definition of a fibroblast, as well as the lack of specific cardiac fibroblast molecular markers and enhancers. Ieda and colleagues demonstrated a unique effect of embryonic cardiac fibroblasts on developing cardiac myocytes.(8) Embryonic cardiac fibroblasts reside within the developing compact myocardium and increase in number over the course of development. They express many components of the extracellular matrix, including fibronectin, collagens, periostin, hyaluronan and proteoglycan link protein 1. Embryonic cardiomyocytes grown on plates enriched with fibronectin, collagen type III, periostin or laminin showed an increase in proliferation. This effect involves β1-integrin signaling and heparin-binding EGF-like growth factor, with induction of downstream ERK and p38MAPK signaling. Interestingly, when embryonic mouse cardiac myocytes were co-cultured with adult cardiac fibroblasts, a hypertrophic rather than a proliferative phenotype was seen, with increased sarcomeric organization and cell size. This suggests that paracrine factors derived from cardiac fibroblasts may influence the phenotype of cardiomyocytes during development in a manner distinct from effects in the adult (Figure 1).
Figure 1. Interactions between cardiac fibroblasts, myocytes and the extracellular matrix in the developing versus adult heart.
In the developing ventricle, embryonic cardiac fibroblasts reside within the outer, compacting myocardium. These fibroblasts express components of the extracellular matrix, including fibronectin and collagen, as well as heparin-binding EGF-like growth factor, that may promote myocyte proliferation.(8) Intracellular signals are activated by myocyte integration of fibroblast-derived paracrine stimuli and signals from matrix proteins to effect coordinated proliferation and organogenesis. In contrast, under biomechanical stress, adult cardiac myocytes respond in a hypertrophic rather than proliferative manner, in part fostered by paracrine signals from neighboring cardiac fibroblasts and interactions with the remodeling extracellular matrix. Again, numerous intracellular signals systems are activated to effect a coordinated hypertrophic program.(163)
Several molecules known to be produced by cardiac fibroblasts have been implicated in cardiac myocyte development, including Fibroblast Growth Factors (FGF). FGF2 and FGF4 induce expression of early cardiac transcription factors, as well as ventricular (but not atrial) specific markers, in the developing chick embryo.(9) The loss of FGF1 arrests multipotent precursor cells in their development toward a cardiac myocyte lineage.(10) Other FGFs have been implicated in Wnt/β-catenin signaling and anterior heart field formation, suggesting that specific FGF family members may influence cardiac morphogenesis in a regional manner.(11) Gp130, a transmembrane protein subunit that serves as a signaling relay for members of the IL-6 family, also plays a prominent role in cardiac development. The Gp130 knockout mouse fails to develop compact myocardium and dies in mid-to-late gestation (reviewed in (12)). Interestingly, members of the IL-6 family that are known to be produced by cardiac fibroblasts, such as cardiotrophin-1 and leukemia inhibitory factor, are not required for cardiac development, (12, 13) and deletion of multiple members of the IL-6 family does not result in cardiac lethality in utero,(14) suggesting functional redundancy in this developmentally critical signaling pathway.
The role of cardiac fibroblasts in cardiomyocyte regeneration in the adult heart is also unclear, although there is support for the concept that fibroblast activity impairs regeneration.(15) Most tissues that regenerate in mammals can heal without extensive fibrosis. For example, the epidermis undergoes healing with minimal scar formation. Critical differences between scar formation in epidermal tissue and in cardiac tissue include myofibroblast apoptosis during epidermal wound evolution in comparison with persistence of activated fibroblasts within injured myocardium.(16, 17) Non-mammalian species with robust regenerative capacity, such as zebrafish and Urodele amphibians, repair wounds with minimal scar formation. Whether regeneration occurs via lineage commitment of a progenitor cell such as in zebrafish myocardial repair(18) or de-differentiation and replication of terminally differentiated resident cells within the heart as in Urodeles,(19) there appears to be a balance between functional regenerative capacity and fibrotic scar formation. It is unclear if the tendency for fibrosis in the mammalian heart after injury is the result of an inherent inability of adult cardiomyocytes to divide, necessitating an exuberant fibrotic response, or if the fibrosis itself prevents cardiomyocytes from adopting a replicative phenotype.
Fibrocytes are bone marrow derived cells that circulate in the blood, express hematopoietic cell surface markers, and can produce extracellular matrix proteins.(20, 21) Fibrocyte cells can differentiate down multiple mesenchymal lineages depending on their molecular microenvironment and may participate in pathological end organ fibrosis.(22–24) They may even be coaxed to manifest properties of mature cardiomyocytes themselves.(25) It has been shown in the context of myocardial infarction that bone-marrow derived cells can invade the myocardium and differentiate into cells with surface markers consistent with a fibroblast phenotype. Mollmann and colleagues used sublethally-irradiated mice with subsequent reconstitution of bone marrow with hematopoietic cells expressing Green Fluorescent Protein (GFP). Upon experimental myocardial infarction via epicardial coronary artery occlusion, the number of GFP+ cardiomyocytes was negligible in relation to the numerous GFP+ fibroblasts and myofibroblasts, as identified by cell surface markers.(26) This process of myofibroblast commitment may be mediated by transforming growth factor-β1 signaling.(27) Although these data suggest that circulating progenitors play a role in the cardiac response to injury, the relative contribution of bone marrow-derived fibroblast cells vs. resident fibroblasts remains unclear.
During early endocardial cushion development, migrating cells of endothelial origin lineage switch to form mesenchyme.(reviewed in (28)) Recent data suggest that this endothelial-to-mesenchymal transition may contribute to the cardiac fibroblast pool in the adult mouse. Zeisberg and colleagues have demonstrated that cells of endothelial origin, during biomechanical overload and under direction of transforming growth factor-β signaling, lose endothelial-specific and gain fibroblast- specific markers and produce proteins such as vimentin, pro-collagen, and α-smooth muscle actin that are typically associated with fibroblasts.(29) How such endothelium-derived cardiac fibroblasts may regulate potential cardiac repair and the biology of cardiac progenitor cells remains to be explored.
Fibroblast - cardiomyocyte paracrine communication
Cardiac fibroblasts may regulate cardiomyocyte phenotype through paracrine hormonal pathways, and it is also likely the cardiomyocytes regulate fibroblast phenotype. There are numerous lines of evidence indicating that cardiac fibroblasts and myocytes release into their local microenvironment proteins that regulate neighboring cells. Although multiple factors have been implicated in this intercellular crosstalk, the following discussion will focus on the best studied of these factors for which the strongest data have been published, including transforming growth factor β1, fibroblast growth factor 2, members of the interleukin-6 family of proteins, and the recently discovered cytokine interleukin-33.
