Abstract
Estrogen stimulates the proliferation of breast cancer cells and the growth of estrogen-responsive tumors. The aromatase enzyme, which converts androgen to estrogen, plays a key role in breast carcinogenesis. The pomegranate fruit, a rich source of ellagitannins (ETs), has attracted recent attention due to its anti-cancer and anti-atherosclerotic properties. On consumption, pomegranate ETs hydrolyze, releasing ellagic acid (EA) which is then converted to 3,8-dihydroxy-6H-dibenzo[b,d]pyran-6-one (‘urolithin’) derivatives by gut microflora. The purpose of this study was to investigate the anti-aromatase activity and inhibition of testosterone-induced breast cancer cell proliferation by ellagitannin (ET)-derived compounds isolated from pomegranates. A panel of ten ET-derived compounds including EA, gallagic acid (GA), and urolithins A and B (and their acetylated, methylated and sulfated analogs prepared in our laboratory) were examined for ability to inhibit aromatase activity and testosterone-induced breast cancer cell proliferation. Using a microsomal aromatase assay, we screened the panel of ET-derived compounds and identified six with anti-aromatase activity. Among these, urolithin B (UB) was shown to most effectively inhibit aromatase activity in a live-cell assay. Kinetic analysis of UB demonstrated mixed inhibition, suggesting more than one inhibitory mechanism. Proliferation assays also determined that UB significantly inhibited testosterone-induced MCF-7aro cell proliferation. The remaining test compounds also exhibited anti-proliferative activity, but to a lesser degree than UB. These studies suggest that pomegranate ET-derived compounds have potential for the prevention of estrogen-responsive breast cancers.
Keywords: aromatase, breast cancer, ellagitannin, pomegranate, proliferation
INTRODUCTION
Treatment of hormone-dependent breast cancers has historically focused on manipulation of the level and/or activity of estrogen. Typically, inhibition of estrogen activity is achievable through antagonism of the estrogen receptor (ER) through selective estrogen receptor modulators (SERMs) [1–3] or estrogen deprivation via inhibition of estrogen synthesis by the aromatase enzyme [4–6]. The aim of chemoprevention research is to identify well-tolerated, highly effective strategies for the prevention of occurrence and re-occurrence of cancer. Currently, interest in a number of fruits high in polyphenolic compounds has been raised due to their reported chemopreventive potential.
Pomegranate juice (Punica granatum L) has been shown to be high in antioxidant activity, which is generally attributed to its high polyphenol content. Pomegranate juice is produced by squeezing of the whole fruit, leading to a high content of polyphenols, among which ellagitannins (ETs) predominate [7]. Pomegranate juice and its purified ETs have been shown to inhibit cancer cell proliferation [8] and inflammatory cell signaling [9] in vitro. Previous work has shown that ETs from pomegranate juice are hydrolyzed to ellagic acid (EA) which is then taken up intact and metabolized by human colonic microflora to 3,8-dihydroxy-6H-dibenzo[b,d]pyran-6-one derivatives (urolithin A and B) [10–13]. These compounds have been shown to inhibit the growth of human prostate and breast cancer cell lines [14]. In vitro studies have demonstrated that urolithin A (UA) and urolithin B (UB) are taken up intact in MCF-7 breast cancer cells and converted to sulfate and glucuronate metabolites. In addition, it has been shown that UB is partially converted to UA in MCF-7 cells [15]. Finally, in vivo studies have shown that pomegranate extract and UA both inhibited the growth of LAPC-4 derived tumors in SCID mice [14].
A recent study by Larrosa et al also demonstrated that urolithins A and B exhibit both estrogenic and anti-estrogenic effects in MCF-7 human breast cancer cells and therefore may have SERM-like qualities [15]. Based on the above information, we investigated the anti-aromatase activity of a panel of ET-derived metabolites and synthetic analogs in both a microsome assay and an in-cell aromatase assay. The kinetics of the interaction was determined and we evaluated the ability of the ET-metabolites to inhibit testosterone-induced cell proliferation in the MCF-7aro aromatase over-expressing breast cancer cell line. These studies bring us closer to an understanding of how the ET-metabolites resulting from pomegranate ingestion can affect the growth of estrogen-responsive breast cancer cells.
