Abstract
Atherothrombotic vascular disease is the major cause of death and disability in obese and diabetic subjects with insulin resistance. Although increased systemic risk factors in the setting of insulin resistance contribute to this problem, it is likely exacerbated by direct effects of insulin resistance on the arterial wall cells that participate in atherosclerosis. A critical process in the progression of atherosclerotic lesions to those that cause clinical disease is necrotic breakdown of plaques. Plaque necrosis, which is particularly prominent in the lesions of diabetics, is caused by the combination of macrophage apoptosis and defective clearance, or efferocytosis, of the apoptotic macrophages. One cause of macrophage apoptosis in advanced plaques is activation of a pro-apoptotic branch of the endoplasmic reticulum stress pathway known as the Unfolded Protein Response (UPR). Macrophages have a functional insulin receptor signal transduction pathway, and down regulation of this pathway in the setting insulin resistance enhances UPR-induced apoptosis. Moreover, other aspects of the obesity/insulin-resistance syndrome may adversely affect efferocytosis. These processes may therefore provide an important mechanistic link among insulin resistance, plaque necrosis, and atherothrombotic vascular disease and suggest novel therapeutic approaches to this expanding health problem.
Keywords: atherosclerosis, insulin resistance, diabetes, macrophages, apoptosis, efferocytosis, plaque necrosis, ER stress, Unfolded Protein Response
The incidence of insulin resistance, metabolic syndrome, and type 2 diabetes is rising rapidly due to the epidemic of obesity in the industrialized world.1 While a number of disease processes are associated with insulin resistance and type 2 diabetes, the leading cause of morbidity and mortality is cardiovascular disease.2 An important factor in accelerated heart disease in type 2 diabetes is likely to be insulin resistance and hyperinsulinemia. For example, the risk of cardiovascular disease is increased in metabolic syndrome, which is characterized by insulin resistance without overt hyperglycemia.3–5 Moreover, rapid weight gain during childhood leads to hyperinsulinemia and increased coronary artery disease risk in adult life.6 Part of the association between insulin resistance and cardiovascular disease is likely related to associated risk factors, including dyslipidemia (increased VLDL, reduced HDL, and possibly altered LDL), hypertension, and a pro-thrombotic state.3 However, insulin resistance may have direct pro-atherogenic effects at the level of the arterial wall, and an emerging concept that will be explored in this review is that insulin resistance in lesional macrophages promotes a series of cellular events critical for advanced plaque progression. After a brief review of atherogenesis, we will focus on new findings related to plaque progression and the role of macrophage insulin resistance that have appeared in the literature since the last review of this topic in this journal in 2007.7
Principles of Atherogenesis
Plaque Initiation and Progression
Atherogenesis begins with the retention of atherogenic lipoproteins in the subendothelium of susceptible areas of the arterial tree.8 In response to these retained lipoproteins, particularly those that undergo atherogenic modifications such as oxidation and aggregation, a series of biological and maladaptive inflammatory responses ensue: (a) monocytes and other inflammatory cells enter the intima; (b) monocytes differentiate into macrophages, which then ingest retained and modified lipoproteins and become cholesteryl ester-loaded foam cells; (c) macrophages and other inflammatory cells contribute to a state of inflammation that fails to properly resolve; and (d) smooth muscle cells populate the intima, leading to collagen synthesis.9–12. At this stage, the plaques are usually asymptomatic due to outward remodeling of the artery to preserve lumenal blood flow and a fibrous cap that protects the lesion from disruption.13, 14 However, some of these plaques, unrelated to plaque size per se, may undergo necrotic breakdown, thinning of the fibrous cap, a heightened state of inflammation, and an accumulation of unesterified cholesterol.13–19. Many of the hallmarks of impaired inflammation resolution are evident in these plaques, including continued entry and poor egress of inflammatory cells, defective clearance of apoptotic cells, and a suppressed fibrotic “scarring” response.12, 20 These so-called “vulnerable plaques” are at risk for plaque disruption through fibrous cap rupture or endothelial erosion, which in turn can trigger acute thrombosis. If the thrombosis is extensive and not quickly resolved, acute vascular occlusion and tissue infarction occurs, leading to acute myocardial infarction, unstable angina, sudden cardiac death, or stroke.
The exact mechanisms of plaque disruption are not known. Cap thinning per se may be caused by a combination of protease-mediated digestion of extracellular matrix molecules, particularly by matrix metalloproteinases (MMPs), and decreased collagen synthesis, perhaps exacerbated by death of the collagen-synthesizing cells in the intima.13 These processes, as well as coagulation and thrombosis, are likely promoted by inflammatory cytokines, many of which are secreted by lesional macrophages.13 Lesional necrosis of vulnerable plaques, which is caused by the combination of macrophage death and defective clearance, or “efferocytosis,” of dead macrophages,21–23 can promote plaque disruption by a number of mechanisms.15, 22, 24–27. For example, although matrix proteases are secreted by living macrophages in lesions, they may also be released by dead and dying macrophages.28 Moreover, lesional necrosis triggers a heightened state of inflammation, which, as mentioned above, promotes MMP secretion, coagulation, and thrombosis.29 Finally, the necrotic core is rich in lipids and poor in cells and extracellular matrix, and the structural properties resulting from this composition are thought to contribute to mechanical stresses in the overlying cap, which may contribute to cap rupture.30 Thus, macrophage death and defective clearance of the dead cells, leading to lesional necrosis, is an important process in the formation of the vulnerable plaque, and, as described in this review, exacerbations of these processes may help explain accelerated atherothrombotic disease in insulin-resistant states.