Transforming Growth Factor β
Transforming growth factor β (TGFβ) regulates the ventricular response to pressure overload as well as injury, including fibrosis and cellular hypertrophy.(30–32) TGFβ exists in three forms, TGFβ1, TGFβ2 and TGFβ3, each encoded by a distinct gene; these forms are intracellular as well as extracellular, residing within the interstitium in an inactive state bound to latent TGFβ binding protein (LTBP). When activated by proteolytic cleavage, TGFβ can bind to cell surface transmembrane receptors to activate Smad-mediated transcriptional events.(33) Ablation of any TGFβ gene results in distinct phenotypic derangements, highlighting the separate roles of the TGFβs in mammalian physiology.(34–36)
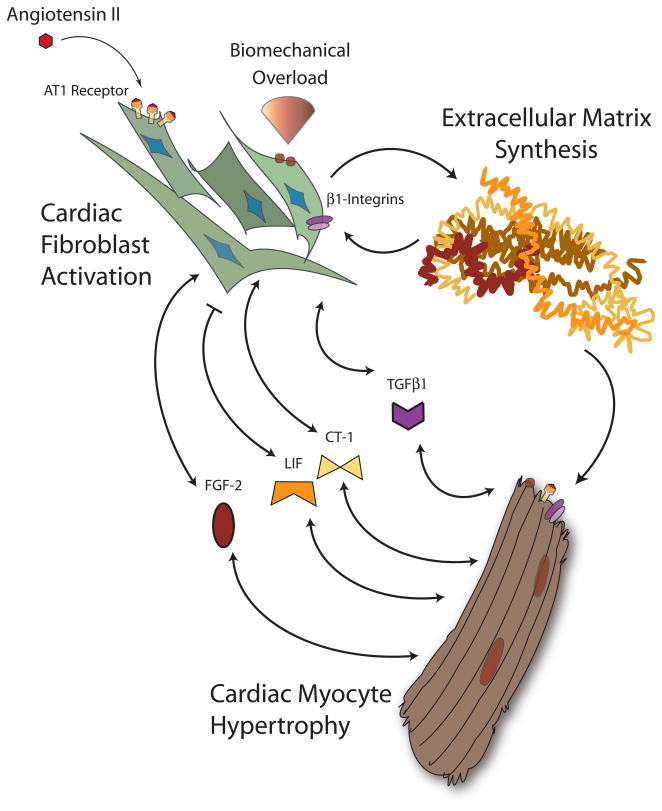
Not only is TGFβ expressed by cardiomyocytes and interstitial cells of both the adult and fetal heart,(37) it is actively released from myocytes and cardiac fibroblasts.(38–41) TGFβ1 is induced and released from cardiomyocytes in response to mechanical stretch,(42) and its expression is upregulated in the context of pressure overload and myocardial infarction. (40, 43, 44) The receptors for TGFβ are found on both ventricular myocytes and fibroblasts.(45) TGFβ1 can elicit myofibroblast transformation(46–49) as well as a marked increase in extracellular protein production. (50, 51) Through the angiotensin type 1 receptor, angiotensin II stimulation of fibroblasts induces TGFβ1,(52, 53) may alter TGFβ1 receptor expression, (54) and increases matrix protein synthesis.(55–57) Importantly, this accumulation of fibroblast-derived extracellular collagen occurs only when fibroblasts are co-cultured with cardiomyocytes.(58–61) Zeisberg and colleagues have documented that in the context of pressure overload, cardiac fibroblasts may arise from endothelial cells in a manner dependent on TGFβ1 and downstream Smad signaling.(29) TGFβ1 has clearly been implicated in cardiomyocyte hypertrophy(62) and may participate in the pathogenesis of human hypertrophic cardiomyopathy.(63–65) TGFβ1 is likely a key mediator of myocyte growth in response to angiotensin II, but in a manner that may require cardiac fibroblasts. Gray and colleagues demonstrated that angiotensin II induces myocyte hypertrophy in co-culture with fibroblasts but not in monoculture preparations; a similar effect was seen with fibroblast-conditioned medium. Of note, the preponderance of angiotensin receptor expression was seen in fibroblasts rather than myocytes.(60) This suggests that the primary target of angiotensin is the cardiac fibroblast, with its ultimate effect on cardiomyocytes occurring in a paracrine fashion via TGFβ (Figure 2). This paracrine effect may not be homogenous throughout the heart. Transgenic expression of constitutively active TGFβ1 in cardiac myocytes results in atrial but not ventricular fibrosis as well as increased ventricular fibroblast apoptosis.(66)
Figure 2. Paracrine bidirectional cardiac fibroblast-myocyte crosstalk during biomechanical strain.
Under biomechanical overload, cardiac fibroblasts and myocytes respond to an altered environment via multiple mechanisms including integrin-extracellular matrix interactions and renin-angiotensin-aldosterone axis activation. Cardiac fibroblasts increase synthesis of matrix proteins and secrete a variety of paracrine factors that can stimulate myocyte hypertrophy. Cardiac myocytes similarly respond by secreting a conglomerate of factors. Hormones such as TGFβ1, FGF-2, and the IL-6 family members LIF and CT-1 have all been implicated in this bidirectional fibroblast-myocyte hormonal crosstalk.
In addition to its effects on fibrosis and hypertrophy, TGFβ has also been shown to coax pluripotent cells towards a cardiomyocyte transcriptional and morphologic phenotype.(67–70) Behfar and colleagues first showed that TGFβ upregulates cardiac transcription factors Nkx2.5 and MEF2C in mouse embryonic stem cells and enhanced the formation of rhythmically contractile embryoid bodies. Stem cells expressing a dominant negative TGFβ failed to differentiate.(67) These observations raise the question of whether fibroblast-myocyte communication may be taking place not only at the level of terminally differentiated cardiomyocytes, but on a cardiomyogenic progenitor cell pool as well.
Fibroblast growth factor-2
Fibroblast growth factor-2, or FGF-2, is an intracellular and extracellular protein synthesized by both myocytes and non-myocytes. Upon release into the interstitial space, FGF-2 binds to heparin sulfate proteoglycans and components of the basement membrane (reviewed in (71)). FGF-2 exists in two forms of different molecular weights (reviewed in (72)). In cardiomyocytes, FGF-2 is induced by adrenergic stimulation and, in a positive feedback loop, by products of its own synthesis.(73, 74) FGF-2 is also upregulated by angiotensin II stimulation and ischemia or hypoxia (Figure 2).(75, 76) FGF-2 lacks a canonical amino-terminal secretory sequence, and the mechanisms by which it is released from myocytes and cardiac fibroblasts are incompletely defined. There is evidence that FGF-2 is released from cells during periods of a loss in cell membrane integrity. This may occur in the setting of toxic or hypoxic cellular injury, programmed cell death, during vesicular “shedding” or during transient, reversible disruptions in the context of cellular contraction.(77–79) Interestingly, embryonic and adult cardiac fibroblasts predominantly express the higher-molecular weight form of FGF-2, which is further upregulated and released upon angiotensin II exposure. High molecular weight FGF-2 induces a fetal gene program and promotes hypertrophy of cardiomyocytes ((80) and reviewed in (81)).
Germline genetic ablation of FGF-2 results in a marked diminution of myocardial hypertrophy in the face of experimental pressure overload.(82) Pellieux and colleagues have documented that FGF-2 null cardiac myocytes respond normally to both angiotensin II and FGF-2. However, FGF-2 deficient fibroblasts are not only deficient in FGF-2 release, but also lack the ability to produce other (unidentified) trophic factors released by wildtype cardiac fibroblasts.(83) These data indicate that FGF-2 is secreted primarily by cardiac fibroblasts, mediating a paracrine, pro-hypertrophic response in neighboring cardiomyocytes. FGF-2 not only acts upon receptors on the surface of target myocytes, but in autocrine fashion also activates fibroblasts themselves to release other pro-hypertrophic factors into the interstitium.
Low molecular weight FGF-2 may mediate a variety of physiologic effects, including the ability to induce stem cell factor (the ligand for the c-kit receptor) and to concentrate c-kit+ cells within the site of experimental infarction.(71) FGF-2 may promote differentiation of embryonic stem cells(84) as well as putative resident stem cells within the myocardium towards a cardiomyocyte phenotype.(85) It may also promote retention of exogenously delivered cardiospheres in regions of infarct.(86) These data suggest that FGF-2 may be part of a conglomerate of fibroblast-derived signals for the homing and differentiation of circulating precursors and/or commitment of resident stem cells towards a myocyte fate.
The Interleukin-6 (IL-6) family: LIF and CT-1
The Interleukin-6 (IL-6) family of peptides can regulate myocyte hypertrophy, and several may be secreted by cardiac fibroblasts. A diverse set of polypeptides with minimal sequence homology, members of the IL-6 family signal through the transmembrane gp130 protein subunit. Both leukemia inhibitor factor (LIF) and cardiotrophin 1 (CT-1) are members of the IL-6 family that are synthesized by cardiac myocytes and fibroblasts and may act as mediators of fibroblast-myocyte crosstalk.(87) LIF induces cardiac myocyte and fibroblast hypertrophy but inhibits the myofibroblast transition and collagen deposition.(88) CT-1 also promotes myocyte hypertrophy but promotes fibroblast proliferation.(89, 90) There is evidence that LIF and CT-1 derived from cardiac fibroblasts mediates the pro-hypertrophic effects of angiotensin II (Figure 2).(91) Sano and colleagues interrogated the expression of the IL-6 family in cultured cardiac fibroblasts after exposure to angiotensin II. LIF and CT-1 were significantly upregulated in contrast to other family molecules. Conditioned medium from angiotensin-stimulated cardiac fibroblasts resulted in phosphorylation of gp130 and STAT3 as well as increased cardiomyocyte cell size. These effects were partially blocked by antisense nucleotides to LIF and CT-1, suggesting that these members of the IL-6 family are part of, but do not comprise in totality, a fibroblast secretory cascade produced upon angiotensin II stimulation which can affect myocyte hypertrophy.
