Abstract
Objective: The aim of the study was to examine serum markers of bone turnover at 6 and 18 months after Roux-en-Y gastric bypass surgery.
Participants: Ten women and 10 men [body mass index (BMI), 50.2 ± 8.4 kg/m2] were studied at 6 months; 10 women and nine men (BMI, 47.2 ± 6.6 kg/m2) were studied at 18 months after surgery.
Main Outcome Measures: Serum osteocalcin, bone specific alkaline phosphatase (BAP), N-telopeptide of type 1 collagen (NTX), PTH, 25-hydroxy vitamin D, and leptin were measured.
Results: BMI was reduced 32.7 ± 6.2% at 6 months after surgery. Serum osteocalcin (6.9 ± 2.4 to 10.9 ± 2.6 ng/ml; P < 0.0001), BAP (14.2 ± 3.7 to 16.4 ± 4.5 ng/ml; P = 0.04), and NTX (10.9 ± 1.7 to 19.6 ± 5.3 nm bone collagen equivalents; P < 0.0001) were increased. Calcium, phosphate, and PTH were unchanged, but 25-hydroxy vitamin D increased (16.0 ± 8.9 vs. 26.9 ± 10.6 ng/ml; P <0.0001). The increase in NTX correlated with reduction in serum leptin (r = 0.58; P = 0.007). BMI was reduced 40.9 ± 7.5% at 18 months after surgery. Serum BAP (17.6 ± 5.3 to 22.2 ± 7.8 ng/ml; P = 0.0017) and NTX (10.8 ± 2.7 to 16.9 ± 5.5 nm bone collagen equivalents; P < 0.0001) were increased. Calcium, phosphate, and PTH were unchanged, but 25-hydroxy vitamin D increased (17.7 ± 7.6 to 25.6 ± 6.8 ng/ml; P < 0.0001). The increase in NTX correlated with reduction in BMI (r = 0.58; P = 0.009) and leptin (r = 0.45; P = 0.04) and the increase in serum 25-hydroxy vitamin D (r = 0.43; P = 0.05). In multiple regression (adjusted model R2 0.263; P = 0.013), reduction in leptin was a significant predictor of increase in NTX (P = 0.016), but changes in BMI and 25-hydroxy vitamin D were not.
Conclusions: Weight loss after bariatric surgery is associated with long-term increase in serum markers of bone turnover. The increase in NTX is related to the decrease in leptin, which may signal caloric restriction to the skeleton.
The increase in N-telopeptide following bariatric surgery is related to the reduction in leptin, a signal of caloric restriction.
Bariatric surgery is the most effective therapy available for severe obesity, achieving substantial and sustained weight loss, significant reductions in comorbid conditions, and prolonged longevity (1,2,3,4). Due to the significantly greater weight loss achieved with bariatric surgery compared with medical intervention, substantially increasing numbers of individuals opt for this treatment (5,6).
Roux-en Y gastric bypass is the most common bariatric operation in the United States. In this procedure, a small gastric pouch is formed, the jejunum is divided at the midsection, and the distal portion of the small intestine is anastomosed to the gastric pouch, thus allowing nutrients to bypass the duodenum and proximal jejunum (7,8,9).
It has been appreciated for more than 50 yr that osteomalacia and osteoporosis are frequent complications of gastric surgery and small bowel malabsorptive disorders. There is now a growing recognition that with increased use of bariatric surgical procedures, and in particular Roux-en-Y gastric bypass, there is an increase in incidence of skeletal disorders in patients treated for obesity. In recent reports significant decreases from presurgery measures in spine (10,11), femoral neck (12,13), and hip (10,11,12,13) bone mineral density have been detected, on average, 1 yr after surgery. These reductions in bone mineral density may not reach the level of clinical significance because of the initial greater bone density in obese subjects (14). However, case reports documenting osteomalacia and osteoporosis at 8 and 12 yr after surgery (15) suggest that the bariatric population is at risk for metabolic bone disease in the long term.