Mechanisms and Consequences of Macrophage Death and Defective Efferocytosis in Advanced Atheromata
To understand how insulin resistance may promote advanced plaque progression in general, and plaque necrosis in particular, it is necessary to review our latest understanding of the mechanisms and consequences of macrophage death in advanced atheromata. A number of hypotheses have been conceived to explain advanced lesional macrophage apoptosis, and undoubtedly more than one mechanism is involved. Examples include growth factor deprivation, toxic cytokines, and oxidized lipids or lipoproteins,31 but there is as yet little proof for these ideas in vivo. Recent mechanistic data in cultured cells and correlative and genetic-causation evidence in vivo support a role for endoplasmic reticulum (ER) stress in advanced lesional macrophage apoptosis and its major consequence, plaque necrosis. As had been previously demonstrated in other models of ER stress-induced apoptosis, macrophages subjected to ER stress undergo apoptosis in a manner that is partially dependent on the CHOP (GADD153) branch of the ER stress pathway known as the Unfolded Protein Response (UPR).32, 33 CHOP-mediated apoptosis can be modeled in cultured macrophages by either potent inducers of ER stress or by the combination of more subtle ER stressors and a “second hit.” An example of an atherosclerosis-relevant inducer of the single-hit ER stress apoptosis is 7-ketocholesterol,33, 34 the most abundant oxysterol in advanced atherosclerotic lesions. Examples of the two-hit model include the combination of low-level ER stressors with pattern recognition receptor (PRR) ligands, such as modified lipoproteins.35, 36 Another example of the two-hit model is incubation of macrophages with atherogenic lipoproteins under conditions of genetic or pharmacologic inhibition of intracellular cholesterol re-esterification.37, 38 In this model, which is often referred to as the “FC” (free cholesterol) model and is designed to mimic FC-loaded macrophages in advanced atheromata,39, 40 the ER stress hit is provided by excess accumulation of unesterified cholesterol in the ER membrane, and the second hit is activation of PRRs by the lipoproteins themselves. The contribution of the second hit to apoptosis involves both amplification of pro-apoptotic pathways and suppression of cell-survival pathways that are activated in ER-stressed cells.36 The tendency of macrophages to undergo apoptosis when subjected to ER stress in combination with PRR activation may have evolved as a host defense mechanism against intracellular organisms that require living macrophages to survive.
While it has been known that activation of the CHOP pathway of the UPR can cause apoptosis, the molecular mechanisms linking CHOP to death execution pathways is poorly understood. Recent work in our laboratories has provided evidence for a calcium-dependent mechanism in ER stress-induced macrophage apoptosis. ER stress in macrophages leads to the release of calcium from the ER lumen into the cytosol.32 The cytosolic calcium chelator BAPTA-AM can block ER stress-induced apoptosis in macrophages, and recent work has shown that a key integrator of cytosolic calcium and death execution in these cells is a calcium-responsive kinase called calcium/calmodulin-dependent protein kinase II (CaMKII).36, 41, 42 Activation of CaMKII leads to multiple death pathways, including induction of the cell-surface death receptor Fas; stimulation of mitochondrial calcium uptake and release of pro-apoptotic cytochrome c from the mitochondria; activation of pro-apoptotic STAT-1; and accumulation of reactive oxygen species through activation of NADPH oxidase.42 CHOP amplifies this calcium-death pathway by leading to activation of IP3 receptors, which are calcium-release channels in the ER membrane.43 The mechanism involves oxidative activation of IP3R by the downstream CHOP transcriptional target, ER oxidase-1α (ERO1α). Net calcium release can also be promoted through inhibition of sarco/endoplasmic reticulum calcium-dependent ATPase (SERCA), which pumps calcium back into the ER lumen. SERCA is inhibited by alterations in the ER membrane by certain ER stressors, such as unesterified cholesterol or saturated fatty acids,44 and SERCA is down-regulated in the setting of insulin resistance, as will be summarized below.
Macrophage apoptosis by itself would not be expected to be detrimental, because apoptotic cells are normally cleared rapidly by phagocytosis (“efferocytosis”) in a manner that prevents post-apoptotic cellular necrosis and that promotes anti-inflammatory processes.27 Indeed, manipulations that accelerate early lesional macrophage apoptosis decrease lesion cellularity and plaque progression, and vice versa,23, 45 suggesting that efferocytosis is very efficient in the early stages of atherogenesis. This principle has been applied recently to a mouse model of type 2 diabetes and early atherosclerosis.46 However, in the later stages of atherosclerosis, macrophage apoptosis is associated with plaque necrosis,23 and there is evidence in humans that efferocytosis is defective in advanced plaques.47 The mechanisms of defective efferocytosis in advanced lesions are not known, but several interesting ideas have been advanced based on in vitro and in vivo observations. For example, oxidized lipids and proteins exist in these plaques, and some of these molecules can competitively inhibit efferocytosis by binding to efferocytosis receptors.48 Thus, to the extent that these oxidized molecules accumulate as lesions progress,10 they may reach a high enough level in advanced atheromata to take on this competitive inhibitory role. In another scenario, the efferocytosis receptor Mertk has been shown to play a role in efferocytosis and plaque necrosis in mouse lesions,49, 50 and inflammation-induced cleavage of this receptor by membrane sheddases51 may contribute to defective clearance of apoptotic cells in advanced plaques. The fact that inflammation increases as lesions progress may offer an explanation as to why this anti-efferocytic process occurs only in advanced plaques.
The concept that ER stress-induced macrophage apoptosis in combination with defective efferocytosis in advanced lesions promotes plaque necrosis is supported by a number of genetic-causation studies in mice and by correlative studies in humans. In fat-fed Apoe−/− or Ldlr−/− mice, ER stress markers are induced as lesions progress.32, 52–56 Most importantly, genetic targeting of CHOP and STAT-1, the pro-apoptotic signaling transducer activated by the CHOP calcium-CaMKII pathway (above), as well as prevention of cholesterol-induced ER damage, inhibit advanced lesional macrophage apoptosis and plaque necrosis.33, 42, 52 Moreover, deletion of two “second-hit” PRRs—SRA and CD36—decreases macrophage apoptosis and plaque necrosis in the lesions of fat-fed Apoe−/− mice.57 In humans, there are close correlations among markers of ER stress, apoptosis, and plaque vulnerability in coronary arteries.55 In terms of efferocytosis, studies have shown an increase in plaque necrosis that correlates with a worsening of lesional efferocytosis in several mouse models in which efferocytosis effectors have been targeted, including Mertk, MFG-E8, transglutaminase-2, and complement factor C1q.49, 58 In summary, in vitro and in vivo evidence support a model in which macrophage apoptosis in advanced lesions, induced in part by a pro-apoptotic ER stress-calcium pathway, plus defective efferocytosis promote plaque necrosis (Figure 1). Because plaque necrosis is strongly associated with disrupted plaques and acute lumenal thrombosis,59 and because plaque necrosis is particularly prominent in atherosclerotic lesions from diabetic subjects, as described in the following section, these insights should be useful in our understanding of and therapeutic approaches to accelerated plaque progression in the setting of insulin resistance.
