Abstract
Context: Pheochromocytomas can usually be confirmed or excluded using currently available biochemical tests of catecholamine excess. Follow-up tests are, nevertheless, often required to distinguish false-positive from true-positive results. The glucagon stimulation test represents one such test; its diagnostic utility is, however, unclear.
Objective: The aim of the study was to determine the diagnostic power of the glucagon test to exclude or confirm pheochromocytoma.
Design, Setting, and Subjects: Glucagon stimulation tests were carried out at three specialist referral centers in 64 patients with pheochromocytoma, 38 patients in whom the tumor was excluded, and in a reference group of 36 healthy volunteers.
Main Outcome Measures: Plasma concentrations of norepinephrine and epinephrine were measured before and after glucagon administration. Several absolute and relative test criteria were used for calculating diagnostic sensitivity and specificity. Expression of the glucagon receptor was examined in pheochromocytoma tumor tissue from a subset of patients.
Results: Larger than 3-fold increases in plasma norepinephrine after glucagon strongly predicted the presence of a pheochromocytoma (100% specificity and positive predictive value). However, irrespective of the various criteria examined, glucagon-provoked increases in plasma catecholamines revealed the presence of the tumor in less than 50% of affected patients. Diagnostic sensitivity was particularly low in patients with pheochromocytomas due to von Hippel-Lindau syndrome. Tumors from these patients showed no significant expression of the glucagon receptor.
Conclusion: The glucagon stimulation test offers insufficient diagnostic sensitivity for reliable exclusion or confirmation of pheochromocytoma. Because of this and the risk of hypertensive complications, the test should be abandoned in routine clinical practice.
The glucagon stimulation test should be abandoned in clinical practice because it offers insufficient diagnostic accuracy for reliable exclusion or confirmation of pheochromocytoma.
Pheochromocytomas and paragangliomas are tumors of chromaffin cells that must be considered in many patients with hypertension as well as others in whom there is suspicion of the tumor due to presence of an adrenal incidentaloma or an underlying hereditary predisposition (1). Because of the significant risks of excess morbidity and mortality, definitive exclusion or confirmation of these tumors is an important concern. Diagnosis in most cases is now relatively easy due to the introduction of new biochemical tests. Measurements of plasma free metanephrines, in particular, offer diagnostic sensitivity of more than 97% (2,3,4,5). Diagnostic specificity is, however, limited. False-positive test results—mainly due to inappropriate sampling conditions, confounding influences of medications, and clinical conditions associated with elevated sympathetic activity—remain a problem (1). Follow-up tests are therefore often required to distinguish true- from false-positive results. The glucagon stimulation test is one potential test for this purpose (6).
Use of the glucagon test to establish a positive result depends on demonstration of a large increment in plasma concentrations of norepinephrine within 3 min of an iv bolus injection of glucagon. Lack of response characterizes negative test results in patients without pheochromocytoma. The test, however, is fraught with numerous methodological problems. In particular, the criteria for establishing positive and negative test results from responses of both norepinephrine and epinephrine have not been adequately defined and tested. For this, the nature of responses of both catecholamines should ideally be established in a reference population, and then tested to establish respective diagnostic sensitivities and specificities in patients with and without the tumor. To date, such an approach has not been used; additionally, the populations of patients studied have been relatively small (7,8,9,10,11). Therefore, it remains unclear how useful the glucagon test is in clinical practice.
In the present study, we examined the responses of both plasma norepinephrine and epinephrine to glucagon in a reference population of healthy volunteers. We then established diagnostic power of the test in groups of patients with confirmed pheochromocytoma compared with patients in whom the tumor was excluded by a combination of negative follow-up and imaging studies.
Subjects and Methods
Subjects
All patients were tested at the National Institutes of Health (NIH, Bethesda, MD), Radboud University Nijmegen Medical Center (Nijmegen, The Netherlands), or the University of Florence (Florence, Italy). A diagnosis of pheochromocytoma was confirmed in 64 patients (30 females, 34 males), aged 36 ± 14 yr (mean ± sd), based on histological examination of surgically resected tumor specimens. This group (tumor-confirmed group) included 26 patients with an apparent sporadic pheochromocytoma, 23 patients with von Hippel-Lindau (VHL) syndrome, 14 patients with multiple endocrine neoplasia type 2 (MEN-2), and one patient with neurofibromatosis type 1.