Interleukin-33
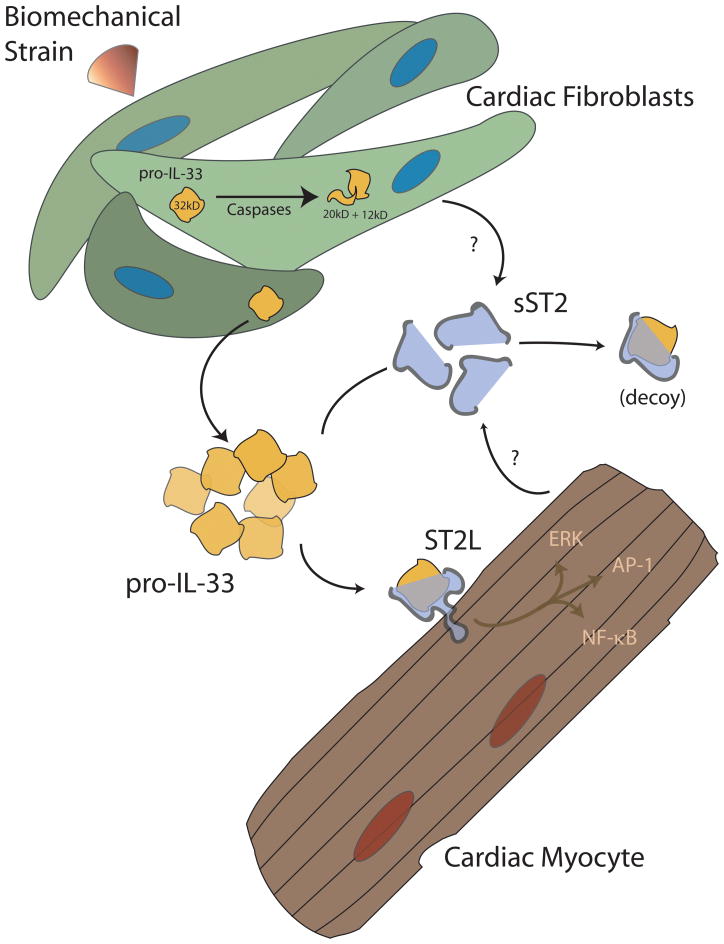
Interleukin-33 (IL-33 or IL-1F11) is a newly discovered member of the IL-1 family that represents a novel paracrine signaling system between fibroblast and myocyte. IL-33 was discovered in 2005 by Schmitz and colleagues by mining the public genomic database for a protein structure common to interleukin-1 and fibroblast growth factor, and the protein identified represented the end of a search for the ligand for the ST2 receptor that lasted two decades.(92) Work by Sanada and colleagues suggests that IL-33 is produced primarily by cardiac fibroblasts, with expression markedly upregulated by cyclic strain.(93) Although initial speculation suggested that IL-33 resembled IL-1 in its requirement for processing by caspase-1, IL-33 appears to be secreted or released in its active state and is instead inactivated via proteolytic cleavage by caspases.(94–96) In the extracellular space, IL-33 binds to one of two differentially transcribed forms of its receptor, ST2. A transmembrane form of ST2 (ST2L) transduces signals that in part converge on NF-κB. A truncated, “soluble” form of ST2 (sST2) is produced by many cell types and appears to act as a “decoy receptor” to sequester IL-33 away from the biologically active pool (Figure 3 and reviewed in (97)). Sanada and colleagues further documented that IL-33 has little effect on resting cardiac myocytes in culture. However, in the presence of pro-hypertrophic stimuli such as phenylephrine or angiotensin II, IL-33 exerts a dose-dependent anti-hypertrophic effect. In vivo, administration of IL-33 results in not only reduced myocyte hypertrophy but also reduced cardiac fibrosis after experimental pressure overload.(93) These data suggest that IL-33 is a fibroblast-derived cytokine with paracrine effects on neighboring cardiomyocyte. IL-33 is also cardioprotective during myocardial ischemia (data unpublished). The effect, if any, that an antifibrotic compound such as IL-33 may have on cardiac myocyte progenitor biology is at this time unaddressed.
Figure 3. The IL33/ST2 system in the heart.
The IL-33/ST2 system is a recently-described cardiac fibroblast-myocyte signaling system. This signaling pathway serves an anti-hypertrophic and cardioprotective mechanism in the face of biomechanical overload. IL-33 is produced by mechanically-loaded cardiac fibroblasts and is possibly inactivated by caspases. Extracellular IL-33 may bind to a soluble “decoy” form of its receptor, sST2, and be removed from the biologically available pool, or bind a transmembrane form, ST2L, on the surface of cardiac myocytes. In the face of pro-hypertrophic stimuli in vitro or pressure overload in vivo, IL-33 appears to confer anti-hypertrophic and anti-fibrotic properties to the myocardium.(93, 97)
Extracellular matrix based fibroblast – cardiomyocyte interactions
Fibroblasts have long been recognized as a major source of non-basement membrane collagen and other proteins of the extracellular space.(98–100) Extracellular matrix proteins, including collagens and fibronectin, signal through cell surface heterodimeric integrin receptors and can therefore act as communicative intermediaries in the dialogue between cardiac fibroblast and myocytes. As in most mammalian tissues, fibrillar collagen is the primary extracellular matrix protein of the normal myocardium. Of the various forms, the cardiac interstitium contains mostly types I and type III collagen, with a greater proportion of type I than III noted in most studies ((101, 102) and reviewed in (103)). In the context of altered loading conditions, the normal balance of collagen subtypes is altered.(104–106) In both end-stage cardiomyopathy as well as in response to cyclic strain in vitro, cardiac fibroblasts not only increase total collagen content of the ventricle, but also increase the ratio of collagen type I to type III.(107–110)
Rat neonatal cardiomyocytes cultured on collagen types I and III display enhanced physical association between cytoskeletal components (specifically filamentous actin), cellular adhesion points (such as vinculin) and the matrix β1-integrin receptors when compared with cultures on a laminin sublayer.(111) Electron microcopy studies of striated muscle grown on various matrices reveal that the composition of those matrices regulates patterns and distributions of both striated myofibrils and focal adhesions. Differences in matrix components can also regulate the physical co-localization of signaling molecules that associate with intracellular membrane-associated proteins.(112) Interestingly, direct inhibition of collagen synthesis disrupts in vitro embryonic cardiac myocyte differentiation, an indication that fibroblast-mediated regulation of matrix could affect cardiomyocyte development and regeneration.(113)
Fibroblast-induced changes in collagen composition represent only one of the only matrix proteins that can pass signals to myocytes. Fibronectin is produced by cardiac fibroblasts and is induced by angiotensin II in an epidermal growth factor dependent manner.(114) Fibronectin interacts with myocyte surface integrins to mediate cellular hypertrophy ((115) and reviewed in (116, 117)). Moreover, fibronectin acts coordinately with secreted extracellular collagens to promote embryonic myocyte proliferation via β1-integrin signaling in a manner that is independent of its effects on cellular adhesion.(8)
Remodeling and maintenance of the extracellular space requires not only synthesis but also coordinated degradation of matrix proteins. The matrix metalloproteinases (MMP) and their tissue inhibitors (TIMPs) are classic participants in matrix homeostasis, and these proteins are produced and secreted by both cardiac myocytes and fibroblasts (reviewed in (118, 119)). In humans, dilated cardiomyopathy as well as LV remodeling after myocardial infarction are accompanied by an increase in MMP activity, and the post-injury maladaptive ventricular phenotype can be abrogated by MMP inhibition in some animal models ((120–125) and reviewed in (126–130)). Collagen fragments produced by the action of MMP-1 on collagen promote fibroblast activation and transition to a myofibroblast phenotype.(131) Creemers and colleagues noted that TIMP-1 null mice, which have an obligate increase in MMP activity, have an exaggerated hypertrophic and dilated ventricular phenotype after myocardial infarction, with hypertrophy of surviving cardiomyocytes and a pronounced loss of fibrillar collagen. (124) Thus, there is an ongoing interplay of matrix synthesis and degradation by both myocytes and fibroblasts both in physiologic and pathophysiologic environments with feedback to both cell types that regulates myocardial physiology and response to injury.