Significant increases in serum markers of bone turnover have been documented in subjects with reduced bone density after bariatric surgery (11,12,13,16). However, these studies have examined markers at short time intervals after surgery, limiting the usefulness of these measures in understanding long-term changes in bone metabolism. Several mechanisms have been proposed to explain effects of bariatric surgery on bone metabolism, including malabsorption of calcium and vitamin D, and reduced mechanical loading of the skeleton. In rodents, the adipose tissue hormone leptin regulates bone remodeling via actions on the central nervous system (17). Thus, changes in bone turnover after bariatric surgery could be induced by the reduction in leptin secretion that results from caloric restriction and weight loss. A role for leptin in regulation of bone metabolism after bariatric surgery has not been addressed in detail.
The aims of this study were to investigate in a sample of men and women undergoing Roux en Y bariatric surgery: 1) serum markers of bone turnover during weight loss (6 months after surgery) and after achieving a body mass index (BMI) close to normal (18 months); 2) serum 25-hydroxy vitamin D (25-OH vitamin D) and PTH to determine whether secondary hyperparathyroidism developed after surgery; and 3) reduction in leptin as a predictor of the change in serum markers of bone turnover.
Patients and Methods
We studied two groups of obese subjects that underwent Roux-en-Y bariatric surgery at St. Vincent Bariatric Center (Carmel, IN). The surgical procedure, performed by three surgeons from the same practice (R.J., B.M.C., C.E.G.), included a 1-ounce gastric pouch, 100-cm Roux, and 50-cm biliopancreatic limb. After surgery, patients were prescribed 1500 mg/d calcium citrate (Citracal; Bayer HealthCare, Morristown, NJ) and 1200 IU/d of vitamin D (three Flintstones complete chewables/day; Bayer HealthCare) and remained on this regimen throughout the study period. All subjects provided informed consent, and the protocol was approved by the Institutional Review Board at Indiana University-Purdue University and at St. Vincent’s Hospital.
Subjects were drawn from a study, begun in January 2005, to evaluate anthropometric and hormonal changes after bariatric surgery. Group 1 included 20 subjects (10 men and 10 women; age, 32.7 ± 7.1 yr; range, 24.0–47.0 yr) evaluated before and approximately 6 months after surgery. Subjects were included in this group if presurgical (obtained January to June, 2005) and postsurgical (obtained at the scheduled 6-month follow-up visit; June to December, 2005) serum samples were available for analysis in December 2005. For the women, the first 10 samples from a total of 46 were included, and for the men the first 10 from a total of 15 samples were used. Postmenopausal women and subjects taking medicines known to affect bone or mineral metabolism (bisphosphonates, estrogen, selective estrogen receptor modulators, corticosteroids) were excluded. Two women in this cohort were African-American; the remaining women and all of the men were European American. Within this group, four subjects were being treated for type 2 diabetes, nine for hypertension, one for hypercholesterolemia, and four for depression. At 6-month follow-up, medicines had been discontinued in two subjects with diabetes, six with hypertension, one with hypercholesterolemia, and four with depression. Doses on medication had been reduced for the remaining two subjects with diabetes, three subjects with hypertension, and two subjects with depression. Subjects in this group are representative of the average subject undergoing bariatric surgery in a private practice.
Group 2 included 19 subjects (nine men and 10 women; age, 46.6 ± 9.0 yr; range, 29.0–60.0 yr) evaluated at baseline and approximately 18 months after bariatric surgery. For inclusion in group 2, the postsurgery BMI had to be less than 35 kg/m2 at approximately 18 months. Subjects were included in this group if presurgical (obtained January to June, 2005) and postsurgical (obtained at the scheduled 18-month follow-up visit; April to November, 2006), serum samples were available for analysis in December 2006. For the women, the first 10 samples from a total of 11 were included, and for the men all nine available samples were used. Within group 2, one woman was African-American; the remaining women and all of the men were European American. Two women were postmenopausal. Seven subjects were being treated for type 2 diabetes, 12 for hypertension, seven for hypercholesterolemia, and six for depression. No subjects were taking medicines known to affect bone or mineral metabolism (bisphosphonates, estrogen, selective estrogen receptor modulators, corticosteroids). At 18-month follow-up, medicines had been discontinued for four subjects with diabetes, 10 with hypertension, six with hypercholesterolemia, and four with depression. Doses on medication had been reduced for the remaining three subjects with diabetes, two with hypertension, and one with hypercholesterolemia. Of the remaining subjects with depression, one was on reduced dose and one on a new treatment.