A group of 38 patients (23 females, 15 males), aged 44 ± 9 yr, with suspected pheochromocytoma in whom the tumor was subsequently excluded, served to establish diagnostic specificity. Pheochromocytoma was excluded in this group (tumor excluded group) by one or more of the following criteria: negative imaging studies; pathological examination of a surgically resected adrenal mass; and normal biochemical test results with lack of the tumor on patient follow-up 2 yr or more after initial testing.
A third group of 36 subjects (19 females, 17 males), aged 41 ± 13 yr, consisting of 31 healthy normotensive volunteers and five patients with primary hypertension served to establish the reference values for plasma catecholamine responses to glucagon (reference group).
Written informed consent was obtained from all patients who were examined at the NIH. In the other centers (Nijmegen, The Netherlands; and Florence, Italy), glucagon tests were carried out in patients under regular clinical care conditions, and the ethical committee approved the use of those data for scientific purposes. The protocol for glucagon tests in healthy subjects (Florence, Italy) was approved by the local ethics committee.
Glucagon provocation testing
Glucagon tests were carried out after an overnight fast. A baseline blood sample was initially taken through a forearm venous cannula after 20 min of supine rest. One milligram of glucagon was then administered iv. A second blood sample was drawn between 2 and 3 min after glucagon administration. In a subset of patients (seven patients with pheochromocytoma, 35 patients without pheochromocytoma, and 14 subjects of the reference group), the time course of plasma norepinephrine and plasma epinephrine responses to glucagon was determined using blood samples collected at 1, 2, 3, and 5 min after glucagon. Blood pressure was monitored throughout testing.
Biochemical assays
Plasma concentrations of catecholamines before and after glucagon were determined using HPLC with electrochemical detection, as described previously (12). The samples of the subjects from Florence were assayed by a radioenzymatic method. Baseline plasma concentrations of free metanephrines were determined using a different HPLC with electrochemical detection procedure (13).
Analysis of glucagon test results
For the subjects in whom multiple blood samples were taken after glucagon, the peak responses of norepinephrine and epinephrine were defined as the highest plasma concentration of each analyte during the first 3 min after glucagon. For all other patients, the peak response was determined from the sample collected between 2 and 3 min after glucagon.
Four criteria were used to define a positive (i.e. abnormal) glucagon test result. Three of the four criteria were based on the glucagon-evoked responses of plasma catecholamines in the reference group: 1) an increase in plasma concentrations of norepinephrine that exceeded the largest absolute increase in the reference group; 2) an increase in plasma norepinephrine and/or epinephrine that exceeded the respective largest absolute increase of analytes in the reference group; and 3) a fold-increase in plasma concentrations of norepinephrine above baseline levels that exceeded the highest fold-increase in the reference group. Additionally, the commonly used definition of a larger than 3-fold increase in plasma concentrations of norepinephrine above baseline levels was examined as the fourth criterion of a positive test result (14).
Sensitivity, specificity, and positive and negative predictive values for the different parameters were calculated as previously described (2).
To establish the utility of the glucagon stimulation test as a follow-up test, diagnostic sensitivity and specificity were also examined in a subgroup of patients who had elevations in plasma normetanephrine and metanephrine that were of insufficient magnitude to unequivocally establish a positive diagnosis (i.e. concentrations above the upper reference intervals, but not high enough to provide 100% positive predictive value—about 4-fold higher than the upper reference intervals). Another subanalysis was carried out in patients with normal baseline plasma concentrations of both norepinephrine and epinephrine. Additionally, separate analyses of diagnostic sensitivity were performed in three subgroups of patients with pheochromocytoma, including those with apparently sporadic tumors and those with tumors associated with MEN-2 and VHL syndrome.