Direct (cell-cell) fibroblast-cardiomyocyte communication and electrical coupling
In addition to interactions between cardiomyocytes and fibroblasts that are mediated by extracellular matrix components and secreted proteins, there is fascinating evidence for important direct cell-to-cell interaction between the two cell types. Chemi-electrical communication between fibroblast and myocytes may occur in a manner similar to the known gap junction-based connections among cardiac myocytes that allow the myocardium to act as a syncytium.
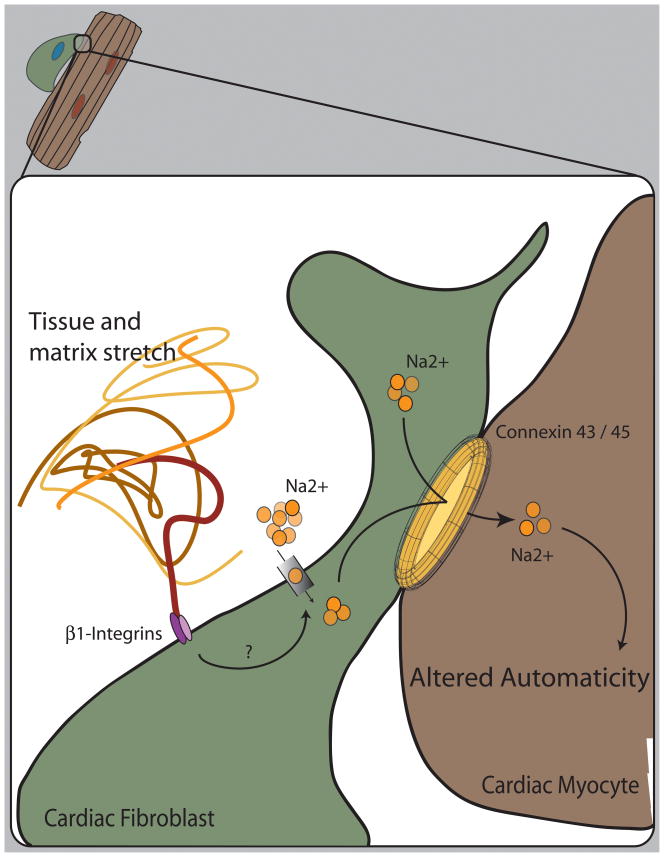
Direct cardiomyocyte cell-cell interactions utilize connexin-43 as the major protein component of gap junctions.(132–134) Cardiac fibroblasts express connexin-43 within gap junctions and appear to utilize connexin-45 when in close proximity to cardiomyocytes, in contrast to connexin-40 when adjacent to other fibroblasts.(135–137) Electrical conductance between fibroblasts and myocytes displays properties of a hemichannel, likely reflecting a mixed pool of gap junctions.(138) Cardiac fibroblasts demonstrate connexin plasticity; under conditions of myocardial injury, fibroblast gap junctions show an alteration in connexin subtype.(139) These data suggest the presence of functional cell-cell interactions between cardiac myocytes and fibroblasts that mediate intercellular communication. In support of this, movement of membrane-impermeant dyes and calcium fluxes has been documented between myocytes and neighboring fibroblasts.(140) Fibroblasts may also facilitate myocyte electrical communication at a distance in a connexin-dependent manner.(141) On a functional level, cardiac fibroblasts in culture acquire the rhythmic depolarization of neighboring cardiomyocytes and mediate myocyte electrical synchrony(142–144) and appear to contribute to myocyte automaticity by altering the depolarization characteristics of the myocyte.(145) Additionally, fibroblasts display changes in ion conductance and cation flux in response to mechanical stretch.(146–149) The combination of myocyte-fibroblast electrical coupling and the fibroblast response to mechanical stimuli may be particularly active in the cardiac atrium at the level of the sinoatrial node, where cardiac fibroblasts are most abundant (Figure 4).(136, 150) (151, 152) Taken together, these data suggest that there is direct cell-to-cell connections between fibroblasts and myocytes that allow a dynamic and environmentally responsive transfer of molecular and ionic signals between these cell types.
Figure 4. Translation of mechanical cues into functional alterations in sinus node automaticity.
Both electrophysiologic and molecular data suggest that cardiac fibroblasts and myocytes are in direct contact and part of a syncytial network afforded by the conglomerate of connexin 43 and 45 hemichannels. Mechanical stretch alters the electrical state of cardiac fibroblasts by activation of a combination of ion selective and non-selective channels.(146, 147) Fibroblasts may acquire and affect the synchronized beating of myocytes in culture. Because fibroblasts are abundant within the region of the sinoatrial node of the mammalian heart, it is plausible that tissue strain, mediated by integrin signaling, affects cardiac fibroblast depolarization and possible intracellular kinase signaling to transmit signals to neighboring cardiac myocytes, altering rhythmic automaticity.
Direct myocyte-fibroblast cell-cell interactions may also contribute to the entry of myocytes into a chronic “hibernating” state. Myocardial hibernation is a phenotype characterized by sarcomere depletion and loss of cytoplasmic structure, specifically the sarcoplasmic reticulum and T-tubules, glycogen accumulation, a preservation of cellular volume, nuclear heterochromatin redistribution, and mitochondrial redistribution. Hibernating mitochondria are functional, with a switch from fatty acid to glucose substrate utilization for ATP production.(153) It has been suggested that the changes of hibernation mimic a more embryonic cellular phenotype, and hence a form of myocyte “dedifferentiation”; these changes can be seen in the context of myocardial ischemia, pressure overload, and atrial fibrillation.(153, 154) In an in vitro model, a dedifferentiated phenotype was induced in cardiomyocytes co-cultured with cardiac fibroblasts, but not in monoculture or in the presence of fibroblast conditioned medium.(155, 156) This influence of fibroblasts on neighboring myocytes may involve activation of the transcription factor GATA4.(157)
Cardiac fibroblast-myocyte interactions in tissue engineering
An area of active research in cardiovascular therapeutics is the attempt to engineer, ex vivo, functional myocardial tissue that may be engrafted onto areas of injured ventricle. Recent data suggests that the inclusion of cardiac fibroblasts in three-dimensional cultures greatly enhances the stability and growth of the nascent myocardium. Cardiac fibroblasts when included in polymer scaffolds seeded with myocytes and endothelial cells have the ability to promote and stabilize vascular structures.(158, 159) Naito and colleagues constructed three dimensional cultures of neonatal rat cell isolates on collagen type I and Matrigel (a basement membrane protein mixture), and isolates of a mixed cell population versus a myocyte-enriched population were compared. The mixed population cultures, which contained a higher fraction of cardiac fibroblasts than the myocyte-enriched cultures, displayed improved contractile force generation and greater inotropic response despite an equivalent overall cell number. Greater vascularity was also seen in the mixed-pool cultures.(160) Building on this, Nichol and colleagues demonstrated that in a self-assembling nanopeptide scaffold, embedded rat neonatal cardiomyocytes exhibit greater cellular alignment and reduced apoptosis when cardiac fibroblasts were included in the initial culture.(161) A similar result was noted when polymer scaffolds were pre-treated with cardiac fibroblasts before myocyte seeding, suggesting a persistent paracrine effect.(162) These data reinforce the concept that engineering functional myocardium, either in situ or ex vivo will require attention to the nature of cell-cell interactions, including fibroblasts.
Conclusion and Future Directions
To date, a broad initial sketch of cardiac fibroblast-myocyte interactions has been drawn. Future studies in this field will better describe these interactions. How do multiple paracrine factors interact to produce a cohesive and coordinated communication scheme? What are the changes in coordinated bidirectional signaling that during development promotes myocyte progenitor proliferation but have different roles in the adult? Might fibroblasts actually be required for improved cardiac repair and regeneration?
Recent studies have begun to apply genetic and cellular fate-mapping techniques to document the origins of cardiac fibroblasts, the dynamic nature of their population, and how that population may be in flux during time of injury or pressure overload. It is crucial to define on a more specific molecular basis the origins and fates of cardiac fibroblasts. Do fibroblasts that have been resident within the ventricle since development fundamentally differ from those that arise from endothelial transition or that infiltrate from the bone marrow during adulthood? Do fibroblasts with these different origins behave differently or take on different roles in the face of ventricular strain or injury?
Our understanding of the nature of the cardiac fibroblast is evolving from the concept of the fibroblast as a bystander that causes unwanted fibrosis to the picture of a more complex role of fibroblasts in the healthy as well as diseased heart. The pathways used by cardiac fibroblasts to communicate with their neighboring myocytes are only partially described, but the data to date indicate that these pathways will be important for cardiac repair and regeneration.