MATERIALS AND METHODS
Reagents and Instruments
All solvents were high performance liquid chromatography (HPLC) grade from Fisher Scientific Co. (Tustin, CA). Formic, and phosphoric acids and chemicals used for syntheses of the urolithin derivatives (2-bromobenzoic, 2-bromo-5-methoxybenzoic and acetic acids, resorcinol, potassium dihydrogen phosphate, etc.) were purchased from Sigma- Aldrich (St. Louis, MO). The HPLC with ultraviolet (UV) detection analyses were carried out on a Waters Alliance 2690 system equipped with a photodiode array detector (Waters Corp., Milford, MA). The HPLC with electrospray ionization mass spectrometry (ESI/MS) system consisted of an LCQ Classic Finnigan system (ThermoFinnigan, San Jose, CA), equipped with an Agilent HP 1100 series HPLC (Santa Clara, CA) system consisting of an autosampler/injector, quaternary pump, column heater, and diode array detector (DAD) with Xcalibur 1.2 software (Finnigan Corp., San Jose, CA).
Test Compounds
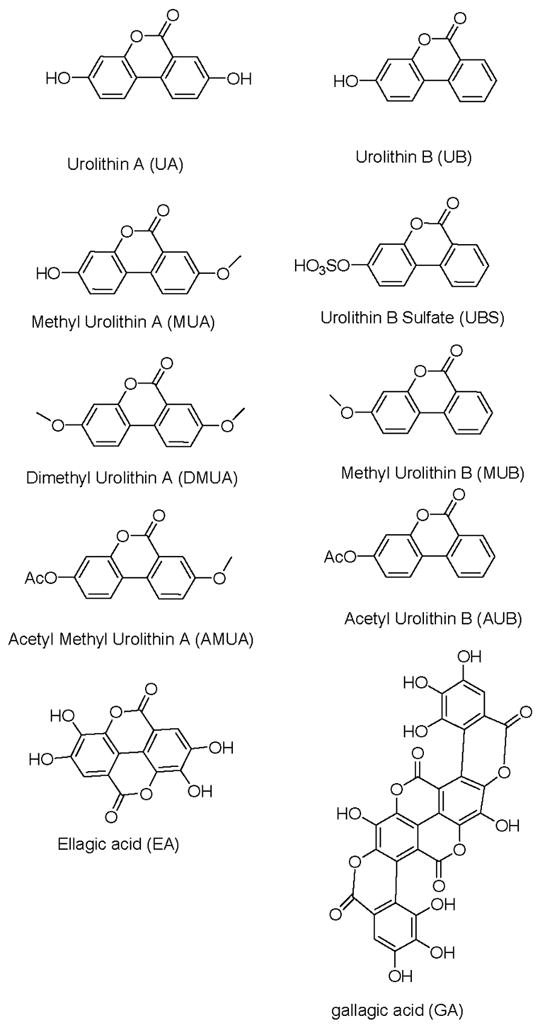
With the exception of ellagic acid (EA; obtained commercially from Sigma-Aldrich) and gallagic acid (GA); isolated in our laboratory from pomegranate fruit extract), urolithins A and B and their synthetic analogs (either methylated or acetylated) were prepared in our laboratory according to methods previously reported [10, 16]. The ET-derived compounds and their analogs included: urolithin A (UA), methylated-UA (MUA), dimethylated-UA (DMUA), acetylated-MUA (AMUA), urolithin B (UB), methylated-UB (MUB), acetylated-UB (AUB) and UB-sulfate (UBS). The structures of the test compounds are shown in Figure 1.
Figure 1. Chemical structures of test compounds.