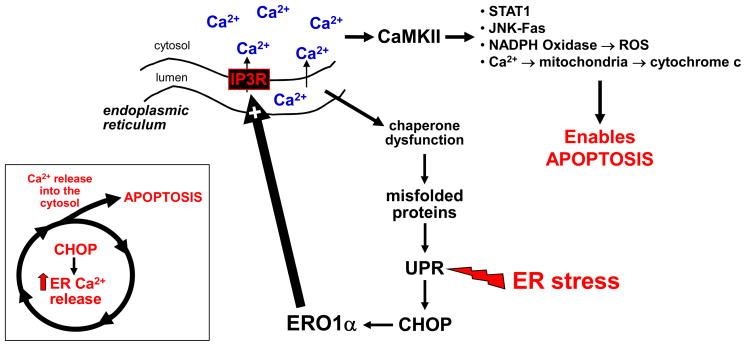
Figure 1.
A CHOP-calcium pathway of ER stress-induced apoptosis in macrophages. A diverse array of ER stress-provoking events, many of which exist in advanced atheromata, trigger the UPR and lead to induction of the downstream effector CHOP. CHOP induces ERO1α, which in turn oxidatively activates IP3R calcium release channels in the ER. IP3R-mediated calcium release begins a pro-apoptotic cascade involving activation of CaMKII by cytosolic calcium and subsequent downstream apoptotic processes, as listed in the figure and as described in the text. In addition, the resulting low level of calcium in the ER lumen likely causes dysfunction of calcium-dependent protein chaperones, which amplifies UPR activation. The central concept is that pro-apoptotic CHOP functions, at least in part, by promoting calcium-induced death as part of a positive feedback cycle (see inset).
Macrophage Death and Plaque Progression in Insulin Resistance
Plaque Necrosis in Human Diabetic Coronary Artery Lesions
It is now well-established that type 2 diabetes and insulin resistance are major risk factors for atherothrombotic vascular disease.3–5 While many theories have arisen to explain this relationship,60, 61 a common endpoint of plaque progression associated with atherothrombotic vascular disease, as mentioned in the previous section, is plaque necrosis. In this context, a number of independent studies have found that advanced atherosclerotic lesions in diabetic subjects are characterized by particularly large necrotic cores when compared to similarly sized lesions from non-diabetic individuals.62–67 For example, Burke et al.62 found that necrotic core size in the coronary arteries of subjects who died suddenly was positively correlated with the presence of diabetes independently of other factors. Similar results were found when coronary atherectomy specimens of diabetics and non-diabetics were compared.63 Nasu et al.66 used virtual histology based on intravascular ultrasound (IVUS) data to assess coronary arterial necrotic cores in non-diabetic and diabetic patients with stable angina and found an approximate 50% increase in the percent area covered by necrotic cores in the diabetic group. Almost identical findings were reported in similar studies conducted by Hong et al.65 in Korea and Pundziute et al.66 in the Netherlands. A prospective study of subjects with coronary artery disease in which radiofrequency data from IVUS was used to assess necrotic core size in coronary arteries found that only diabetes and age were associated positively with necrotic core size in logistic regression analysis.67 These collective data raise the issue as to whether the cellular events described in the previous sections, particularly advanced lesional macrophage apoptosis and/or defective efferocytosis, are enhanced in the setting of diabetes, leading to increased plaque necrosis and, ultimately, accelerated atherothrombotic vascular disease.
The Effect of Insulin Resistance on Macrophage Death Pathways
In view of the role of insulin resistance in diabetic heart disease and the larger necrotic cores in the coronary arteries of diabetic subjects, we and others have examined how insulin resistance at the level of the macrophage affects mechanisms and consequences of macrophage death in vitro and in vivo. Macrophages have insulin receptors, and acute exposure of the cells to insulin in vitro results in phosphorylation of the insulin receptor, insulin receptor substrate-2 (IRS-2), and Akt, leading, among other responses, to nuclear exclusion and inactivation of FoxO transcription factors.7, 68 Moreover, pre-treatment of macrophages in vitro with high-dose insulin leads to down-regulation of their insulin receptors and suppression of insulin receptor signaling, which is also observed in freshly isolated macrophages from insulin-resistant mice, such as the hyperinsulinemic leptin-deficient ob/ob mouse.68 Thus, macrophages show the hallmarks of “insulin resistance” at a cellular level in the setting of high insulin concentrations.
Macrophages rendered insulin resistant through pre-incubation with insulin, genetic deletion of the insulin receptor, or pharmacologic inhibition of insulin signaling, and macrophages freshly isolated from hyperinsulinemic mice, show an increase in the levels of the scavenger receptor SRA.7, 68 As mentioned in the previous section, SRA can serve as a “second-hit” PRR in ER stress-induced macrophage apoptosis both in vitro and in advanced lesional macrophage death and plaque necrosis in vivo. In this regard, insulin-resistant macrophages show markedly enhanced apoptosis in vitro when exposed to ER stress conditions plus an SRA-mediated second hit, as is the case with macrophages loaded with lipoprotein-derived unesterified cholesterol.56, 69, 70
ER stress in macrophages triggers compensatory cell-survival pathways, notably those activated by Akt and NF-κB, and apoptosis is temporally correlated with a down-regulation of these pathways and can be accelerated by their inhibition.36, 71, 72 Moreover, Akt deficiency in Apoe−/− mice was shown to enhance lesional macrophage apoptosis and inflammation and plaque progression.73 In this context, an important observation was that phosphorylation of Akt is suppressed in ER-stressed, insulin-resistant macrophages.69, 71 Consistent with a decrease in Akt phosphorylation, Senokuchi et al.71 found an increase in nuclear FoxO1 in insulin-resistant, ER-stressed macrophages, which normally translocates to the cytoplasm in response to Akt-dependent phosphorylation.74 Moreover, macrophages genetically lacking FoxO1, 3 and 4, were resistant to ER stress-induced apoptosis.71 However, FoxO-overexpression experiments indicated that nuclear localization of these transcription factors was not by itself sufficient for macrophage apoptosis but rather led to an enhancement of apoptosis in the setting of ER stress. The apoptosis-enhancing mechanism of FoxO1 is directly related to the role of another compensatory cell-survival factor in ER-stressed macrophages, namely NF-κB.71, 72, 75, 76 In ER-stressed macrophages, FoxO1 induces the expression of the NF-κB inhibitor IκBε and thereby enhances apoptosis.71
Importantly, insulin resistance potentiates the ER stress response itself.77 ER stress in macrophages leads to activation of the mitogen-activated protein kinase ERK,76 and Liang et al.77 found that this response was blunted in insulin-resistant macrophages. Additional studies revealed that the MEK-ERK pathway induces SERCA,77 which, as explained above, can abrogate ER stress by replenishing ER lumenal calcium stores and can protect macrophages from ER stress-induced apoptosis by lowering cytosolic calcium levels. Thus, the blunted MEK-ERK-SERCA pathway in insulin-resistant macrophages exacerbates the ER stress response and the calcium-mediated apoptosis pathway described above, and restoration of MEK1 in these cells is protective against both ER stress and apoptosis.77
In summary, mechanistic studies using various cell culture models of insulin-sensitive and insulin-resistant macrophages, including primary macrophages freshly harvested from ob/ob mice, have revealed an integrated pathway of cell signaling events responsible for the increased apoptotic response to ER stress in the setting of insulin resistance. Key among these events are those related to the compromise of compensatory cell survival pathways and the exacerbation of pro-apoptotic calcium signaling pathways (Figure 2).