Expression of the glucagon receptor
Tumor specimens from seven patients with MEN-2 and -11 with VHL syndrome were examined for expression of the glucagon receptor by quantitative PCR. In brief, RNA was extracted from frozen samples of tumor tissue and reversibly transcribed to cDNA using random hexamers. Real-time quantitative PCR (TaqMan PCR) with a 7000 Sequence Detector (Applied Biosystems, Foster City, CA) was used for quantification of glucagon receptor mRNA. Glucagon receptor forward primer: 5′-TGC ACT GCA CCC GCA AT-3′; glucagon receptor reverse primer: 5′-GCA CGG AGC TGG CTT TCA-3′; glucagon receptor TaqMan probe: 5′(FAM)-CGC GAA TCT GTT TGC GTC CTT CG-(TAMRA)3′.
Specimens from six patients with MEN-2 and -4 with VHL syndrome were also examined for expression of the glucagon receptor by immunohistochemistry. Primary antibodies for the glucagon receptor (rabbit polyclonal antiglucagon receptor, 1:100) were from Novus Biologicals (Littleton, CO), and those for tyrosine hydroxylase (mouse monoclonal antityrosine hydroxylase, 1:250) were from ImmunoStar, Inc. (Hudson, WI). Complete details on the procedures for quantitative PCR and immunohistochemstry can be obtained from Thanh-Truc Huynh (E-mail: huynht@mail.nih.gov).
Statistical analyses
Baseline data for plasma concentrations of catecholamines and metanephrines are presented as median values and ranges. Where appropriate, data were log-transformed before statistical analysis. Differences between groups were compared by ANOVA with Scheffé’s test. Changes in plasma concentrations of catecholamines after glucagon were examined using paired t tests. A two-tailed P value of <0.05 was considered to be statistically significant.
Results
Baseline plasma concentrations of catecholamines and metanephrines
Baseline plasma concentrations of norepinephrine or epinephrine or both catecholamines were elevated in 49 of the 64 patients with pheochromocytoma, whereas plasma concentrations of normetanephrine or metanephrine or both metabolites were elevated in 63 patients. Patients with pheochromocytoma had 180 to 360% higher (P < 0.0001) median baseline plasma concentrations of norepinephrine than those without pheochromocytoma (Table 1). Median baseline plasma concentrations of epinephrine were 60 to 90% higher in patients with pheochromocytoma than in subjects without the tumor, but this difference only reached significance (P = 0.016) for the comparison with the reference group. The pheochromocytoma-associated percentage elevations in median baseline plasma concentrations of normetanephrine and metanephrine were larger (P < 0.0001) than respective elevations of norepinephrine and epinephrine. Median baseline plasma concentrations of normetanephrine in patients with pheochromocytoma were 620 to 660% higher (P < 0.0001) than in subjects without the tumor (compared with only 180 to 360% for norepinephrine), whereas plasma concentrations of metanephrine were 70 to 190% higher (P < 0.0001) than in subjects without the tumor (compared with 60 to 90% for epinephrine).
Table 1.
Baseline and peak plasma concentrations of catecholamines after glucagon administration in the reference group and groups of patients with confirmed and excluded pheochromocytoma
| URI (nmol/liter) | Baseline values (nmol/liter)
|
Peak values (nmol/liter)
|
|||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Reference group | |||||
| Norepinephrine | 2.90 | 1.17 | 0.58–9.24 | 1.45 | 0.66–8.19 |
| Epinephrine | 0.50 | 0.10 | 0.01–0.52 | 0.28 | 0.01–1.50 |
| Normetanephrine | 0.61 | 0.35 | 0.18–0.50 | ||
| Metanephrine | 0.31 | 0.10 | 0.04–0.21 | ||
| Tumor excluded | |||||
| Norepinephrine | 2.90 | 1.96 | 0.59–6.15 | 2.59 | 0.72–7.50 |
| Epinephrine | 0.50 | 0.12 | 0.01–0.75 | 0.31 | 0.02–6.73 |
| Normetanephrine | 0.61 | 0.37 | 0.09–1.33 | ||
| Metanephrine | 0.31 | 0.17 | 0.03–0.63 | ||
| Tumor confirmed | |||||
| Norepinephrine | 2.90 | 5.40 | 0.57–41.3 | 8.36 | 1.14–120.5 |
| Epinephrine | 0.50 | 0.19 | 0.02–6.9 | 0.61 | 0.03–30.9 |
| Normetanephrine | 0.61 | 2.66 | 0.28–173 | ||
| Metanephrine | 0.31 | 0.29 | 0.04–20 | ||
URI, Upper reference intervals are shown for baseline values of norepinephrine, epinephrine, normetanephrine, and metanephrine.