Body weight was measured to the nearest 0.1 kg using a platform digital scale, and standing height was obtained to the nearest 0.1 cm using a stadiometer. The presurgical serum sample was obtained after an overnight fast on the morning of surgery. The postsurgical sample was obtained after a fast of 4.9 ± 4.1 h (range, 40 min to 12 h) in group 1 and 6.8 ± 4.4 h (range, 15 min to 12 h) in group 2.
Assays
Serum osteocalcin was analyzed by RIA [coefficient of variation (CV), 8.9%; Nichols Institute Diagnostics, San Juan Capistrano, CA]. Serum bone specific alkaline phosphatase (BAP) (CV, 4.1%; Quidel, San Diego, CA) and cross-linked N-telopeptide of type 1 collagen (NTX) (CV, 7.5%; Osteomark; Ostex International, Inc., Seattle, WA) were measured by ELISA. Intact PTH was measured by two-site immunoassay (CV, 9.7%, Nichols Institute Diagnostics), and 25-OH vitamin D was analyzed by protein binding assay (CV, 8.1; DiaSorin, Stillwater, MN). Serum adiponectin was measured by ELISA (CV, 7.4%) and serum leptin by RIA (CV, 3.4%; Linco Inc., St. Charles, MO). Serum calcium, phosphate, creatinine, albumin, total protein, glucose, triglyceride, total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were measured using a Roche COBAS MIRA Clinical Analyzer. Baseline and postsurgery samples for each subject were run in duplicate in the same assay.
Statistical analyses
All variables were expressed as the mean ± sd. A prestudy power analysis was based on Coates et al. (12), who observed a 174 ± 168% increase in urinary NTX at 3 months postsurgery in 15 subjects. Assuming the same effect size and variability in the prestudy power analysis for the current study, a significant difference (P = 0.05) would be detected with 83.2% power in 10 subjects. Combining both genders (20 subjects/group) provided 98.2% power to detect significant differences. To calculate percentage change in BMI after surgery, the percentage change for each individual was calculated, and these values were averaged across the study group. Unpaired Student’s t tests were used to examine sex differences, and paired t tests were used to detect differences before and after weight loss. Given the borderline statistical significance of some of the univariate comparisons in Tables 1 and 2, we considered the effect of an adjustment for multiple comparisons on the interpretation of our results (Bonferroni, new threshold <0.003). Even with this adjustment, the main results and interpretation were unchanged. Thus, no adjustments for type I error were made. Pearson correlation coefficients were used to examine the relationship between change (postsurgical minus presurgical value) in serum markers of bone turnover and BMI, 25-OH vitamin D, leptin, and adiponectin. Multiple regression analysis was carried out to determine the predictors of change in NTX after surgery. Inspection of the data indicated that the decrease in leptin from baseline to 6 months (group 1) was not statistically different from the decrease in leptin from baseline to 18 months (group 2), and that the increase in NTX in both groups (6-month minus baseline or 18-month minus baseline) was not statistically different; therefore the two datasets were combined. Independent variables tested included BMI, leptin, and 25-OH vitamin D. All statistical tests were considered significant at P < 0.05. Analyses were performed using StatView for Macintosh (SAS Institute, Inc., Cary, NC).
Table 1.