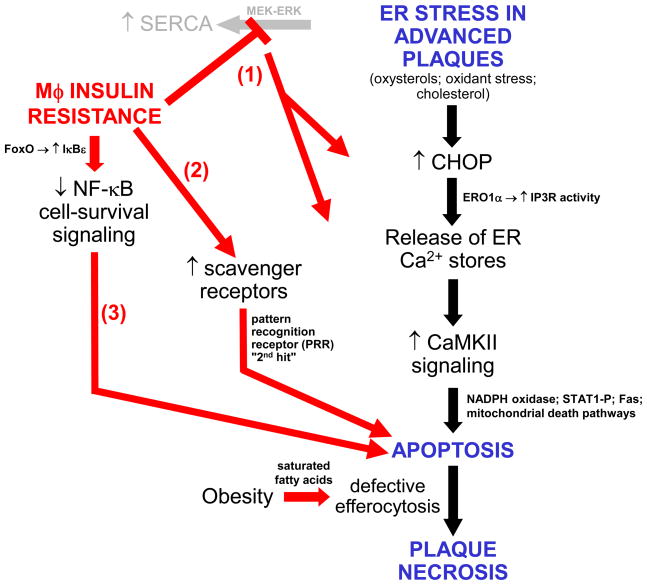
Figure 2.
Cellular-molecular mechanisms by which macrophage insulin resistance promotes ER stress-induced macrophage apoptosis and advanced plaque progression. At least three pro-apoptotic processes are enhanced in ER stressed macrophages: (1) ER stress normally activates a compensatory MEK-ERK-SERCA pathway to lower cytoplasmic calcium and replenish ER lumenal stores. This pathway is blocked in the setting of insulin resistance, leading to enhanced activation of calcium-mediated apoptotic pathways (increased cytosolic calcium) and further UPR-CHOP activation (decreased ER lumenal calcium); (2) pattern recognition receptors like scavenger receptors are up-regulated in insulin-resistant macrophages, and, when activated, are synergistic with ER stress in inducing apoptosis (“2nd hit” concept); (3) Increased nuclear FoxO in insulin-resistant macrophages induces IκBε, thereby suppressing a compensatory NF-κB cell-survival pathway. In addition to these pro-apoptotic processes, increased levels of saturated fatty acids in the setting obesity compromise the ability of macrophages to engulf apoptotic cells. Apoptotic cells that are not efficiently cleared become secondarily necrotic and, over time, accumulate into necrotic cores in advanced plaques. These necrotic cores, which are particularly large in diabetic atheromata, are thought to contribute to plaque disruption. See text for details.
The Effect of Macrophage Insulin Resistance on Murine Atherosclerosis
To test relevance of enhanced ER stress-induced apoptosis in insulin-resistant macrophages in vivo, irradiated Ldlr−/− mice were transplanted with bone marrow from Insr+/+ or Insr−/− mice.69 It should be noted that this proof-of-concept model represents the most extreme form of “insulin resistance.” After recovery of the graft, the mice were fed a high-fat diet, and lesions were analyzed for overall area and, most importantly, plaque morphology. Consistent with the in vitro data, the advanced lesions of the Insr−/−→ Ldlr−/− mice fed the diet for 12 weeks had more apoptotic cells, particularly in macrophage-rich regions of the plaque, and more plaque necrosis than those of the Insr+/+ → Ldlr−/− control mice. Overall lesion area, the less important endpoint for the hypothesis being tested, showed no change after 8 weeks of diet and only a modest increase after 12 weeks. Baumgartl et al.78 used the cre-lox system to create Apoe−/− with macrophage-targeted deficiency of insulin receptors. After 4 months on a high-fat diet, these mice had a modest decrease in lesion area compared with control Apoe−/− mice. Apoptotic cells and necrotic areas were not quantified. Immortalized macrophages derived from these mice had a marked reduction in LPS-induced interleukin-6 (IL-6) secretion. The authors also tested the effects of global and bone marrow-derived IRS-2 deficiency in fat-fed Apoe−/− mice. In the holo-knockout model, lesion area was modestly increased, and in the bone marrow transplant model, lesion area was modestly decreased. Plaque morphology was not quantified. The authors interpreted these data as showing that myeloid-derived insulin receptors suppress atherosclerosis by blunting the inflammatory response.78 Senokuchi et al.71 also observed decreased inflammatory responses during ER stress in insulin-resistant macrophages. In that study, reduced NF-κB responses led to both increased apoptosis, as noted in the previous section, and decreased expression of some inflammatory genes. In summary, a careful comparison of Han et al.69 and Baumgartl et al.78 reveal a common finding of relatively modest effects of macrophage insulin resistance on overall lesion size, with subtle differences between the two studies perhaps arising from differences in genetic background (mixed vs. inbred C67Bl/6J), diets used (Western-type diet vs. the pro-inflammatory high-cholesterol/bile salt diet), and stage of lesion development. As noted, those specific features of atherosclerotic lesions related to the novel concept that insulin-resistant macrophages are more susceptible to apoptosis, i.e., advanced lesional macrophage apoptosis and plaque necrotic area, were assessed in only one of the two studies, and the data supported that concept.69
Our laboratories have been working with another model of advanced lesional apoptosis and plaque necrosis in mice that may relate to the findings above. During certain types of ER stress, macrophages respond with activation of a compensatory cell-survival pathway in which the MAP kinase p38α enhances phosphorylation/activation of Akt, a potent survival signal in these cells (see previous section).79 In essence, this ER stress-activated pathway delays or suppresses apoptosis, but eventually the survival pathway gets overwhelmed, and apoptosis ensues. As predicted by this concept, we found that gene-targeting of macrophage p38α partially impedes Akt activation and promotes ER stress-induced apoptosis both in vitro and in advanced plaques in fat-fed Ldlr−/− mice. Because insulin resistance also partially impedes Akt signaling, we reasoned that the two pathways might be additive. Indeed, treatment of macrophages from Insr−/−;Ldlr−/− mice with an ER stressor plus a p38 inhibitor enhanced apoptosis to a very high level, i.e., above the high level already seen when these macrophages are subjected to ER stress alone79 (see previous section). These findings further demonstrate the importance of defective Akt signaling, a critical component of intact insulin receptor signaling, in ER stress-induced macrophage apoptosis and raise caution about the use of p38 inhibitors, currently under development as anti-inflammatory agents in a number of diseases including type 2 diabetes,80 in insulin-resistance subjects.