Effects of glucagon administration
In the subset of patients with pheochromocytoma (n = 7) in whom the time course of plasma catecholamine levels after glucagon could be assessed, peak levels were reached at 2 min (data not shown). At 5 min, plasma levels already had decreased substantially, but not to baseline values.
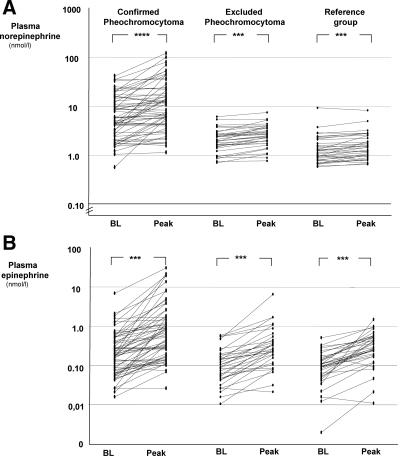
Administration of glucagon resulted in significant (P < 0.0001) increases in plasma concentrations of both norepinephrine and epinephrine in all three groups of subjects (Fig. 1). The increases were not consistent for all subjects in any group. There was considerable overlap in peak plasma concentrations of both norepinephrine and epinephrine among all three groups. The degree of overlap in peak plasma concentrations was larger for epinephrine than for norepinephrine (Fig. 1).
Figure 1.
Line graphs showing plasma concentrations of norepinephrine (A) and epinephrine (B) at baseline (BL) and after glucagon (Peak) in individual patients with and without pheochromocytoma, and in the reference group. ***, P < 0.0001 increase compared with baseline; ****, P < 0.00001.
Increases of plasma concentrations of norepinephrine, expressed in terms of absolute increments, were more pronounced in patients with pheochromocytoma than in the two groups of subjects without pheochromocytoma (Fig. 2). The median increment of plasma norepinephrine concentrations in patients with pheochromocytoma was 1.17 nmol/liter, compared with smaller increments of 0.42 nmol/liter in patients in whom the tumor was excluded (P < 0.0001) and 0.16 nmol/liter in the reference group (P < 0.0001). Nevertheless, there was considerable overlap of the absolute increments in plasma norepinephrine concentrations among the three groups. Similarly, relative increases in plasma norepinephrine (i.e. fold or percentage increases after glucagon relative to baseline values), also showed considerable overlap, such that when expressed in this manner the responses of norepinephrine to glucagon did not differ significantly among the three groups.
Figure 2.
Dot plots showing changes in plasma concentrations of norepinephrine (A and B) and of epinephrine (C and D) in individual patients with and without pheochromocytoma, and in the reference group. Absolute increments (Δ increases) in plasma concentrations of norepinephrine and epinephrine after glucagon (plasma concentrations after glucagon minus baseline plasma concentrations before glucagon) are shown in panels A and C, respectively, whereas relative increments (fold-increases) in plasma norepinephrine and epinephrine levels after glucagon (peak plasma concentrations divided by baseline concentrations) are shown in panels B and D, respectively. *, P < 0.05; ***, P < 0.0001 difference in responses of indicated groups.
The degree of overlap among the three groups of subjects was larger for the responses of plasma epinephrine to glucagon than for the responses of norepinephrine (Fig. 2). Median increments in plasma concentrations of epinephrine were 0.30 nmol/liter in patients with pheochromocytoma, compared with 0.19 nmol/liter in those in whom the tumor was excluded, and 0.17 nmol/liter in the reference group. Absolute increases in plasma epinephrine after glucagon in the patients with pheochromocytoma were larger (P < 0.05) than the increases in the reference group, but not compared with the group in whom the tumor was excluded. The relative increments in plasma epinephrine concentrations after glucagon did not differ significantly among patients with and without pheochromocytoma or subjects in the reference group (Fig. 2).
In two patients with pheochromocytoma, the rise in blood pressure necessitated the administration of phentolamine. One other patient with a history of hypertensive paroxysms, but in whom pheochromocytoma was subsequently excluded, suffered a hypertensive crisis within the first few minutes after glucagon. This was followed by a long-lasting period of impaired vision in one eye, a serious adverse event, but with full recovery after several months.