Cell Culture
The ER-positive aromatase-overexpressing MCF-7 cell line, MCF-7aro, was developed previously in our laboratory as previously described [17]. The cells were cultured in Eagle’s minimum essential medium (MEM) (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) and the selection antibiotic G418 (500 μg/ml) (UBS Corporation, Cleveland, OH). Cells were incubated at 37°C with 5% CO2 and maintained in the linear phase of growth.
Placental Microsome Assay
This assay was performed utilizing human placental microsomes prepared from human placenta as previously described [18]. The assay mixture consisted of 20 μg placental microsome, 100 nmol/L [3H]-androstenedione (specific activity 37 MBq/mol), 10 μM progesterone, 1 g/L bovine serum albumin and 67 mM potassium phosphate buffer, pH 7.4 and 12 mM NADPH. Controls consisted of a blank (reaction that is started and stopped immediately), a positive control (water) and a vehicle control (DMSO in equal amount to the highest concentration of test product). Concentrations of test products were ten times higher in this assay than the in-cell assay, because the chemicals are relatively hydrophobic and are possibly taken up through the cell membrane. Therefore, the actual concentration in cells could be higher than that added to the microsomal mixture. The reaction was run for 20 minutes at 22°C and the enzymatic activity terminated with 5% trichloroacetic acid. The supernatant was extracted with dextran-treated charcoal. The suspension was centrifuged and an aliquot collected and counted for radioactivity. Aromatase activity was calculated as pmol [3H]-H2O formed/minute/mg protein. Analyses were performed in triplicate and the data are expressed as mean ± SEM.
Aromatase “in cell” Assay
MCF-7aro cells were cultured in 6-well plates in MEM overnight. The following day the medium was changed to phenol red free MEM with 10% charcoal dextran-treated (cdFBS) for 24 hours. The medium was removed and fresh cdFBS medium alone, with DMSO control or the test compounds (0 – 4.7μM) was added plus 0.1 mM [3H]-androstenedione and 500 nM progesterone for three hours. The supernatant was removed and extracted with dextran-treated charcoal. The suspension was centrifuged (700 × g for 7 minutes) and an anliquot of the supernatant was collected and counted for radioactivity. The cells were solubilized with 1 mL of 0.5 mM NaOH. Protein concentration was determined by the Bradford assay. Aromatase activity was expressed as pmol [3H]-H2O formed/hour. Analyses were performed in triplicate and the data are expressed as mean ± SEM.
Cell Proliferation Assay
Proliferation was measured utilizing the CellTiter-Glo® Luminescent Cell Viability Assay (Technical Bulletin # 288, Promega Corp., Madison, WI). MCF-7aro cells were plated in 96 well plates in MEM overnight. The following day the medium was removed and changed to phenol red free MEM with 10% charcoal-dextran stripped fetal bovine serum (cdFBS) for 24 hours. Cells were treated with 100 μL cdFBS MEM alone, cdFBS MEM with 1 nM testosterone or cdFBS with test samples (0 – 4.7μM) in the presence of 1 nM testosterone and incubated for 48-hour drug exposure duration. After 48 hours, the reagent was added and results were read on an Orion Microplate Luminometer (Bertholds Detection Systems, Pforzheim, Germany). All plates had control wells containing medium without cells to obtain a value for background luminescence which was subtracted from the test sample readings. This experiment was also carried out using estradiol (1 nM) in the place of testosterone. Data are expressed as percentage of untreated cells, mean ± SE for three replications.
Kinetic determinations
The inhibition kinetic studies for UB were performed using the placental microsome assay. The concentration range of [3H]-androstenedione ranged from 0 to 200 nM. The rate of the reaction was determined to be linear in this range previously in our laboratory [19–21]. Analyses were performed in triplicate and the data are expressed as mean ± SEM.