Two models of global insulin resistance have also shown an effect on plaque necrosis. A recent study examining Western diet-fed ob/ob;Ldlr−/− mice, which have obesity and insulin resistant secondary to leptin deficiency, showed an increase in necrotic core size compared to similarly fed Ldlr−/− mice.81 As explained below, the mechanism not only involves increased susceptibility to apoptosis but also defective efferocytosis in the macrophages of these mice. Hsueh and colleagues82 compared 3 mo/old and 12 mo/old Ldlr−/− mice fed a high-fat diet for 3 months. The older mice developed worse insulin resistance and worse atherosclerosis than the younger mice, and the lesions in the older mice appeared to be associated with a marked increase in plaque necrosis. The insulin-resistant older mice had a blunted anti-oxidant response that might be caused by a defective DJ-1—Nrf2 anti-oxidant pathway,83 and a higher lesional expression of the NADPH oxidase subunit, p47. Atherosclerosis and plaque morphology were improved by treating the mice with the NADPH oxidase inhibitor and anti-oxidant, apocynin. One implication of these findings is that aging, a major risk factor for cardiovascular disease in humans,84 may interact with insulin resistance to promote plaque necrosis, and in this regard it is interesting to note that aging is associated with both enhanced ER stress and defective efferocytosis.85, 86 Second, a critical downstream pro-apoptotic effector of ER stress and ER calcium release is activation of NADPH oxidase, and, given the pathways described in Figure 2, this response may be further enhanced in the setting of insulin resistance. Although vitamin E has not been shown to be effective in decreasing cardiovascular risk in humans,87 more targeted anti-oxidants, such as NADPH oxidase inhibitors, in the specific setting of insulin resistance and possibly aging, may be more mechanistically justified and have more promise.
How Insulin Resistance Might Affect Efferocytosis
The increase in plaque necrosis in diabetic lesions raises the important issue as to whether efferocytosis is defective in these lesions and, if so, how this is mechanistically linked to insulin resistance. For example, defective phosphatidylinositol 3-kinase signaling in the setting of insulin resistance could, in theory, lead to a defect in efferocytosis in general and a specific defect in Mertk-mediated efferocytosis in particular.88, 89 Using an in situ assay that quantifies the percentage of apoptotic cells that have been engulfed by phagocytic macrophages vs. not associated with phagocytes,49 Li et al.81 found that the aortic root lesions of Western diet-fed ob/ob;Ldlr−/− mice had evidence of defective efferocytosis and, as predicted, increased plaque necrosis compared with lesions of Western diet-fed Ldlr−/− mice. In vitro studies showed that primary macrophages isolated from ob/ob mice have a defect in efferocytosis that was associated with defective PI3 kinase activity, but those from Insr−/− mice do not. Further studies revealed that the key defect in ob/ob macrophages was an increase in the saturated fatty acid:unsaturated fatty acid ratio in the macrophage membranes, perhaps through “stiffening” the plasma membrane to the point where phagocytosis is compromised.90 The efferocytosis defect on ob/ob macrophage could be corrected by treating the cells with the omega-3 polyunsaturated fatty acid eicosapentanoic acid (EPA), and similar results were found when macrophages were harvested from EPA-fed ob/ob mice. Most importantly, lesional efferocytosis was improved in ob/ob;Ldlr−/− mice by EPA feeding, which interestingly has also been associated with protection from heart disease in humans.91 The precise mechanism of how saturated fatty acid impair efferocytosis and how EPA improves it is still under investigation, as are other possible links between insulin resistance and clearance of apoptotic cells. Nonetheless, we can begin to imagine an integrated model in which direct effects of insulin resistance on advanced lesional macrophage apoptosis, combined with defective efferocytosis caused by systemic fatty acid defects in the setting of insulin resistance, can at least partially explain the large neurotic cores and accelerated thrombotic vascular disease in diabetics (Figure 2).
Conclusions and Future Directions
This review focused on one key feature of type 2 diabetes, insulin resistance; one type of lesional cell, the macrophage; and one overall context of atherosclerosis, advanced plaque progression. Even within this focused area of research, more work is needed to further define mechanisms whereby insulin resistance affects specific signaling pathways involved in the panoply of atherosclerosis-relevant macrophage activities, including, interaction with lipoproteins and intracellular metabolism of lipoprotein-derived lipids; inflammation and the resolution thereof; stress responses, including oxidative, heat shock, and ER stress; secretion of proteases, pro-coagulant molecules, and other factors involved in plaque progression; phagocytosis, efferocytosis, and antigen presentation; apoptosis-cell survival balance; and interaction with other cells and extracellular matrix. Moreover, it is likely that insulin resistance affects these processes differently in different subsets of macrophages and in other types of myeloid cells, notably dendritic cells, mast cells, and neutrophils. A limitation of our in vivo studies has been the lack of a mouse model that fully recapitulates features of human plaque disruption and athero-thrombosis,92 and so further developments to improve mouse models of diabetic atherothrombotic vascular disease is an important goal. Nonetheless, it is becoming clear that key morphologic features of such plaques are worsened by ER stress33 and insulin resistance in macrophages.69
Beyond the specific areas of plaque macrophages, insulin resistance, and advanced plaque progression, other areas of focus may offer additional clues as to why heart disease is enhanced in type 2 diabetes.61 For example, decreased insulin signaling in endothelial cells, through impaired Akt signaling, is also likely to have important pro-atherogenic consequences through decreased eNOS activity and increased expression of inflammatory genes and VCAM-1.73 In the liver, hyperinsulinemia and insulin signaling may increase VLDL secretion while having the opposite effects on LDL receptor expression.93 The other major feature of type 2 diabetes, hyperglycemia, may promote plaque instability by enhancing the inflammatory response in macrophages through effects on plasma triglyceride-rich lipoproteins and free fatty acids.94 Hyperglycemia may also cause endothelial cell abnormalities, including oxidative stress and RAGE-induced inflammation, that promote the earlier stages of atherogenesis.95, 96 Interestingly, there are recent data suggesting that hyperglycemia may exert some of its pro-atherogenic effects in endothelial cells through FoxO1 and also through the induction of ER stress.97, 98 These hyperglycemia-endothelial cell studies, together with the insulin resistance-macrophage studies described in this review, raise the interesting possibility that hyperglycemia may affect mostly the earlier stages of atherogenesis, while insulin resistance has its greatest effect on promoting advanced plaque progression. In this context, a recent analysis of the Veterans Affairs Diabetes Trial found that intensive glucose lowering reduced cardiovascular events in diabetics with a coronary artery calcium store < 100 (multivariable hazard ratio [HR] = 0.08, p=0.03), but not in those with a calcium score >100 (HR = 0.74, p=0.21).99 Smooth muscle cells, a key cell type in the generation of the “protective” fibrous cap in advanced lesions, and platelets, the final effector of acute vascular occlusion, may be affected by insulin resistance, hyperglycemia, or fatty acid abnormalities, which provide additional opportunities for investigation.61 Continued progress in these areas will provide a more complete understanding of how multiple features of diabetes promotes heart disease.