Acknowledgments
none
Sources of Funding: This work was supported by grants from the National Institutes of Health (HL092930 and AG032977).
Non-standard Acronyms and Abbreviations
- CT-1
Cardiotrophin 1
- CCN2
Connective tissue growth factor
- FGF-2
Fibroblast growth factor-2
- GFP
Green Fluorescent Protein
- IL-6
Interleukin-6
- IL-33
Interleukin-33
- LTBP
Latent TGFβ binding protein
- LIF
Leukemia inhibitor factor
- MMP
Matrix metalloproteinases
- ST2L
ST2, transmembrane form
- sST2
ST2, soluble form
- TIMPs
Tissue inhibitors of MMP
- TGFβ
Transforming growth factor β
Footnotes
Subject Codes: [6], [110], [108], [154]
Disclosures: Brigham and Women’s Hospital has filed for patents on IL-33 and ST2, with Dr. Lee listed as an inventor.
References
- 1.Brilla CG, Maisch B, Weber KT. Myocardial collagen matrix remodelling in arterial hypertension. Eur Heart J. 1992;13(Suppl D):24–32. doi: 10.1093/eurheartj/13.suppl_d.24. [DOI] [PubMed] [Google Scholar]
- 2.Weber KT, Brilla CG. Myocardial fibrosis and the renin-angiotensin-aldosterone system. J Cardiovasc Pharmacol. 1992;20(Suppl 1):S48–54. [PubMed] [Google Scholar]
- 3.Weber KT, Brilla CG, Campbell SE, Zhou G, Matsubara L, Guarda E. Pathologic hypertrophy with fibrosis: the structural basis for myocardial failure. Blood Press. 1992;1:75–85. doi: 10.3109/08037059209077497. [DOI] [PubMed] [Google Scholar]
- 4.Weber KT, Brilla CG, Janicki JS, Reddy HK, Campbell SE. Myocardial fibrosis: role of ventricular systolic pressure, arterial hypertension, and circulating hormones. Basic Res Cardiol. 1991;86(Suppl 3):25–31. doi: 10.1007/978-3-662-30769-4_3. [DOI] [PubMed] [Google Scholar]
- 5.Weber KT, Jalil JE, Janicki JS, Pick R. Myocardial collagen remodeling in pressure overload hypertrophy. A case for interstitial heart disease. Am J Hypertens. 1989;2:931–940. doi: 10.1093/ajh/2.12.931. [DOI] [PubMed] [Google Scholar]
- 6.Crabos M, Roth M, Hahn AW, Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signaling systems and gene expression. J Clin Invest. 1994;93:2372–2378. doi: 10.1172/JCI117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villarreal FJ, Kim NN, Ungab GD, Printz MP, Dillmann WH. Identification of functional angiotensin II receptors on rat cardiac fibroblasts. Circulation. 1993;88:2849–2861. doi: 10.1161/01.cir.88.6.2849. [DOI] [PubMed] [Google Scholar]
- 8.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Sanchez C, Climent V, Schoenwolf GC, Alvarez IS, Garcia-Martinez V. Induction of cardiogenesis by Hensen’s node and fibroblast growth factors. Cell Tissue Res. 2002;309:237–249. doi: 10.1007/s00441-002-0567-2. [DOI] [PubMed] [Google Scholar]
- 10.Dell’Era P, Ronca R, Coco L, Nicoli S, Metra M, Presta M. Fibroblast growth factor receptor-1 is essential for in vitro cardiomyocyte development. Circ Res. 2003;93:414–420. doi: 10.1161/01.RES.0000089460.12061.E1. [DOI] [PubMed] [Google Scholar]
- 11.Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wollert KC, Chien KR. Cardiotrophin-1 and the role of gp130-dependent signaling pathways in cardiac growth and development. J Mol Med. 1997;75:492–501. doi: 10.1007/s001090050134. [DOI] [PubMed] [Google Scholar]
- 13.Oppenheim RW, Wiese S, Prevette D, Armanini M, Wang S, Houenou LJ, Holtmann B, Gotz R, Pennica D, Sendtner M. Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J Neurosci. 2001;21:1283–1291. doi: 10.1523/JNEUROSCI.21-04-01283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtmann B, Wiese S, Samsam M, Grohmann K, Pennica D, Martini R, Sendtner M. Triple knock-out of CNTF, LIF, and CT-1 defines cooperative and distinct roles of these neurotrophic factors for motoneuron maintenance and function. J Neurosci. 2005;25:1778–1787. doi: 10.1523/JNEUROSCI.4249-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palatinus JA, Rhett JM, Gourdie RG. Translational lessons from scarless healing of cutaneous wounds and regenerative repair of the myocardium. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Kiani MF, Postlethwaite AE, Weber KT. Infarct scar as living tissue. Basic Res Cardiol. 2002;97:343–347. doi: 10.1007/s00395-002-0365-8. [DOI] [PubMed] [Google Scholar]
- 18.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 19.Ausoni S, Sartore S. From fish to amphibians to mammals: in search of novel strategies to optimize cardiac regeneration. J Cell Biol. 2009;184:357–364. doi: 10.1083/jcb.200810094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 21.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 23.Hong KM, Burdick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. Faseb J. 2005;19:2029–2031. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- 24.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda K. Regeneration of cardiomyocytes from bone marrow: Use of mesenchymal stem cell for cardiovascular tissue engineering. Cytotechnology. 2003;41:165–175. doi: 10.1023/A:1024882908173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leone AM, Rutella S, Bonanno G, Abbate A, Rebuzzi AG, Giovannini S, Lombardi M, Galiuto L, Liuzzo G, Andreotti F, et al. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26:1196–1204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 27.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 28.Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 30.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 31.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1) Mol Genet Metab. 2000;71:418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 32.Leask A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res. 2007;74:207–212. doi: 10.1016/j.cardiores.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. Faseb J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 34.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson NL, Flanders KC, Smith JM, Ellingsworth LR, Roberts AB, Sporn MB. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989;108:661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eghbali M. Cellular origin and distribution of transforming growth factor-beta in the normal rat myocardium. Cell Tissue Res. 1989;256:553–558. doi: 10.1007/BF00225603. [DOI] [PubMed] [Google Scholar]
- 39.Lee AA, Delhaas T, McCulloch AD, Villarreal FJ. Differential responses of adult cardiac fibroblasts to in vitro biaxial strain patterns. J Mol Cell Cardiol. 1999;31:1833–1843. doi: 10.1006/jmcc.1999.1017. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi N, Calderone A, Izzo NJ, Jr, Maki TM, Marsh JD, Colucci WS. Hypertrophic stimuli induce transforming growth factor-beta 1 expression in rat ventricular myocytes. J Clin Invest. 1994;94:1470–1476. doi: 10.1172/JCI117485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villareal RP, Lee VV, Elayda MA, Wilson JM. Coronary artery bypass surgery versus coronary stenting: risk-adjusted survival rates in 5,619 patients. Tex Heart Inst J. 2002;29:3–9. [PMC free article] [PubMed] [Google Scholar]
- 42.van Wamel AJ, Ruwhof C, van der Valk-Kokshoorn LJ, Schrier PI, van der Laarse A. Stretch-induced paracrine hypertrophic stimuli increase TGF-beta1 expression in cardiomyocytes. Mol Cell Biochem. 2002;236:147–153. doi: 10.1023/a:1016138813353. [DOI] [PubMed] [Google Scholar]
- 43.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson NL, Bazoberry F, Speir EH, Casscells W, Ferrans VJ, Flanders KC, Kondaiah P, Geiser AG, Sporn MB. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1:91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- 45.Engelmann GL, Grutkoski PS. Coordinate TGF-beta receptor gene expression during rat heart development. Cell Mol Biol Res. 1994;40:93–104. [PubMed] [Google Scholar]
- 46.Lijnen P, Petrov V. Transforming growth factor-beta 1-induced collagen production in cultures of cardiac fibroblasts is the result of the appearance of myofibroblasts. Methods Find Exp Clin Pharmacol. 2002;24:333–344. doi: 10.1358/mf.2002.24.6.693065. [DOI] [PubMed] [Google Scholar]