RESULTS
Inhibition of aromatase by ET-derived compounds
To determine the anti-aromatase activity of test compounds, we utilized the placental microsome aromatase assay. Initial studies with the test compounds utilized a range of concentrations to determine the correct range for further study. The range of 0 – 47 μM was determined to be appropriate. As can be seen from Figure 2, when they are graphed at an equal concentration (47 μM), the compounds showed varying potency. MUB, UBS, UB, AUB, UA and MUA significantly inhibited aromatase activity in this assay (p ≤ 0.01) where AMUA, DMUA and the polyphenols GA and EA showed no significant anti-aromatase activity compared to vehicle-treated controls. These results revealed which metabolites of pomegranate ingestion demonstrate anti-aromatase activity in vitro.
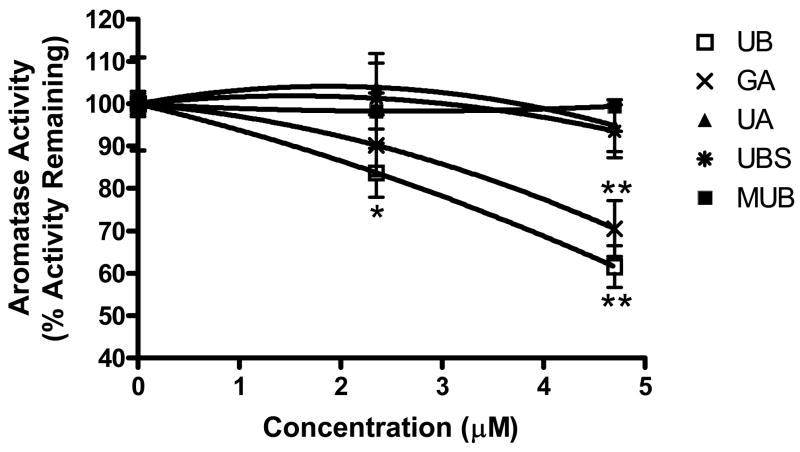
Figure 2. Inhibition of aromatase by urolithins.
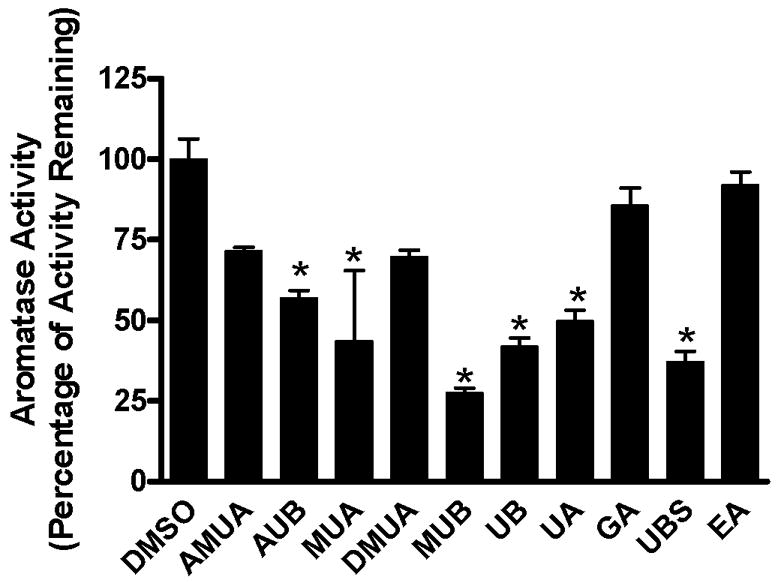
In vitro human placental aromatase assay was performed in the presence of 0.1 μM 3H-androstenedione and urolithin compounds (47 μM). The aromatase activity of vehicle-treated microsomes was set at 100%. The measurements were performed in triplicate. Data is expressed as percentage of activity remaining, mean ± SEM, p ≤ 0.01.
Aromatase inhibition in MCF-7aro cells treated with ET-derived compounds
To determine the inhibitory action of the compounds in a live cell system, active compounds identified in the microsome assay were tested in the MCF-7aro, aromatase over-expressing cell line. The cells were cultured in medium alone, medium plus vehicle control or medium plus increasing concentrations of urolithin compounds (Figure 3). As can be seen from the figure, UB significantly inhibited aromatase activity at 2.35μM (p ≤ 0.05) and 4.7μM (p ≤ 0.01) in the in-cell assay. In addition, GA also significantly inhibited aromatase activity at 4.7μM (p ≤ 0.01). This result shows that although a number of the other test compounds were active in the microsomal assay, UB was the most active in the cell-based assay.