The ultimate goal of these studies is to complement our current efforts at identifying and treating systemic risk factors that promote cardiovascular disease in diabetics. Despite the relative success of this strategy, risk is still very high,100, and the tremendous scale of this epidemic is such that overall risk will still be high even if compliance is improved and the experimental modalities prove useful. Further understanding of the specific mechanisms of increased vascular disease in diabetics, particularly at the molecular level in arterial wall cells, may be a promising approach for further eradication in the future—and one that should be additive or even synergistic with reduction of lipid and other systemic risk factors. One approach is to increase insulin sensitivity in diabetic macrophages, such has been demonstrated recently using a PPARγ activator in vivo68 and 1,25(OH)2 vitamin D in vitro.101 Another approach is to develop agents to prevent ER stress or downstream pro-apoptotic processes in macrophages by pharmacologic means, e.g., through the use of chemical chaperones102 or inhibitors of the calcium-mediated pro-apoptotic pathway.103, 104 Moreover, in view of the importance of defective efferocytosis in the generation of plaque necrosis and the ob/ob efferocytosis study described above, experimental therapeutic modalities designed to enhance efferocytosis58, 105 may be particularly useful in diabetics. Delivery of such drugs to plaques might be facilitated by specific vehicles targeted to plaques,106 while clinical assessment in phase 2 and phase 3 studies could be assisted by imaging techniques such as carotid MRI, that have the capacity to measure important plaque features such as necrotic core area and cap thickness.107 Studies in these areas occurring in parallel with ongoing efforts at systemic risk reduction offer the best chance to curb the growing epidemic of diabetes-associated atherothrombotic vascular disease.
Acknowledgments
The authors gratefully acknowledge the outstanding members of our laboratories who contributed to the studies described herein, including Seongah Han, Chien-Ping Liang, Takafumi Senokuchi, Suzhao Li, Tracie Seimon, Gang Li, Jenelle Timmins, Lale Ozcan, Edward Thorp, Rebecca Haeusler, Jun Tanaka, and Li Qiang.
Sources of Funding
This work was supported by National Institutes of Health Grants HL087123, HL075662, and HL054591 and US Army Medical Research and Materiel Command (USAMRMC) grant W81XWH-06-1-0212.
Nonstandard Abbreviations and Acronyms
- UPR
unfolded protein response
- VLDL
very low-density lipoprotein
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- MMP
matrix metalloproteinase
- ER
endoplasmic reticulum
- CHOP
CEBP-homologous protein
- GADD
growth arrest and DNA damage
- PRR
pattern recognition receptor
- FC
free cholesterol
- TLR
toll-like receptor
- SAR
type A scavenger receptor
- BAPTA-AM
acetoxymethyl ester of 1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- CaMKII
calcium/calmodulin-dependent protein kinase II
- STAT
signal transducer and activator of transcription
- IP3
inositol 1,4,5-triphosphate
- ERO1α
ER oxidase-1α
- SERCA
sarco/endoplasmic reticulum calcium-dependent ATPase
- Mertk
c-mer tyrosine kinase
- Apoe
apolipoprotein E
- Ldlr
LDL receptor
- MFG-E8
milk fat globule epidermal growth factor 8
- IVUS
intravascular ultrasound
- IRS-2
insulin receptor substrate-2
- ERK
extracellular signal-regulated kinases
- MEK
MAPK/ERK kinase
- Insr
insulin receptor
- EPA
eicosapentanoic acid
- eNOS
endothelial nitric oxide synthase
- VCAM
vascular cells adhesion molecule
- PPAR
peroxisome proliferator-activated receptor
Footnotes
Disclosures
None.