Diagnostic utility of the glucagon stimulation test
Irrespective of the various criteria used to define a positive test result, the diagnostic sensitivity of the glucagon stimulation test remained less than 50% (Table 2). Values for diagnostic specificity varied between 87 and 100%. Thus, the corresponding positive predictive values ranged between 86 and 100% with negative predictive values of 51% or less.
Table 2.
Sensitivities, specificities, and positive and negative predictive values for different parameters of plasma norepinephrine and epinephrine at a cutoff level as established by the highest value in the reference group
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|
| Δ Plasma norepinephrine (>1.25 nmol/liter) | 48% (31/64) | 92% (35/38) | 91% (31/34) | 51% (35/68) |
| Δ Plasma norepinephrine (>1.25 nmol/liter) and/or Δ plasma epinephrine (>1.46 nmol/liter) | 48% (31/64) | 87% (33/38) | 86% (31/36) | 50% (33/66) |
| Fold-increase over baseline norepinephrine (>2.03 times) | 34% (22/64) | 97% (37/38) | 96% (22/23) | 46% (37/81) |
| Fold-increase over baseline norepinephrine (>3.0 times) | 22% (14/64) | 100% (38/38) | 100% (14/14) | 43% (38/88) |
The highest diagnostic sensitivity of the glucagon test (48%) was defined by increases in plasma concentrations of norepinephrine after glucagon of more than 1.25 nmol/liter (Table 2). The additional consideration of plasma epinephrine responses to glucagon was of no value in increasing diagnostic sensitivity, but instead led to a decrease in diagnostic specificity from 92 to 87%. Larger than 3-fold increases in plasma concentrations of norepinephrine after glucagon yielded the highest diagnostic specificity of 100% with the corresponding maximum positive predictive value; however, this definition of a positive test result was associated with a value for diagnostic sensitivity of only 22%. Diagnostic accuracy associated with the different diagnostic criteria ranged from 51% (more than three times baseline plasma norepinephrine) to 65% (increase in plasma norepinephrine of >1.25 nmol/liter). Because of the near complete overlap in fold-increases in plasma epinephrine among the three groups (Fig. 2), there was no additional value in considering this variable among the criteria for defining a positive glucagon test result.
There were clear differences in responses to glucagon between patients with VHL syndrome and MEN-2. Among these two groups of patients with pheochromocytoma, the glucagon test yielded negative test results according to all four criteria in 20 of the 23 VHL patients (diagnostic sensitivity = 13%) compared with four of the 14 MEN-2 patients (diagnostic sensitivity = 71%).
Diagnostic sensitivity did not improve when the analysis was limited to those 15 patients who had normal baseline plasma concentrations of norepinephrine and epinephrine. Nine of these 15 patients showed normal glucagon test results (i.e. false-negative results) according to all four criteria, resulting in a diagnostic sensitivity of 40%. Excluding the six VHL patients from this subanalysis yielded a diagnostic sensitivity of 66%.
Restricting the analysis to the 35 patients with pheochromocytoma who had elevations of plasma normetanephrine and/or metanephrine that were not large enough to unequivocally confirm the tumor (i.e. concentrations higher than the upper reference limits but less then four times the upper reference limit) also did not result in improved diagnostic sensitivity. Glucagon test results were normal (i.e. false-negative results) in 23 of these 35 patients according to all four predefined criteria, whereas in 12 patients glucagon test results were positive according to at least three criteria. Thus, diagnostic sensitivity in this group was only 34%, but it increased to 63% after excluding the 16 VHL patients.