Figure 3. “In Cell” inhibitory effect of urolithin compounds on aromatase in MCF-7aro cells.
MCF-7aro cells were maintained in MEM medium and switched to serum-free medium upon assay. Tritiated androstenedione (0.1 μM) and urolithins (0, 2.35 and 4.7μM) were administered and incubated for 3 hours. Values are means ± SEM, n = 3, *p ≤ 0.05, **p ≤ 0.01.
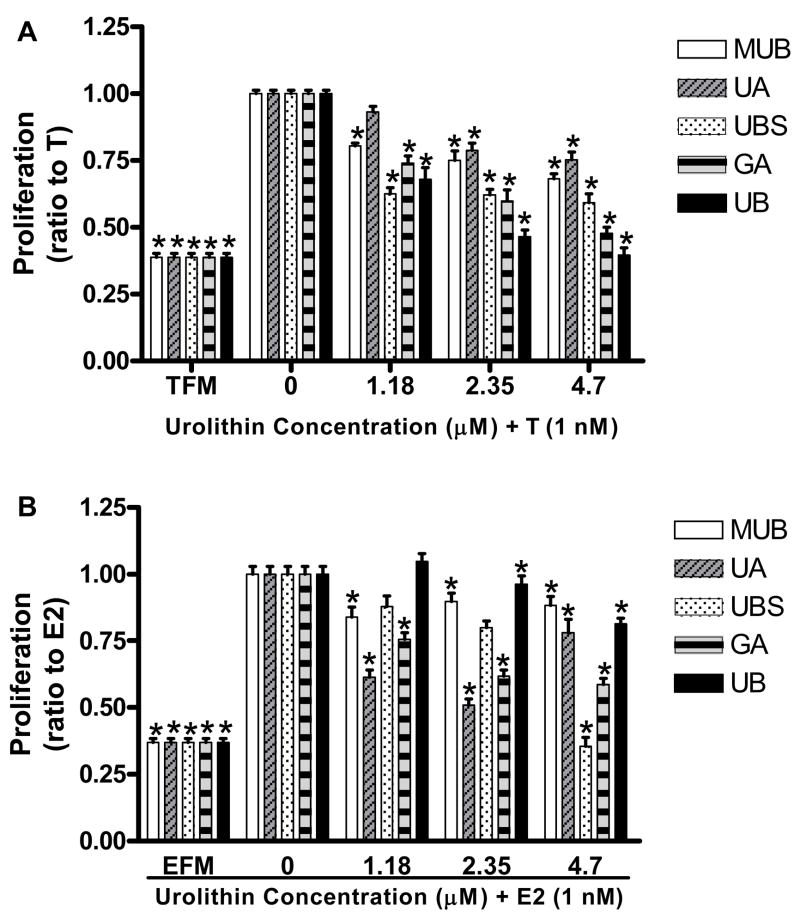
Anti-proliferative activity of ET-derived compounds in MCF-7aro cells
In the cell, testosterone is converted to estrogen via a reaction catalyzed by the aromatase enzyme; therefore we investigated the inhibition of both estrogen and testosterone-induced proliferation by ET-derived compounds in the MCF-7aro cell line. Results against testosterone-induced proliferation showed that UB had the highest antiproliferative activity at all test concentrations (p ≤ 0.01), which would be expected as it was the most effective inhibitor of aromatase using the in-cell assay. GA had the second highest activity (p ≤ 0.01), UBS, UA and MUB showed a mild response (p ≤ 0.01) (Figure 4A).
Figure 4. Effect of urolithin compounds on MCF-7aro cell proliferation.