References
- 1.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Coady S, Sorlie PD, D’Agostino RB, Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, D’Agostino R, Jr, Mykkanen L, Tracy R, Howard B, Rewers M, Selby J, Savage PJ, Saad MF. Insulin sensitivity in subjects with type 2 diabetes. Relationship to cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 1999;22:562–568. doi: 10.2337/diacare.22.4.562. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 7.Liang CP, Han S, Senokuchi T, Tall AR. The macrophage at the crossroads of insulin resistance and atherosclerosis. Circ Res. 2007;100:1546–1555. doi: 10.1161/CIRCRESAHA.107.152165. [DOI] [PubMed] [Google Scholar]
- 8.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 10.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 11.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature Rev Immunol. 2010 doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004;13:125–138. doi: 10.1016/S1054-8807(04)00004-3. [DOI] [PubMed] [Google Scholar]
- 14.Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, Finn AV, Gold HK. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–1391. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke AP, Virmani R, Galis Z, Haudenschild CC, Muller JE. 34th Bethesda Conference: Task force #2--What is the pathologic basis for new atherosclerosis imaging techniques? J Am Coll Cardiol. 2003;41:1874–1886. doi: 10.1016/s0735-1097(03)00359-0. [DOI] [PubMed] [Google Scholar]
- 16.Kruth HS. Localization of unesterified cholesterol in human atherosclerotic lesions. Demonstration of filipin-positive, oil-red-O-negative particles. Am J Pathol. 1984;114:201–208. [PMC free article] [PubMed] [Google Scholar]
- 17.Guyton JR, Klemp KF. Development of the atherosclerotic core region. Chemical and ultrastructural analysis of microdissected atherosclerotic lesions from human aorta. Arterioscler Thromb. 1994;14:1305–1314. doi: 10.1161/01.atv.14.8.1305. [DOI] [PubMed] [Google Scholar]
- 18.Small DM. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988;8:103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- 19.Lundberg B. Chemical composition and physical state of lipid deposits in atherosclerosis. Atherosclerosis. 1985;56:93–110. doi: 10.1016/0021-9150(85)90087-5. [DOI] [PubMed] [Google Scholar]
- 20.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer HE. The role of macrophages in atherosclerosis. Hamatol Bluttransfus. 1981;27:137–142. doi: 10.1007/978-3-642-81696-3_15. [DOI] [PubMed] [Google Scholar]
- 22.Ball RY, Stowers EC, Burton JH, Cary NR, Skepper JN, Mitchinson MJ. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-s. [DOI] [PubMed] [Google Scholar]
- 23.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 24.Libby P, Clinton SK. The role of macrophages in atherogenesis. Curr Opin Lipidol. 1993;4:355–363. [Google Scholar]
- 25.Hegyi L, Skepper JN, Cary NRB, Mitchinson MJ. Foam cell apoptosis and the development of the lipid core of human atherosclerosis. J Pathol. 1996;180:423–429. doi: 10.1002/(SICI)1096-9896(199612)180:4<423::AID-PATH677>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 27.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J Clin Invest. 2001;108:957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 29.Young JL, Libby P, Schonbeck U. Cytokines in the pathogenesis of atherosclerosis. Thromb Haemost. 2002;88:554–567. [PubMed] [Google Scholar]
- 30.Ohayon J, Finet G, Gharib AM, Herzka DA, Tracqui P, Heroux J, Rioufol G, Kotys MS, Elagha A, Pettigrew RI. Necrotic core thickness and positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol. 2008;295:H717–H727. doi: 10.1152/ajpheart.00005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabas I. Apoptosis and plaque destabilization: the role of macrophage apoptosis induced by cholesterol. Cell Death & Differentiation. 2004;11:S12–S16. doi: 10.1038/sj.cdd.4401444. [DOI] [PubMed] [Google Scholar]
- 32.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 33.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metabolism. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O’dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeVries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao PM, Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem. 2000;275:23807–23813. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- 38.Yao PM, Tabas I. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J Biol Chem. 2001;276:42468–42476. doi: 10.1074/jbc.M101419200. [DOI] [PubMed] [Google Scholar]
- 39.Shio H, Haley NJ, Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. III. Intracellular localization of cholesterol and cholesteryl ester. Lab Invest. 1979;41:160–167. [PubMed] [Google Scholar]
- 40.Katz SS, Shipley GG, Small DM. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976;58:200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim WS, Timmins JM, Seimon TA, Sadler A, Kolodgie FD, Virmani R, Tabas I. STAT1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links endoplasmic reticulum stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Ge M, Ciani L, Kuriakose G, Westover E, Dura M, Covey D, Freed JH, Maxfield FR, Lytton J, Tabas I. Enrichment of endoplasmic reticulum with cholesterol inhibits SERCA2b activity in parallel with increased order of membrane lipids. Implications for depletion of ER calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem. 2004;279:37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 45.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 46.Secchiero P, Candido R, Corallini F, Zacchigna S, Toffoli B, Rimondi E, Fabris B, Giacca M, Zauli G. Systemic tumor necrosis factor-related apoptosis-inducing ligand delivery shows antiatherosclerotic activity in apolipoprotein E-null diabetic mice. Circulation. 2006;114:1522–1530. doi: 10.1161/CIRCULATIONAHA.106.643841. [DOI] [PubMed] [Google Scholar]
- 47.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 48.Chang M-K, Bergmark C, Laurila A, Horkko S, Han K-H, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: Evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of Apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.it-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 51.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng B, Zhang D, Kuriakose G, Devlin CM, Kockx M, Tabas I. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:10423–10428. doi: 10.1073/pnas.1732494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 54.Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 55.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 56.Sanson M, Auge N, Vindis C, Muller C, Bando Y, Thiers JC, Marachet MA, Zarkovic K, Sawa Y, Salvayre R, Negre-Salvayre A. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: prevention by oxygen-regulated protein 150 expression. Circ Res. 2009;104:328–336. doi: 10.1161/CIRCRESAHA.108.183749. [DOI] [PubMed] [Google Scholar]
- 57.Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, varez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukocyte Biol. 2009 doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 60.Plutzky J, Viberti G, Haffner S. Atherosclerosis in type 2 diabetes mellitus and insulin resistance: mechanistic links and therapeutic targets. J Diabetes Complications. 2002;16:401–415. doi: 10.1016/s1056-8727(02)00202-7. [DOI] [PubMed] [Google Scholar]
- 61.D’Souza A, Hussain M, Howarth FC, Woods NM, Bidasee K, Singh J. Pathogenesis and pathophysiology of accelerated atherosclerosis in the diabetic heart. Mol Cell Biochem. 2009 doi: 10.1007/s11010-009-0148-8. [DOI] [PubMed] [Google Scholar]
- 62.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 63.Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, Fallon JT. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102:2180–2184. doi: 10.1161/01.cir.102.18.2180. [DOI] [PubMed] [Google Scholar]