- 47.Eghbali M, Tomek R, Woods C, Bhambi B. Cardiac fibroblasts are predisposed to convert into myocyte phenotype: specific effect of transforming growth factor beta. Proc Natl Acad Sci U S A. 1991;88:795–799. doi: 10.1073/pnas.88.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sigel AV, Centrella M, Eghbali-Webb M. Regulation of proliferative response of cardiac fibroblasts by transforming growth factor-beta 1. J Mol Cell Cardiol. 1996;28:1921–1929. doi: 10.1006/jmcc.1996.0185. [DOI] [PubMed] [Google Scholar]
- 49.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butt RP, Bishop JE. Mechanical load enhances the stimulatory effect of serum growth factors on cardiac fibroblast procollagen synthesis. J Mol Cell Cardiol. 1997;29:1141–1151. doi: 10.1006/jmcc.1996.0347. [DOI] [PubMed] [Google Scholar]
- 51.Butt RP, Laurent GJ, Bishop JE. Collagen production and replication by cardiac fibroblasts is enhanced in response to diverse classes of growth factors. Eur J Cell Biol. 1995;68:330–335. [PubMed] [Google Scholar]
- 52.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 53.Lee AA, Dillmann WH, McCulloch AD, Villarreal FJ. Angiotensin II stimulates the autocrine production of transforming growth factor-beta 1 in adult rat cardiac fibroblasts. J Mol Cell Cardiol. 1995;27:2347–2357. doi: 10.1016/s0022-2828(95)91983-x. [DOI] [PubMed] [Google Scholar]
- 54.Chen K, Mehta JL, Li D, Joseph L, Joseph J. Transforming growth factor beta receptor endoglin is expressed in cardiac fibroblasts and modulates profibrogenic actions of angiotensin II. Circ Res. 2004;95:1167–1173. doi: 10.1161/01.RES.0000150369.68826.2f. [DOI] [PubMed] [Google Scholar]
- 55.Sadoshima J, Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 56.Schorb W, Booz GW, Dostal DE, Conrad KM, Chang KC, Baker KM. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ Res. 1993;72:1245–1254. doi: 10.1161/01.res.72.6.1245. [DOI] [PubMed] [Google Scholar]
- 57.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 58.Sarkar S, Vellaichamy E, Young D, Sen S. Influence of cytokines and growth factors in ANG II-mediated collagen upregulation by fibroblasts in rats: role of myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H107–117. doi: 10.1152/ajpheart.00763.2003. [DOI] [PubMed] [Google Scholar]
- 59.MacLellan WR, Brand T, Schneider MD. Transforming growth factor-beta in cardiac ontogeny and adaptation. Circ Res. 1993;73:783–791. doi: 10.1161/01.res.73.5.783. [DOI] [PubMed] [Google Scholar]
- 60.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352–363. doi: 10.1016/s0008-6363(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 61.Pathak M, Sarkar S, Vellaichamy E, Sen S. Role of myocytes in myocardial collagen production. Hypertension. 2001;37:833–840. doi: 10.1161/01.hyp.37.3.833. [DOI] [PubMed] [Google Scholar]
- 62.Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002;109:787–796. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsybouleva N, Zhang L, Chen S, Patel R, Lutucuta S, Nemoto S, DeFreitas G, Entman M, Carabello BA, Roberts R, et al. Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy. Circulation. 2004;109:1284–1291. doi: 10.1161/01.CIR.0000121426.43044.2B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li RK, Li G, Mickle DA, Weisel RD, Merante F, Luss H, Rao V, Christakis GT, Williams WG. Overexpression of transforming growth factor-beta1 and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation. 1997;96:874–881. doi: 10.1161/01.cir.96.3.874. [DOI] [PubMed] [Google Scholar]
- 65.Patel R, Lim DS, Reddy D, Nagueh SF, Lutucuta S, Sole MJ, Zoghbi WA, Quinones MA, Roberts R, Marian AJ. Variants of trophic factors and expression of cardiac hypertrophy in patients with hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2000;32:2369–2377. doi: 10.1006/jmcc.2000.1267. [DOI] [PubMed] [Google Scholar]
- 66.Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 67.Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. Faseb J. 2002;16:1558–1566. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 68.Li TS, Hayashi M, Ito H, Furutani A, Murata T, Matsuzaki M, Hamano K. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation. 2005;111:2438–2445. doi: 10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]
- 69.Gwak SJ, Bhang SH, Yang HS, Kim SS, Lee DH, Lee SH, Kim BS. In vitro cardiomyogenic differentiation of adipose-derived stromal cells using transforming growth factor-beta1. Cell Biochem Funct. 2009;27:148–154. doi: 10.1002/cbf.1547. [DOI] [PubMed] [Google Scholar]
- 70.Lim JY, Kim WH, Kim J, Park SI. Involvement of TGF-beta1 signaling in cardiomyocyte differentiation from P19CL6 cells. Mol Cells. 2007;24:431–436. [PubMed] [Google Scholar]
- 71.Kardami E, Detillieux K, Ma X, Jiang Z, Santiago JJ, Jimenez SK, Cattini PA. Fibroblast growth factor-2 and cardioprotection. Heart Fail Rev. 2007;12:267–277. doi: 10.1007/s10741-007-9027-0. [DOI] [PubMed] [Google Scholar]
- 72.Liao S, Bodmer J, Pietras D, Azhar M, Doetschman T, Schultz Jel J. Biological functions of the low and high molecular weight protein isoforms of fibroblast growth factor-2 in cardiovascular development and disease. Dev Dyn. 2009;238:249–264. doi: 10.1002/dvdy.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jimenez SK, Sheikh F, Jin Y, Detillieux KA, Dhaliwal J, Kardami E, Cattini PA. Transcriptional regulation of FGF-2 gene expression in cardiac myocytes. Cardiovasc Res. 2004;62:548–557. doi: 10.1016/j.cardiores.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 74.Detillieux KA, Meij JT, Kardami E, Cattini PA. alpha1-Adrenergic stimulation of FGF-2 promoter in cardiac myocytes and in adult transgenic mouse hearts. Am J Physiol. 1999;276:H826–833. doi: 10.1152/ajpheart.1999.276.3.H826. [DOI] [PubMed] [Google Scholar]
- 75.Peifley KA, Winkles JA. Angiotensin II and endothelin-1 increase fibroblast growth factor-2 mRNA expression in vascular smooth muscle cells. Biochem Biophys Res Commun. 1998;242:202–208. doi: 10.1006/bbrc.1997.7940. [DOI] [PubMed] [Google Scholar]
- 76.Endoh M, Pulsinelli WA, Wagner JA. Transient global ischemia induces dynamic changes in the expression of bFGF and the FGF receptor. Brain Res Mol Brain Res. 1994;22:76–88. doi: 10.1016/0169-328x(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 77.Taverna S, Ghersi G, Ginestra A, Rigogliuso S, Pecorella S, Alaimo G, Saladino F, Dolo V, Dell’Era P, Pavan A, et al. Shedding of membrane vesicles mediates fibroblast growth factor-2 release from cells. J Biol Chem. 2003;278:51911–51919. doi: 10.1074/jbc.M304192200. [DOI] [PubMed] [Google Scholar]
- 78.Clarke MS, Caldwell RW, Chiao H, Miyake K, McNeil PL. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ Res. 1995;76:927–934. doi: 10.1161/01.res.76.6.927. [DOI] [PubMed] [Google Scholar]
- 79.Kaye D, Pimental D, Prasad S, Maki T, Berger HJ, McNeil PL, Smith TW, Kelly RA. Role of transiently altered sarcolemmal membrane permeability and basic fibroblast growth factor release in the hypertrophic response of adult rat ventricular myocytes to increased mechanical activity in vitro. J Clin Invest. 1996;97:281–291. doi: 10.1172/JCI118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang ZS, Jeyaraman M, Wen GB, Fandrich RR, Dixon IM, Cattini PA, Kardami E. High- but not low-molecular weight FGF-2 causes cardiac hypertrophy in vivo; possible involvement of cardiotrophin-1. J Mol Cell Cardiol. 2007;42:222–233. doi: 10.1016/j.yjmcc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 81.Schneider MD, McLellan WR, Black FM, Parker TG. Growth factors, growth factor response elements, and the cardiac phenotype. Basic Res Cardiol. 1992;87(Suppl 2):33–48. doi: 10.1007/978-3-642-72477-0_4. [DOI] [PubMed] [Google Scholar]