MCF-7aro cells were seeded in 96-well plates and maintained in MEM supplemented with 10% charcoal dextran-treated serum. Proliferation was measured after 48 hours under the influence of testosterone (A) or estrogen (B) + urolithins at the indicated concentrations using the CellTiter-Glo® Luminescent Cell Viability Assay. “TFM” = testosterone free medium, “T” = testosterone, “EFM” = estrogen free medium, “E2” = estrogen. Values are expressed as mean ± SEM, n = 9, asterisk indicates significant difference from hormone alone (0) group (p ≤ 0.01).
In the estrogen-induced proliferation assay, UBS was the most potent inhibitor (p ≤ 0.01), with GA again the second most potent inhibitor. UA, MUB and UB all showed a mild response, with UB only showing a significant inhibition at the highest dose (p ≤ 0.01) (Figure 4B).
The results suggest that UB likely inhibits the proliferation of MCF7aro cells primarily through aromatase inhibition where the other test compounds may affect an aromatase-independent mechanism such as direct antagonism of estrogen receptor (ER) signaling or a combination of aromatase-dependent and independent mechanisms.
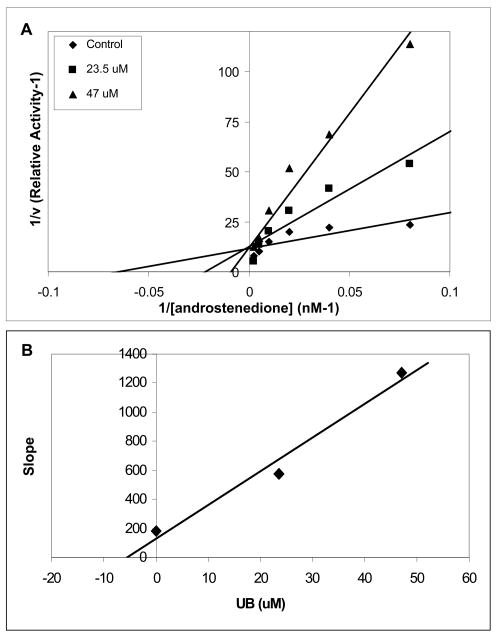
Kinetic analysis of aromatase inhibition with urolithin B (UB)
To determine the nature of aromatase inhibition by UB, enzyme kinetic analysis was performed using the human placental microsome assay. Three concentrations (0, 23.5 and 47 μM) of UB were administered to the cells along with increasing concentrations of 3H-androstenedione (0 – 200 nM). The Lineweaver-Burk plot shows that the presence of UB increased the Km value and slope with no change in the Vmax. This indicates that urolithin B is a competitive inhibitor with respect to the substrate, androstenedione (Figure 5A). An approximate Kι value of 5 μM was determined from the secondary plot (slope versus [I]) (Figure 5B).
Figure 5. Aromatase inhibition kinetic profile by urolithin B compound.
The enzyme assay was performed in the presence of 0, 23.5 and 47μM of urolithin B with increasing concentrations of [3H]-androstenedione (0 – 200 nM). The assay was performed in triplicate. (A) Lineweaver-Burk plot (1/v versus 1/ [androstenedione] (B) secondary plot (slope versus [UB]) used to determine Ki value for UB.
DISCUSSION
Six of the ET-derived compounds, MUB, UBS, UB, AUB, UA and MUA exhibited significant anti-aromatase activity as measured in the microsome assay. However, this activity did not reach that of known aromatase inhibitor drugs previously tested in our laboratory [22]. The test compounds AMUA, GA and EA showed no significant anti-aromatase activity in this assay. It is possible that a combination of these products may produce a more potent effect, as in vivo they would not be found circulating in isolation. For example, a study by Kim et al showed that polyphenol-rich fractions of pomegranate inhibited aromatase activity in a microsomal assay up to 51% [23], suggesting that combinations of polyphenols may be more effective than individual compounds. Future studies to examine the synergistic effects of these compounds would answer this question.