- 64.Nasu K, Tsuchikane E, Katoh O, Fujita H, Surmely JF, Ehara M, Kinoshita Y, Tanaka N, Matsubara T, Asakura Y, Asakura K, Terashima M, Suzuki T. Plaque characterisation by Virtual Histology intravascular ultrasound analysis in patients with type 2 diabetes. Heart. 2008;94:429–433. doi: 10.1136/hrt.2007.118950. [DOI] [PubMed] [Google Scholar]
- 65.Hong YJ, Jeong MH, Choi YH, Ko JS, Lee MG, Kang WY, Lee SE, Kim SH, Park KH, Sim DS, Yoon NS, Yoon HJ, Kim KH, Park HW, Kim JH, Ahn Y, Cho JG, Park JC, Kang JC. Plaque characteristics in culprit lesions and inflammatory status in diabetic acute coronary syndrome patients. JACC Cardiovasc Imaging. 2009;2:339–349. doi: 10.1016/j.jcmg.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Pundziute G, Schuijf JD, Jukema JW, van Werkhoven JM, Nucifora G, Decramer I, Sarno G, Vanhoenacker PK, Reiber JH, Wijns W, Bax JJ. Type 2 diabetes is associated with more advanced coronary atherosclerosis on multislice computed tomography and virtual histology intravascular ultrasound. J Nucl Cardiol. 2009;16:376–383. doi: 10.1007/s12350-008-9046-9. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Garcia HM, Serruys PW, Mintz GS, Saito S, Klaus V, Margolis P, Carlier S, Goedhart D, Schwartz R. Synergistic effect of cardiovascular risk factors on necrotic core in coronary arteries: a report from the global intravascular radiofrequency data analysis registry. JACC Cardiovasc Imaging. 2009;2:629–636. doi: 10.1016/j.jcmg.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metabolism. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 71.Senokuchi T, Liang CP, Seimon TA, Han S, Matsumoto M, Banks AS, Paik JH, Depinho RA, Accili D, Tabas I, Tall AR. Forkhead transcription factors (FoxOs) promote apoptosis of insulin-resistant macrophages during cholesterol-induced endoplasmic reticulum stress. Diabetes. 2008;57:2967–2976. doi: 10.2337/db08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thorp E, Kuriakose G, Shah YM, Gonzalez FJ, Tabas I. Pioglitazone increases macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions of nondiabetic low-density lipoprotein receptor-null mice. Circulation. 2007;116:2182–2190. doi: 10.1161/CIRCULATIONAHA.107.698852. [DOI] [PubMed] [Google Scholar]
- 73.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 75.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 76.Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 77.Liang CP, Han S, Li G, Senokuchi T, Tabas I, Tall AR. Defective MEK signaling and SERCA activity contribute to increased apoptosis in insulin resistant macrophages. Diabetes. 2008;57:A198. [Google Scholar]
- 78.Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab. 2006;3:247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seimon TA, Wang Y, Han S, Senokuchi T, Schrijvers DM, Kuriakose G, Tall AR, Tabas IA. Macrophage deficiency of p38alpha MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J Clin Invest. 2009;119:886–898. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schindler JF, Monahan JB, Smith WG. p38 pathway kinases as anti-inflammatory drug targets. J Dent Res. 2007;86:800–811. doi: 10.1177/154405910708600902. [DOI] [PubMed] [Google Scholar]
- 81.Li S, Sun Y, Thorp E, Liang CP, Han S, Jehle A, Viswanathan S, Kanter J, Li R, Welch CL, Hasty AH, Bornfeldt KE, Tabas I, Tall AR. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and its reversal by a fish oil diet. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 83.Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;10:879–891. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- 84.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 85.Naidoo N. The endoplasmic reticulum stress response and aging. Rev Neurosci. 2009;20:23–37. doi: 10.1515/revneuro.2009.20.1.23. [DOI] [PubMed] [Google Scholar]
- 86.Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol. 2008;152:448–455. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams KJ, Fisher EA. Oxidation, lipoproteins, and atherosclerosis: which is wrong, the antioxidants or the theory? Curr Opin Clin Nutr Metab Care. 2005;8:139–146. doi: 10.1097/00075197-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 88.Hu B, Punturieri A, Todt J, Sonstein J, Polak T, Curtis JL. Recognition and phagocytosis of apoptotic T cells by resident murine tissue macrophages require multiple signal transduction events. J Leukoc Biol. 2002;71:881–889. [PMC free article] [PubMed] [Google Scholar]
- 89.Hall MO, Agnew BJ, Abrams TA, Burgess BL. The phagocytosis of os is mediated by the PI3-kinase linked tyrosine kinase receptor, mer, and is stimulated by GAS6. Adv Exp Med Biol. 2003;533:331–336. doi: 10.1007/978-1-4615-0067-4_41. [DOI] [PubMed] [Google Scholar]
- 90.Calder PC, Bond JA, Harvey DJ, Gordon S, Newsholme EA. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem J. 1990;269:807–814. doi: 10.1042/bj2690807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leaf A. Historical overview of n-3 fatty acids and coronary heart disease. Am J Clin Nutr. 2008;87:1978S–1980S. doi: 10.1093/ajcn/87.6.1978S. [DOI] [PubMed] [Google Scholar]
- 92.Rosenfeld ME, Carson KG, Johnson JL, Williams H, Jackson CL, Schwartz SM. Animal models of spontaneous plaque rupture: the holy grail of experimental atherosclerosis research. Curr Atheroscler Rep. 2002;4:238–242. doi: 10.1007/s11883-002-0025-3. [DOI] [PubMed] [Google Scholar]
- 93.Han S, Liang CP, Westerterp M, Senokuchi T, Welch CL, Wang Q, Matsumoto M, Accili D, Tall AR. Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J Clin Invest. 2009;119:1029–1041. doi: 10.1172/JCI36523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johansson F, Kramer F, Barnhart S, Kanter JE, Vaisar T, Merrill RD, Geng L, Oka K, Chan L, Chait A, Heinecke JW, Bornfeldt KE. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci U S A. 2008;105:2082–2087. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 96.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tanaka J, Li Q, Banks AS, Welch CL, Matsumoto M, Kitamura T, Ido-Kitamura Y, Depinho RA, Accili D. Foxo1 links hyperglycemia to LDL oxidation and eNOS dysfunction in vascular endothelial cells. Diabetes. 2009 doi: 10.2337/db09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khan MI, Pichna BA, Shi Y, Bowes AJ, Werstuck GH. Evidence Supporting a Role for Endoplasmic Reticulum Stress in the Development of Atherosclerosis in a Hyperglycaemic Mouse Model. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2569. [DOI] [PubMed] [Google Scholar]
- 99.Reaven PD, Moritz TE, Schwenke DC, Anderson RJ, Criqui M, Detrano R, Emanuele N, Kayshap M, Marks J, Mudaliar S, Rao RH, Shah JH, Goldman S, Reda DJ, McCarren M, Abraira C, Duckworth W. Intensive Glucose Lowering Therapy Reduces Cardiovascular Disease Events in VADT Participants with Lower Calcified Coronary Atherosclerosis. Diabetes. 2009 doi: 10.2337/db09-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Preis SR, Pencina MJ, Hwang SJ, D’Agostino RB, Sr, Savage PJ, Levy D, Fox CS. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the framingham heart study. Circulation. 2009;120:212–220. doi: 10.1161/CIRCULATIONAHA.108.846519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, Bernal-Mizrachi L, Bernal-Mizrachi C. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 103.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 104.Yu J, Weiwer M, Linhardt RJ, Dordick JS. The role of the methoxyphenol apocynin, a vascular NADPH oxidase inhibitor, as a chemopreventative agent in the potential treatment of cardiovascular diseases. Curr Vasc Pharmacol. 2008;6:204–217. doi: 10.2174/157016108784911984. [DOI] [PubMed] [Google Scholar]
- 105.Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR, Godson C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 106.Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25:667–680. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 107.Silvera SS, Aidi HE, Rudd JH, Mani V, Yang L, Farkouh M, Fuster V, Fayad ZA. Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]