- 82.Schultz JE, Witt SA, Nieman ML, Reiser PJ, Engle SJ, Zhou M, Pawlowski SA, Lorenz JN, Kimball TR, Doetschman T. Fibroblast growth factor-2 mediates pressure-induced hypertrophic response. J Clin Invest. 1999;104:709–719. doi: 10.1172/JCI7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pellieux C, Foletti A, Peduto G, Aubert JF, Nussberger J, Beermann F, Brunner HR, Pedrazzini T. Dilated cardiomyopathy and impaired cardiac hypertrophic response to angiotensin II in mice lacking FGF-2. J Clin Invest. 2001;108:1843–1851. doi: 10.1172/JCI13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawai T, Takahashi T, Esaki M, Ushikoshi H, Nagano S, Fujiwara H, Kosai K. Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circ J. 2004;68:691–702. doi: 10.1253/circj.68.691. [DOI] [PubMed] [Google Scholar]
- 85.Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest. 2005;115:1724–1733. doi: 10.1172/JCI23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takehara N, Tsutsumi Y, Tateishi K, Ogata T, Tanaka H, Ueyama T, Takahashi T, Takamatsu T, Fukushima M, Komeda M, et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52:1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 87.Matsui H, Fujio Y, Kunisada K, Hirota H, Yamauchi-Takihara K. Leukemia inhibitory factor induces a hypertrophic response mediated by gp130 in murine cardiac myocytes. Res Commun Mol Pathol Pharmacol. 1996;93:149–162. [PubMed] [Google Scholar]
- 88.Wang F, Trial J, Diwan A, Gao F, Birdsall H, Entman M, Hornsby P, Sivasubramaniam N, Mann D. Regulation of cardiac fibroblast cellular function by leukemia inhibitory factor. J Mol Cell Cardiol. 2002;34:1309–1316. doi: 10.1006/jmcc.2002.2059. [DOI] [PubMed] [Google Scholar]
- 89.Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, Vernallis AB, Heath JK, Pennica D, Wood WI, et al. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J Biol Chem. 1996;271:9535–9545. doi: 10.1074/jbc.271.16.9535. [DOI] [PubMed] [Google Scholar]
- 90.Tsuruda T, Jougasaki M, Boerrigter G, Huntley BK, Chen HH, D’Assoro AB, Lee SC, Larsen AM, Cataliotti A, Burnett JC., Jr Cardiotrophin-1 stimulation of cardiac fibroblast growth: roles for glycoprotein 130/leukemia inhibitory factor receptor and the endothelin type A receptor. Circ Res. 2002;90:128–134. doi: 10.1161/hh0202.103613. [DOI] [PubMed] [Google Scholar]
- 91.Sano M, Fukuda K, Kodama H, Pan J, Saito M, Matsuzaki J, Takahashi T, Makino S, Kato T, Ogawa S. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem. 2000;275:29717–29723. doi: 10.1074/jbc.M003128200. [DOI] [PubMed] [Google Scholar]
- 92.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 93.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 96.Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009;284:19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bashey RI, Donnelly M, Insinga F, Jimenez SA. Growth properties and biochemical characterization of collagens synthesized by adult rat heart fibroblasts in culture. J Mol Cell Cardiol. 1992;24:691–700. doi: 10.1016/0022-2828(92)93383-u. [DOI] [PubMed] [Google Scholar]
- 99.Eghbali M, Blumenfeld OO, Seifter S, Buttrick PM, Leinwand LA, Robinson TF, Zern MA, Giambrone MA. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol. 1989;21:103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- 100.Eghbali M, Czaja MJ, Zeydel M, Weiner FR, Zern MA, Seifter S, Blumenfeld OO. Collagen chain mRNAs in isolated heart cells from young and adult rats. J Mol Cell Cardiol. 1988;20:267–276. doi: 10.1016/s0022-2828(88)80059-2. [DOI] [PubMed] [Google Scholar]
- 101.Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem. 1990;96:1–14. doi: 10.1007/BF00228448. [DOI] [PubMed] [Google Scholar]
- 102.Medugorac I, Jacob R. Characterisation of left ventricular collagen in the rat. Cardiovasc Res. 1983;17:15–21. doi: 10.1093/cvr/17.1.15. [DOI] [PubMed] [Google Scholar]
- 103.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 104.Diez J, Hernandez M. Is the extracellular degradation of collagen type I fibers depressed in spontaneously hypertensive rats with myocardial fibrosis? Circulation. 1996;94:2998. [PubMed] [Google Scholar]
- 105.Diez J, Panizo A, Gil MJ, Monreal I, Hernandez M, Pardo Mindan J. Serum markers of collagen type I metabolism in spontaneously hypertensive rats: relation to myocardial fibrosis. Circulation. 1996;93:1026–1032. doi: 10.1161/01.cir.93.5.1026. [DOI] [PubMed] [Google Scholar]
- 106.Poobalarahi F, Baicu CF, Bradshaw AD. Cardiac myofibroblasts differentiated in 3D culture exhibit distinct changes in collagen I production, processing, and matrix deposition. Am J Physiol Heart Circ Physiol. 2006;291:H2924–2932. doi: 10.1152/ajpheart.00153.2006. [DOI] [PubMed] [Google Scholar]
- 107.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 108.Pauschinger M, Knopf D, Petschauer S, Doerner A, Poller W, Schwimmbeck PL, Kuhl U, Schultheiss HP. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation. 1999;99:2750–2756. doi: 10.1161/01.cir.99.21.2750. [DOI] [PubMed] [Google Scholar]
- 109.Bishop JE, Greenbaum R, Gibson DG, Yacoub M, Laurent GJ. Enhanced deposition of predominantly type I collagen in myocardial disease. J Mol Cell Cardiol. 1990;22:1157–1165. doi: 10.1016/0022-2828(90)90079-h. [DOI] [PubMed] [Google Scholar]
- 110.Carver W, Nagpal ML, Nachtigal M, Borg TK, Terracio L. Collagen expression in mechanically stimulated cardiac fibroblasts. Circ Res. 1991;69:116–122. doi: 10.1161/01.res.69.1.116. [DOI] [PubMed] [Google Scholar]
- 111.Hilenski LL, Terracio L, Borg TK. Myofibrillar and cytoskeletal assembly in neonatal rat cardiac myocytes cultured on laminin and collagen. Cell Tissue Res. 1991;264:577–587. doi: 10.1007/BF00319047. [DOI] [PubMed] [Google Scholar]
- 112.Di Felice V, Cappello F, Montalbano A, Ardizzone NM, De Luca A, Macaluso F, Amelio D, Cerra MC, Zummo G. HSP90 and eNOS partially co-localize and change cellular localization in relation to different ECM components in 2D and 3D cultures of adult rat cardiomyocytes. Biol Cell. 2007;99:689–699. doi: 10.1042/BC20070043. [DOI] [PubMed] [Google Scholar]
- 113.Fisher SA, Periasamy M. Collagen synthesis inhibitors disrupt embryonic cardiocyte myofibrillogenesis and alter the expression of cardiac specific genes in vitro. J Mol Cell Cardiol. 1994;26:721–731. doi: 10.1006/jmcc.1994.1087. [DOI] [PubMed] [Google Scholar]
- 114.Moriguchi Y, Matsubara H, Mori Y, Murasawa S, Masaki H, Maruyama K, Tsutsumi Y, Shibasaki Y, Tanaka Y, Nakajima T, et al. Angiotensin II-induced transactivation of epidermal growth factor receptor regulates fibronectin and transforming growth factor-beta synthesis via transcriptional and posttranscriptional mechanisms. Circ Res. 1999;84:1073–1084. doi: 10.1161/01.res.84.9.1073. [DOI] [PubMed] [Google Scholar]
- 115.Laser M, Willey CD, Jiang W, Cooper Gt, Menick DR, Zile MR, Kuppuswamy D. Integrin activation and focal complex formation in cardiac hypertrophy. J Biol Chem. 2000;275:35624–35630. doi: 10.1074/jbc.M006124200. [DOI] [PubMed] [Google Scholar]
- 116.Hynes RO. Fibronectins. Sci Am. 1986;254:42–51. doi: 10.1038/scientificamerican0686-42. [DOI] [PubMed] [Google Scholar]
- 117.Farhadian F, Contard F, Corbier A, Barrieux A, Rappaport L, Samuel JL. Fibronectin expression during physiological and pathological cardiac growth. J Mol Cell Cardiol. 1995;27:981–990. doi: 10.1016/0022-2828(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 118.Manso AM, Elsherif L, Kang SM, Ross RS. Integrins, membrane-type matrix metalloproteinases and ADAMs: potential implications for cardiac remodeling. Cardiovasc Res. 2006;69:574–584. doi: 10.1016/j.cardiores.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 119.Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res. 2000;46:214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 120.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 121.Spinale FG, Coker ML, Krombach SR, Mukherjee R, Hallak H, Houck WV, Clair MJ, Kribbs SB, Johnson LL, Peterson JT, et al. Matrix metalloproteinase inhibition during the development of congestive heart failure: effects on left ventricular dimensions and function. Circ Res. 1999;85:364–376. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- 122.Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L. Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res. 1998;82:482–495. doi: 10.1161/01.res.82.4.482. [DOI] [PubMed] [Google Scholar]