Expression of the glucagon receptor in tumors from VHL and MEN-2 patients
In the subset of tumors from the seven MEN-2 patients and 11 VHL patients, mRNA expression for the glucagon receptor was substantially higher (P < 0.01) in tumors from MEN-2 patients (median, 1,585; range, 169–10,960 relative units) than from the VHL patients (median, 25; range, 0–2,957 relative units). Representative immunohistochemical stainings for tyrosine hydroxylase and the glucagon receptor in single tumor specimens from one patient with MEN-2 and another with VHL syndrome are shown in Fig. 3. Among the tumors from the six patients with MEN-2 and the four patients with VHL syndrome, all showed intense staining for tyrosine hydroxylase. All tumors from MEN-2 patients also showed a scattered pattern of staining for the glucagon receptor, with 30 to 70% of tyrosine hydroxylase-positive cells also showing staining for the glucagon receptor. In contrast, there was negligible specific staining for the glucagon receptor in tyrosine hydroxylase-positive cells of tumor specimens from the four VHL patients.
Figure 3.
Immunohistochemical staining of tyrosine hydroxylase (TH; panels A and C) and of the glucagon receptor (GR; panels B and D) in representative samples of pheochromocytomas from patients with MEN-2 (A and B) and VHL syndrome (C and D).
Discussion
The results of this study clearly show that the glucagon test used as a follow-up test has insufficient diagnostic sensitivity to reliably exclude pheochromocytoma. Our analysis indicated that irrespective of the criteria used to define a positive test result, the diagnosis would be missed in more than 50% and up to 80% of patients suspected of harboring the tumor. This applies in particular to those patients for whom this test has been proposed to be most useful, namely patients with normal plasma or urinary catecholamines (11,15). Of more relevance to current recommendations that initial diagnostic testing should include measurements of plasma free or urinary fractionated metanephrines (16), we also established that diagnostic sensitivity of the glucagon test remains low when applied to patients with borderline elevations of plasma metanephrines that are of insufficient magnitude to unequivocally confirm pheochromocytoma.
As developed and described by Lawrence in 1965 (6), use of the glucagon test to diagnose pheochromocytoma initially depended on findings of excessive blood pressure responses to glucagon compared with the blood pressure response to a cold pressor test. Use of plasma catecholamine responses as diagnostic end-points for the glucagon test followed improvements in assay methodologies that enabled measurements of catecholamines in plasma (7,15). Several subsequent reports indicated that the glucagon test can be particularly useful for establishing the diagnosis of pheochromocytoma in patients where other biochemical test results produce inconclusive results (10,11,17). The largest of these previously published studies was by Grossman et al. (11), who reported on 31 patients with pheochromocytoma and 72 symptomatic patients in whom the tumor had been excluded. Sensitivity of the test was reported at 81% and specificity at 100%. The high diagnostic specificity indicated potential utility of the test for conclusive confirmation of pheochromocytoma.
Optimism about the glucagon test has, however, been tempered by other reports indicating several shortcomings (17,18,19,20). Notably, there has been disagreement about whether iv glucagon can produce increases in plasma catecholamines in the absence of a pheochromocytoma. Although some investigators have indicated that glucagon is normally without affect on plasma concentrations of norepinephrine and epinephrine (15), others have reported small increases largely limited to epinephrine (9,10,11), and still others reported substantial increases in both norepinephrine and epinephrine (19).
The present study of the glucagon test—to date the largest of its kind and the first to include a reference population—clearly establishes that iv glucagon produces significant increases of both plasma norepinephrine and epinephrine, irrespective of the presence of a pheochromocytoma. In all patients without pheochromocytoma, including subjects of the reference group, the absolute increments of plasma epinephrine were of similar magnitude to those of plasma norepinephrine. More importantly, both the relative (i.e. fold-increases) and absolute increments of plasma epinephrine did not differ among subjects with and without pheochromocytoma. Thus, consideration of glucagon-evoked increases in plasma epinephrine, in addition to norepinephrine, provides no additional utility in improving the diagnostic accuracy of the test beyond consideration of norepinephrine responses alone. In fact, the additional consideration of epinephrine responses leads to decreased diagnostic specificity.
Our findings that relative increments (i.e. fold-increases) in plasma norepinephrine also did not differ between patients with and without pheochromocytoma indicate a further short-coming in the usual use of glucagon-induced fold increases in plasma norepinephrine to define a positive test result. Consequently, use of absolute increments to define a positive test result, as determined by the maximum increment of plasma norepinephrine in our reference population, provided higher diagnostic sensitivity than use of fold-increases.