Of the urolithin compounds active in the microsomal assay, UB showed the highest anti-aromatase activity compared to the other compounds tested in the in-cell assay. This result may be due to a greater absorption of UB into the cells. A recent study by Larrosa et al demonstrated that both UA and UB were taken up intact in MCF-7 cells and metabolized to their sulfate and glucuronide forms, however, UB uptake was shown to be greater than that of UA [15]. This result could explain the lower activity of UA in the live cell system. In addition to the differences in UB and UA absorption into the cells, urolithin B-sulfate and other isomers have been detected in the media but not the cell lysate in significant amounts, suggesting that the other forms of UA and UB were not as active in the in-cell assay perhaps because they must first undergo conversion to UB [15]. The proliferation assays were done in a longer time frame (48 hours), and more activity was seen than the shorter term (3 hours) in-cell aromatase assay, suggesting longer term exposure could increase conversion and absorption.
In addition, the flavones chrysin and apigenin are identical in structure with the exception of an additional hydroxyl group on apigenin. In an aromatase assay, this small difference changed the EC50 of apigenin to 20 μM where chrysin was 7 μM, illustrating this point [24]. Because MCF-7 is the parental line of the MCF-7aro cells utilized in our study, we suggest that urolithin B absorption is similar in both cells lines and the decreased uptake of UA may account for its lack of aromatase inhibition in the in-cell assay. We further tested UB to determine the nature of its inhibition of aromatase by kinetic analysis utilizing the microsome assay. Results indicated that UB is a competitive inhibitor of aromatase, with an approximate Kι of 5 μM.
Inhibition of testosterone-induced cell proliferation was observed with UB treatment as would be expected from the aromatase assay results. This suggests that the inhibition of aromatase, and therefore estrogen production in the cell, is one mechanism through which UB may inhibit breast cancer cell proliferation. However, because the MCF-7aro cell line also contains a functional estrogen receptor (ER), the antiproliferative effect may also be due in part to direct antagonism of the ER. To determine the roles of both aromatase and ER signaling in the antiproliferative mechanisms of urolithins in this cell line, proliferation assays using estrogen as an inducer were done. In this assay, UB showed very low activity, further suggesting its role as an inhibitor of aromatase.
Interestingly, the inhibition of testosterone-induced proliferation was also observed in cells treated with UA, GA, MUB and UBS, suggesting that these ET-derived compounds may inhibit breast cancer cell growth through aromatase-independent mechanisms. In the estrogen-induced proliferation assay, UBS and GA showed the highest anti-proliferative activity, suggesting that UBS may antagonize ER-signaling. GA on the other hand, had activity in both testosterone and estrogen-induced proliferation assays, suggesting multiple roles for this compound. Although these compounds may not be as readily taken up by the cells as UB, it is possible that their antiproliferative activity could be due conversion to other compounds in the medium. Future studies into the fate of these compounds and their effects on the cell cycle and cell signaling will be carried out to elucidate their mechanisms of action.
The ingestion of pomegranate juice can lead to concentrations of circulating urolithins reaching up to 18 μM in blood. Taken together with the results of current studies and reports of the presence of UA and UB in the blood and urine of human subjects following pomegranate ingestion, the results of these analyses suggest that pomegranate intake may be a viable strategy for the chemoprevention of breast cancer.
Acknowledgments
This research was supported by National Institutes of Health Grants ES08258 and CA44735.