- 123.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 124.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284:H364–371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 125.Felkin LE, Birks EJ, George R, Wong S, Khaghani A, Yacoub MH, Barton PJ. A quantitative gene expression profile of matrix metalloproteinases (MMPS) and their inhibitors (TIMPS) in the myocardium of patients with deteriorating heart failure requiring left ventricular assist device support. J Heart Lung Transplant. 2006;25:1413–1419. doi: 10.1016/j.healun.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 126.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89:201–210. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- 127.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006;69:604–613. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 128.Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart Fail Rev. 2004;9:7–19. doi: 10.1023/B:HREV.0000011390.44039.b7. [DOI] [PubMed] [Google Scholar]
- 129.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 130.Lee RT. Matrix metalloproteinase inhibition and the prevention of heart failure. Trends Cardiovasc Med. 2001;11:202–205. doi: 10.1016/s1050-1738(01)00113-x. [DOI] [PubMed] [Google Scholar]
- 131.Tyagi SC, Kumar SG, Alla SR, Reddy HK, Voelker DJ, Janicki JS. Extracellular matrix regulation of metalloproteinase and antiproteinase in human heart fibroblast cells. J Cell Physiol. 1996;167:137–147. doi: 10.1002/(SICI)1097-4652(199604)167:1<137::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 132.Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987;105:2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luke RA, Beyer EC, Hoyt RH, Saffitz JE. Quantitative analysis of intercellular connections by immunohistochemistry of the cardiac gap junction protein connexin43. Circ Res. 1989;65:1450–1457. doi: 10.1161/01.res.65.5.1450. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y, Kanter EM, Laing JG, Aprhys C, Johns DC, Kardami E, Yamada KA. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun Adhes. 2008;15:289–303. doi: 10.1080/15419060802198736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 137.Oyamada M, Kimura H, Oyamada Y, Miyamoto A, Ohshika H, Mori M. The expression, phosphorylation, and localization of connexin 43 and gap-junctional intercellular communication during the establishment of a synchronized contraction of cultured neonatal rat cardiac myocytes. Exp Cell Res. 1994;212:351–358. doi: 10.1006/excr.1994.1154. [DOI] [PubMed] [Google Scholar]
- 138.Rook MB, Jongsma HJ, de Jonge B. Single channel currents of homo- and heterologous gap junctions between cardiac fibroblasts and myocytes. Pflugers Arch. 1989;414:95–98. doi: 10.1007/BF00585633. [DOI] [PubMed] [Google Scholar]
- 139.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res. 2004;62:415–425. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 140.Chilton L, Giles WR, Smith GL. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol. 2007;583:225–236. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 142.Mark GE, Strasser FF. Pacemaker activity and mitosis in cultures of newborn rat heart ventricle cells. Exp Cell Res. 1966;44:217–233. doi: 10.1016/0014-4827(66)90427-7. [DOI] [PubMed] [Google Scholar]
- 143.Goshima K. Synchronized beating of and electrotonic transmission between myocardial cells mediated by heterotypic strain cells in monolayer culture. Exp Cell Res. 1969;58:420–426. doi: 10.1016/0014-4827(69)90523-0. [DOI] [PubMed] [Google Scholar]
- 144.Goshima K. Formation of nexuses and electrotonic transmission between myocardial and FL cells in monolayer culture. Exp Cell Res. 1970;63:124–130. doi: 10.1016/0014-4827(70)90339-3. [DOI] [PubMed] [Google Scholar]
- 145.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 146.Kamkin A, Kiseleva I, Lozinsky I, Scholz H. Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res Cardiol. 2005;100:337–345. doi: 10.1007/s00395-005-0529-4. [DOI] [PubMed] [Google Scholar]
- 147.Kamkin A, Kiseleva I, Isenberg G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc Res. 2003;57:793–803. doi: 10.1016/s0008-6363(02)00775-7. [DOI] [PubMed] [Google Scholar]
- 148.Kamkin A, Kiseleva I, Isenberg G, Wagner KD, Gunther J, Theres H, Scholz H. Cardiac fibroblasts and the mechano-electric feedback mechanism in healthy and diseased hearts. Prog Biophys Mol Biol. 2003;82:111–120. doi: 10.1016/s0079-6107(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 149.Kamkin A, Kiseleva I, Wagner KD, Lozinsky I, Gunther J, Scholz H. Mechanically induced potentials in atrial fibroblasts from rat hearts are sensitive to hypoxia/reoxygenation. Pflugers Arch. 2003;446:169–174. doi: 10.1007/s00424-003-1032-0. [DOI] [PubMed] [Google Scholar]
- 150.Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol. 1994;79:943–956. doi: 10.1113/expphysiol.1994.sp003819. [DOI] [PubMed] [Google Scholar]
- 151.Shiraishi I, Takamatsu T, Minamikawa T, Onouchi Z, Fujita S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation. 1992;85:2176–2184. doi: 10.1161/01.cir.85.6.2176. [DOI] [PubMed] [Google Scholar]
- 152.De Maziere AM, van Ginneken AC, Wilders R, Jongsma HJ, Bouman LN. Spatial and functional relationship between myocytes and fibroblasts in the rabbit sinoatrial node. J Mol Cell Cardiol. 1992;24:567–578. doi: 10.1016/0022-2828(92)91041-3. [DOI] [PubMed] [Google Scholar]
- 153.Vanoverschelde JL, Wijns W, Borgers M, Heyndrickx G, Depre C, Flameng W, Melin JA. Chronic myocardial hibernation in humans. From bedside to bench. Circulation. 1997;95:1961–1971. doi: 10.1161/01.cir.95.7.1961. [DOI] [PubMed] [Google Scholar]
- 154.Ausma J, Wijffels M, van Eys G, Koide M, Ramaekers F, Allessie M, Borgers M. Dedifferentiation of atrial cardiomyocytes as a result of chronic atrial fibrillation. Am J Pathol. 1997;151:985–997. [PMC free article] [PubMed] [Google Scholar]
- 155.Driesen RB, Verheyen FK, Dispersyn GD, Thone F, Lenders MH, Ramaekers FC, Borgers M. Structural adaptation in adult rabbit ventricular myocytes: influence of dynamic physical interaction with fibroblasts. Cell Biochem Biophys. 2006;44:119–128. doi: 10.1385/CBB:44:1:119. [DOI] [PubMed] [Google Scholar]
- 156.Dispersyn GD, Geuens E, Ver Donck L, Ramaekers FC, Borgers M. Adult rabbit cardiomyocytes undergo hibernation-like dedifferentiation when co-cultured with cardiac fibroblasts. Cardiovasc Res. 2001;51:230–240. doi: 10.1016/s0008-6363(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 157.Zaglia T, Dedja A, Candiotto C, Cozzi E, Schiaffino S, Ausoni S. Cardiac interstitial cells express GATA4 and control dedifferentiation and cell cycle re-entry of adult cardiomyocytes. J Mol Cell Cardiol. 2009;46:653–662. doi: 10.1016/j.yjmcc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 158.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 159.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 160.Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, Eschenhagen T, Zimmermann WH. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114:I72–78. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 161.Nichol JW, Engelmayr GC, Jr, Cheng M, Freed LE. Co-culture induces alignment in engineered cardiac constructs via MMP-2 expression. Biochem Biophys Res Commun. 2008;373:360–365. doi: 10.1016/j.bbrc.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Radisic M, Park H, Martens TP, Salazar-Lazaro JE, Geng W, Wang Y, Langer R, Freed LE, Vunjak-Novakovic G. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. J Biomed Mater Res A. 2008;86:713–724. doi: 10.1002/jbm.a.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]