The present findings of significant responses of norepinephrine and epinephrine to glucagon that were independent of the presence of a pheochromocytoma are consistent with other findings showing that glucagon can evoke catecholamine release from both isolated pheochromocytoma tumor cells and adrenal medullary chromaffin cells (21). This evoked release appears dependent on the presence of the glucagon receptor (22). As we further show here, differences in the expression of the glucagon receptor in pheochromocytomas likely explain the variable catecholamine responses to glucagon and the correspondingly poor diagnostic sensitivity of the glucagon test among patients with pheochromocytoma. More specifically, the notably higher rate of false-negative responses to glucagon in VHL patients (87%) compared with MEN-2 patients (29%) can be explained by the relative lack of expression of the glucagon receptor in tumors from VHL patients.
Although sensitivity of the glucagon test is insufficient for reliable exclusion of pheochromocytoma, specificity of the test is excellent, a finding in agreement with the results of a previous study (11). It might be argued that the high diagnostic specificity of the glucagon test makes it useful as a secondary test to confirm pheochromocytoma in patients in whom initial biochemical tests yield equivocal results. However, it must also be considered that an unequivocal diagnosis of pheochromocytoma using the glucagon test could only be reached in at most one third of patients with the tumor; in all other patients with and without tumors the diagnosis would remain unclear, making the test unacceptable for clinical practice. To be effective, follow-up tests should ideally have both higher diagnostic sensitivity and specificity than the initial screening test. As previously described, the clonidine suppression test, when combined with measurements of normetanephrine, has nearly 100% sensitivity and specificity, making this an excellent follow-up test (23). Nevertheless, even before that particular test is implemented, due consideration should also be given to other simpler to implement biochemical tests that can be performed after excluding possible causes of false-positive test results (24).
Finally, as reviewed elsewhere (25), use of the glucagon test is not without risk. This was illustrated in the present study by severe hypertensive reactions in three patients, including one patient who did not harbor a pheochromocytoma. Importantly, in that latter patient, the hypertensive crisis was associated with a severe adverse event, involving impaired vision in one eye that only slowly resolved over several months. Thus, the risks of the test are not insubstantial and should be balanced against any benefits for diagnosis of suspected pheochromocytomas.
In summary, the glucagon stimulation test offers insufficient diagnostic sensitivity for reliable exclusion of pheochromocytoma or confirmation of disease in all patients with the tumor. Taking this into account, along with advances in other biochemical testing strategies and the risk of hypertensive complications, we conclude that there is no justification for this test in routine clinical practice.
Footnotes
No grants/fellowships were received for this study.
Disclosure Summary: J.W.M.L. and G.E. received royalties for publication of a book on pheochromocytoma (entity <$10,000). The other authors have nothing to declare.
First Published Online November 6, 2009
Abbreviations: MEN-2, Multiple endocrine neoplasia type 2; VHL, von Hippel-Lindau.
References
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K 2005 Pheochromocytoma. Lancet 366:665–675 [DOI] [PubMed] [Google Scholar]
- Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G 2002 Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 287:1427–1434 [DOI] [PubMed] [Google Scholar]
- Hickman PE, Leong M, Chang J, Wilson SR, McWhinney B 2009 Plasma free metanephrines are superior to urine and plasma catecholamines and urine catecholamine metabolites for the investigation of pheochromocytoma. Pathology 41:173–177 [DOI] [PubMed] [Google Scholar]
- Václavík J, Stejskal D, Lacnák B, Lazárová M, Jedelský L, Kadalová L, Janosová M, Frysák Z, Vlcek P 2007 Free plasma metanephrines as a screening test for pheochromocytoma in low-risk patients. J Hypertens 25:1427–1431 [DOI] [PubMed] [Google Scholar]