Change in metabolic characteristics 6 months after bariatric surgery in group 1
| Baseline | 6 months | P value | |
|---|---|---|---|
| Age (yr) | 32.7 ± 7.1 (24.0–47.0) | ||
| BMI (kg/m2) | 50.2 ± 8.4 (42.3–74.6) | 33.8 ± 1.4 (26–54) | <0.0001 |
| Osteocalcin (ng/ml) | 6.9 ± 2.4 (3.9–11.4) | 10.9 ± 2.6 (5.9–14.6) | <0.0001 |
| BAP (ng/ml) | 14.2 ± 3.7 (7.2–21.7) | 16.4 ± 4.5 (9.2–26.0) | 0.04 |
| NTX (nm BCE) | 10.9 ± 1.7 (7.9–13.6) | 19.6 ± 5.3 (12.3–29.0) | <0.0001 |
| Calcium (mg/dl) | 9.4 ± 0.5 (8.4–10.8) | 9.4 ± 0.4 (8.9–10.7) | 0.65 |
| Phosphate (mg/dl) | 3.6 ± 0.9 (2.5–6.1) | 3.7 ± 0.5 (2.8–5.0) | 0.31 |
| PTH (pg/ml) | 33.6 ± 12.0 (20.6–67.2) | 30.5 ± 7.5 (21.4–52.8) | 0.30 |
| 25-OH vitamin D (ng/ml) | 16.0 ± 8.9 (5.0–38.2) | 26.9 ± 10.6 (5.0–53.8) | <0.0001 |
| Creatinine (mg/dl) | 1.0 ± 0.2 (0.7–1.5) | 0.9 ± 0.1 (0.7–1.2) | 0.03 |
| Albumin (g/dl) | 4.6 ± 0.4 (4.0–5.8) | 4.4 ± 0.3 (4.0–5.6) | 0.13 |
| Total protein (g/dl) | 7.3 ± 0.6 (6.2–8.8) | 7.1 ± 0.5 (6.3–8.8) | 0.16 |
| Glucose (mg/dl) | 101.7 ± 23.2 (75.0–147.0) | 86.2 ± 14.8 (66.0–128.0) | 0.01 |
| Triglyceride (mg/dl) | 176.0 ± 78.3 (48.0–365.0) | 87.5 ± 26.3 (45.0–140.0) | <0.0001 |
| Total cholesterol (mg/dl) | 185.7 ± 37.3 (119.0–250.0) | 152.3 ± 17.0 (122.0–182.0) | 0.0002 |
| LDL cholesterol (mg/dl) | 124.7 ± 35.8 (57.0–180.0) | 95.3 ± 20.2 (61.0–126.0) | 0.0006 |
| HDL cholesterol (mg/dl) | 44.2 ± 10.2 (18.5–64.9) | 43.7 ± 8.3 (29.9–63.5) | 0.74 |
| Leptin (ng/ml) | 60.8 ± 21.3 (18.5–106.9) | 14.3 ± 8.4 (2.3–33.1) | <0.0001 |
| Adiponectin (μg/ml) | 4.8 ± 2.6 (2.0–11.5) | 7.3 ± 2.7 (3.2–12.1) | 0.0002 |
Values are presented as mean ± sd (range). Paired t test comparison at baseline and after weight loss. BCE, Bone collagen equivalents.
Table 2.