References
- 1.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 4.Morandi P, Rouzier R, Altundag K, et al. The role of aromatase inhibitors in the adjuvant treatment of breast carcinoma: the M. D. Anderson Cancer Center evidence-based approach. Cancer. 2004;101(7):1482–9. doi: 10.1002/cncr.20522. [DOI] [PubMed] [Google Scholar]
- 5.O’Shaughnessy J. A decade of letrozole: FACE. Breast Cancer Res Treat. 2007;105 Suppl 1:67–74. doi: 10.1007/s10549-007-9702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boccardo F, Rubagotti A, Aldrighetti D, et al. Switching to an aromatase inhibitor provides mortality benefit in early breast carcinoma: pooled analysis of 2 consecutive trials. Cancer. 2007;109(6):1060–7. doi: 10.1002/cncr.22513. [DOI] [PubMed] [Google Scholar]
- 7.Seeram NP, Zhang Y, Reed J, Krueger C, Vaya J. Pomegranate Phytochemicals. In: Schulman R, Seeram NP, Heber D, editors. Pomegranates Ancient Roots to Modern Medicine. Boca Raton, FL: Taylor & Francis Group; 2006. pp. 3–30. [Google Scholar]
- 8.Seeram NP, Adams LS, Henning SM, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16(6):360–7. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Adams LS, Seeram NP, Aggarwal BB, et al. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54(3):980–5. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- 10.Seeram NP, Henning SM, Zhang Y, et al. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136(10):2481–5. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 11.Cerda B, Espin JC, Parra S, Martinez P, Tomas-Barberan FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004;43(4):205–20. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- 12.Cerda B, Soto C, Albaladejo MD, et al. Pomegranate juice supplementation in chronic obstructive pulmonary disease: a 5-week randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2006;60(2):245–53. doi: 10.1038/sj.ejcn.1602309. [DOI] [PubMed] [Google Scholar]
- 13.Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum l.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem. 2006;54(23):8956–61. doi: 10.1021/jf061674h. [DOI] [PubMed] [Google Scholar]
- 14.Seeram NP, Aronson WJ, Zhang Y, et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55(19):7732–7. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 15.Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54(5):1611–20. doi: 10.1021/jf0527403. [DOI] [PubMed] [Google Scholar]
- 16.Ghosal SLJ, Singh S, Kumar Y, Shilajit F. Chemistry of two bioactive benzopyrone metabolites. J Chem Res Synop. 1989;11:350–351. [Google Scholar]
- 17.Sun XZ, Zhou D, Chen S. Autocrine and paracrine actions of breast tumor aromatase. A three-dimensional cell culture study involving aromatase transfected MCF-7 and T-47D cells. J Steroid Biochem Mol Biol. 1997;63(1–3):29–36. doi: 10.1016/s0960-0760(97)00068-x. [DOI] [PubMed] [Google Scholar]
- 18.Grube BJ, Eng ET, Kao YC, Kwon A, Chen S. White button mushroom phytochemicals inhibit aromatase activity and breast cancer cell proliferation. J Nutr. 2001;131(12):3288–93. doi: 10.1093/jn/131.12.3288. [DOI] [PubMed] [Google Scholar]
- 19.Kao YC, Cam LL, Laughton CA, Zhou D, Chen S. Binding characteristics of seven inhibitors of human aromatase: a site-directed mutagenesis study. Cancer Res. 1996;56(15):3451–60. [PubMed] [Google Scholar]
- 20.Chen S, Kao YC, Laughton CA. Binding characteristics of aromatase inhibitors and phytoestrogens to human aromatase. J Steroid Biochem Mol Biol. 1997;61(3–6):107–15. [PubMed] [Google Scholar]
- 21.Kao YC, Zhou C, Sherman M, Laughton CA, Chen S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ Health Perspect. 1998;106(2):85–92. doi: 10.1289/ehp.9810685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Cho M, Karlsberg K, Zhou D, Yuan YC. Biochemical and biological characterization of a novel anti-aromatase coumarin derivative. J Biol Chem. 2004;279(46):48071–8. doi: 10.1074/jbc.M406847200. [DOI] [PubMed] [Google Scholar]
- 23.Kim ND, Mehta R, Yu W, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat. 2002;71(3):203–17. doi: 10.1023/a:1014405730585. [DOI] [PubMed] [Google Scholar]
- 24.Hong Y, Chen S. Aromatase inhibitors: structural features and biochemical characterization. Ann N Y Acad Sci. 2006;1089:237–51. doi: 10.1196/annals.1386.022. [DOI] [PubMed] [Google Scholar]