- Sawka AM, Jaeschke R, Singh RJ, Young Jr WF 2003 A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 88:553–558 [DOI] [PubMed] [Google Scholar]
- Lawrence AM 1967 Glucagon provocative test for pheochromocytoma. Ann Int Med 66:1091–1096 [DOI] [PubMed] [Google Scholar]
- Sheps SG, Maher FT 1968 Histamine and glucagon tests in diagnosis of pheochromocytoma. JAMA 205:895–859 [PubMed] [Google Scholar]
- Bravo EL, Tarazi RC, Gifford RW, Stewart BH 1979 Circulating and urinary catecholamines in pheochromocytoma: diagnostic and pathophysiologic implications. N Engl J Med 301:682–686 [DOI] [PubMed] [Google Scholar]
- Bernini GP, Vivaldi MS, Argenio GF, Moretti A, Sgrò M, Salvetti A 1997 Frequency of pheochromocytoma in adrenal incidentalomas and utility of the glucagon test for the diagnosis. J Endocrinol Invest 20:65–71 [DOI] [PubMed] [Google Scholar]
- Levinson PD, Hamilton BP, Mersey JH, Kowarski AA 1983 Plasma normetanephrine and epinephrine responses to glucagon in patients with suspected pheochromocytomas. Metabolism 32:998–1001 [DOI] [PubMed] [Google Scholar]
- Grossman E, Goldstein DS, Hoffman A, Keiser HR 1991 Glucagon and clonidine testing in the diagnosis of pheochromocytoma. Hypertension 17:733–741 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ 1986 Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem 32:2030–2033 [PubMed] [Google Scholar]
- Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ 1993 Determination of plasma metanephrines by liquid chromatography with electrochemical detection. Clin Chem 39:97–103 [PubMed] [Google Scholar]
- Bravo EL, Tagle R 2003 Pheochromocytoma: state of the art and future prospects. Endocr Rev 24:539–553 [DOI] [PubMed] [Google Scholar]
- Sebel EF, Hull RD, Kleerekoper M, Stokes GS 1974 Responses to glucagon in hypertensive patients with and without pheochromocytoma. Am J Med Sci 267:337–343 [DOI] [PubMed] [Google Scholar]
- Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, Grossman AB, Kimura N, Mannelli M, McNicol AM, Tischler AS 2007 Pheochromocytoma: recommendations for clinical practice from the First International Symposium. Nat Clin Pract Endocrinol Metab 3:92–102 [DOI] [PubMed] [Google Scholar]
- Bernheim C, Jaillon P, Graisely B, Cloarec M, Debray J, Cheymol G 1978 Merits of the assay of plasma catecholamines in a glucagon test to diagnosis of pheochromocytoma (author’s translation). Nouv Presse Med 7:553–555 [PubMed] [Google Scholar]
- Kuchel O, Hamet P, Buu NT, Larochelle P 1981 Basis of false-positive glucagon tests for pheochromocytoma. Clin Pharmacol Ther 29:687–694 [DOI] [PubMed] [Google Scholar]
- McLoughlin MJ, Langer B, Wilson DR 1981 Life-threatening reaction to glucagon in a patient with pheochromocytoma. Radiology 140:841–845 [DOI] [PubMed] [Google Scholar]
- Bisschop PH, Corssmit EP, Baas SJ, Serlie MJ, Endert E, Wiersinga WM, Fliers E 2009 Evaluation of endocrine tests. C: glucagon and clonidine test in phaeochromocytoma. Neth J Med 67:91–95 [PubMed] [Google Scholar]
- Nakai T, Yamada R, Baba M, Komiya E 1980 A reevaluation of the glucagon provocative test for pheochromocytoma–on the in vitro release of catecholamine from the adrenal medulla or pheochromocytoma tissue, and on the effect of intravenous glucagon on urinary catecholamine excretion and blood pressure. Nippon Naibunpi Gakkai Zasshi 56:855–867 [DOI] [PubMed] [Google Scholar]
- Levey GS, Weiss SR, Ruiz E 1975 Characterization of the glucagon receptor in a pheochromocytoma. J Clin Endocrinol Metab 40:720–723 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, Pacak K 2003 Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab 88:2656–2666 [DOI] [PubMed] [Google Scholar]
- Algeciras-Schimnich A, Preissner CM, Young Jr WF, Singh RJ, Grebe SK 2008 Plasma chromogranin A or urine fractionated metanephrines follow-up testing improves the diagnostic accuracy of plasma fractionated metanephrines for pheochromocytoma. J Clin Endocrinol Metab 93:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Rivers G, Rosas AL, Quezado Z, Manger WM, Pacak K 2007 Adverse drug reactions in patients with phaeochromocytoma. Drug Safety 30:1031–1062 [DOI] [PubMed] [Google Scholar]