Change in metabolic characteristics 18 months after bariatric surgery in group 2
| Baseline | 18 months | P Value | |
|---|---|---|---|
| Age (yr) | 46.6 ± 9.0 (29.0 – 60.0) | ||
| BMI (kg/m2) | 47.2 ± 6.6 (39.5–60.0) | 27.6 ± 3.0 (23–33) | <0.0001 |
| BAP (ng/ml) | 17.6 ± 5.3 (9.9–30.4) | 22.2 ± 7.8 (11.8–41.1) | 0.0017 |
| NTX (nm BCE) | 10.8 ± 2.7 (7.1–18.5) | 16.9 ± 5.5 (10.5–33.7) | <0.0001 |
| Calcium (mg/dl) | 9.8 ± 0.9 (8.3–12.8) | 9.2 ± 0.6 (7.9–10.3) | 0.03 |
| Phosphate (mg/dl) | 3.7 ± 0.8 (2.5–5.7) | 3.9 ± 0.5 (3.0–4.6) | 0.45 |
| PTH (pg/ml)a | 36.3 ± 23.6 (12.3–75.0) | 45.3 ± 16.1 (26.5–77.8) | 0.11 |
| 25-OH vitamin D (ng/ml) | 17.7 ± 7.6 (5.3–36.7) | 25.6 ± 6.8 (13.7–39.5) | <0.0001 |
| Creatinine (mg/dl) | 1.1 ± 0.3 (0.7–1.8) | 1.0 ± 0.2 (0.7–1.6) | 0.10 |
| Albumin (g/dl) | 4.8 ± 0.7 (3.9–7.0) | 4.4 ± 0.4 (3.8–5.1) | 0.05 |
| Total protein (g/dl) | 7.7 ± 1.2 (6.5–11.7) | 7.1 ± 0.8 (5.6–8.7) | 0.04 |
| Glucose (mg/dl) | 92.1 ± 16.3 (63.0–115.0) | 85.7 ± 27.9 (48.0–155.0) | 0.37 |
| Triglyceride (mg/dl) | 187.8 ± 72.3 (78.0–339.0) | 88.8 ± 25.7 (50.0–134.0) | <0.0001 |
| Total cholesterol (mg/dl) | 195.7 ± 53.1 (126.0–320.0) | 160.4 ± 36.8 (120.0–274.0) | 0.0098 |
| LDL cholesterol (mg/dl) | 133.9 ± 50.3 (74.0–260.0) | 92.8 ± 27.0 (63.0–165.0) | 0.0024 |
| HDL cholesterol (mg/dl) | 59.9 ± 14.9 (32.9–87.2) | 70.7 ± 17.7 (45.4–123.4) | 0.0036 |
| Leptin (ng/ml) | 54.9 ± 21.0 (18.8–90.2) | 7.4 ± 6.4 (1.1–28.9) | <0.0001 |
Values are presented as mean ± sd (range). Paired t test comparison at baseline and after weight loss. Osteocalcin and adiponectin not measured. BCE, Bone collagen equivalents.
Values for five women and four men only.
Results
Group 1
At the time of surgery, men had a greater BMI [54.1 ± 10.5 (range, 42.3–74.6) vs. 46.4 ± 2.6 (range, 42.4–50.1) kg/m2; P = 0.04] and were older than the women [38.8 ± 7.3 (range, 26–47) vs. 32.5 ± 5.7 (range, 24–41) yr; P = 0.05]. Women had higher HDL cholesterol [50.9 ± 6.7 (range, 41.5–64.9) vs. 37.5 ± 8.7 (range, 18.5–48.0) mg/dl; P = 0.001]. There were no significant differences between women and men in calcium, phosphate, PTH, osteocalcin, BAP, NTX, creatinine, albumin, and total protein, which were all in the normal range. Serum 25-OH vitamin D was not significantly different between men and women, although three women and three men met the definition of deficiency (<10 ng/ml). Women and men had equivalent fasting glucose, triglyceride, total cholesterol, LDL cholesterol, leptin, and adiponectin.
At 6 months after surgery, there was a 33.1 ± 7.4% and 32.3 ± 5.0% reduction in BMI in the men and women, respectively. There was a significant reduction in serum creatinine, glucose, triglyceride, total cholesterol, LDL cholesterol, and leptin. HDL cholesterol, albumin, and total protein were unchanged. Adiponectin significantly increased (Table 1). There was no significant difference between women and men in the extent of change of these variables (data not shown).
After 6-month weight loss, there was an increase in serum osteocalcin, BAP, and NTX (Table 1), with the largest percentage increase occurring in NTX (82.7 ± 12.6%). There was no change in calcium, phosphate, or PTH, but 25-OH vitamin D was increased (Table 1). Despite vitamin D supplementation, the group on average remained in the insufficiency range with only one man and six women achieving 25-OH vitamin D above 30 ng/ml. One woman with presurgery vitamin D deficiency (<10 ng/ml) remained so at 6 months after surgery.
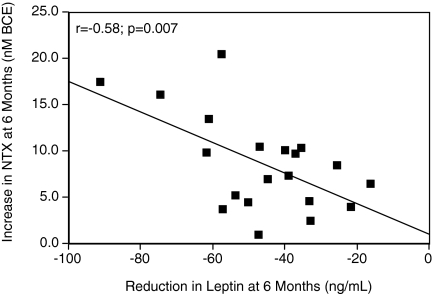
The increase in serum NTX with weight loss was significantly correlated with the reduction in serum leptin (Fig. 1). The increase in NTX was not significantly correlated with change in BMI, 25-OH Vitamin D, or adiponectin (Table 3). The increase in BAP was significantly correlated with the increase in 25-OH vitamin D.
Figure 1.
The increase in serum NTX is significantly correlated with the decrease in leptin at 6 months after surgery. BCE, Bone collagen equivalents.
Table 3.
Pearson correlations (r) for the increase in serum markers of bone turnover at 6 months and change in selected anthropometric and metabolic variables
| Osteocalcin | BAP | NTX | |
|---|---|---|---|
| BMI | −0.21 (0.37) | 0.10 (0.67) | −0.24 (0.32) |
| 25-OH vitamin D | −0.21 (0.37) | −0.44 (0.05) | 0.05 (0.85) |
| Leptin | −0.24 (0.32) | −0.10 (0.69) | −0.58 (0.007) |
| Adiponectin | 0.05 (0.82) | −0.13 (0.58) | 0.08 (0.75) |
Values in parentheses show the significance of the correlation.
Group 2
At the time of surgery, there was no significant difference between the women and men in age or BMI. Calcium, phosphate, PTH, BAP, NTX, creatinine, albumin, and total protein were not significantly different between men and women and were within the normal range. Mean serum 25-OH vitamin D was higher in the women than men [21.3 ± 7.8 (range, 7.6–36.7) vs. 13.6 ± 5.2 (range, 5.3–21.7) ng/ml; P = 0.02], although one woman and two men had deficient levels of serum 25-OH vitamin D (<10 ng/ml). One woman had a serum 25-OH vitamin D level above 30 ng/dl. There was no significant gender difference in glucose, triglycerides, cholesterol, or leptin.
At 18 months after surgery, women had achieved a 41.3 ± 5.4% reduction in BMI, and men had a 40.4 ± 9.7% reduction. Weight loss was associated with a significant reduction in total protein, albumin, triglyceride, total cholesterol, LDL cholesterol, HDL cholesterol, and leptin. Serum glucose and creatinine were unchanged (Table 2. There was no significant difference between women and men in the extent of change of these variables (data not shown).
Serum BAP and NTX increased at 18 months after surgery (Table 2). The 28.8 ± 7.1% increase in BAP and 58.9 ± 9.5% increase in NTX were not significantly different between men and women. There was no change in phosphate or PTH. There was a significant reduction in calcium, total protein, and albumin, but all remained in the normal range (Table 2). Serum 25-OH vitamin D significantly increased but remained below 30 ng/ml. Four women and one man achieved a 25-OH vitamin D above 30 ng/ml.
The increase in serum NTX was significantly correlated with the reduction in serum leptin, the reduction in BMI, and increase in serum 25-OH vitamin D (Table 4). The increase in BAP was not significantly correlated with change in BMI, leptin, or 25-OH vitamin D.
Table 4.
Pearson correlations (r) for the increase in serum markers of bone turnover at 18 months and change in selected anthropometric and metabolic variables
| BAP | NTX | |
|---|---|---|
| BMI | −0.33 (0.16) | −0.58 (0.009) |
| 25-OH vitamin D | 0.38 (0.10) | 0.43 (0.05) |
| Leptin | −0.18 (0.47) | −0.45 (0.04) |
Values in parentheses show the significance of the correlation.
Change in leptin predicts change in NTX
Multiple regression analysis with change in NTX after bariatric surgery as the dependent variable was performed using combined data from both study groups (n = 39). The independent variables included the change in BMI, leptin, and 25-OH vitamin D. The adjusted model R2 for this analysis was 0.263 (P = 0.013). Decrease in leptin was a significant predictor of the increase in serum NTX after surgery (β = −0.435; P = 0.016). Neither the decrease in BMI (β = −0.046; P = 0.79) nor the increase in serum 25-OH vitamin D (β = 0.133; P = 0.39) was a significant predictor of change in NTX.
Discussion
In this study we found that BAP and NTX were significantly increased at both 6 and 18 months after Roux-en-Y bariatric surgery, and to the same extent in both sexes. Using various biochemical markers of bone turnover, previous studies have also found an increase after Roux-en-Y surgery (11,12,13,16,18). However, we are the first to show that at 18 months after surgery, serum NTX and BAP continue to remain increased. Importantly, the BMI at 18 months after surgery was in the normal to overweight range, suggesting that bone remodeling continued at an increased rate, although weight loss had slowed and/or stopped. Thus, it is likely that the increased bone turnover is not due solely to mechanical unloading of the skeleton unless turnover lags well behind the loss of weight. It will be important to establish when markers of bone turnover return to baseline values with the achievement of normal weight. It is unclear from our studies whether the long-term increase in bone turnover represents an adverse effect of surgery with the development of metabolic bone disease, or whether it represents a physiological adjustment by the skeleton to the very large changes in body weight. However, if surgery stimulates bone remodeling that continues despite achievement of normal weight, bone disease could develop in the long term as suggested by some case studies (15).
A common observation after bariatric surgery has been the development of secondary hyperparathyroidism (19), which has been invoked to explain the increase in markers of bone resorption and decrease in bone density. Several studies suggest that hyperparathyroidism secondary to vitamin D insufficiency may be present in 25 to 40% of obese subjects before surgery (19,20,21,22,23,24). Thus, it is not clear whether bariatric surgery increases the prevalence of hyperparathyroidism or only exacerbates the condition. In our study, none of the subjects presented at the time of surgery with hyperparathyroidism (PTH >66 pg/ml) despite the fact that serum 25-OH vitamin D was low for the group, and eight subjects met a criterion of vitamin D deficiency (<10 ng/ml). There was no increase in PTH at 6 or 18 months after surgery. Fleischer et al. (13) recently reported a small but significant increase in serum PTH at 3 months after Roux-en-Y surgery, although the elevated PTH remained in the normal range and returned to presurgery levels at 6 and 12 months. Although we cannot rule out an acute increase in PTH before 6 months after surgery, elevated PTH is not an explanation for the increase in markers of bone turnover observed in our study.
Supplementing subjects in this study with 1200 IU vitamin D after surgery resulted in a significant increase in serum 25-OH vitamin D. Previous studies have found that 800 IU vitamin D does not increase 25-OH vitamin D after Roux-en-Y surgery (13,21), but in agreement with our findings, higher intakes do increase serum 25-OH vitamin D (25). However, supplementation and the resulting elevation in serum 25-OH vitamin D did not prevent the increase in bone turnover observed in our study.
The adipocyte hormones leptin and adiponectin have been studied for effects on bone metabolism. Adiponectin stimulates osteoblast proliferation in vitro (26) and adenoviral overexpression in mice promotes bone formation in vivo (27). Thus, an increase in adiponectin with weight loss might be expected to have a stimulatory effect on bone. Although adiponectin was significantly increased after 6 months of weight loss, the correlations between change in adiponectin and increase in serum markers of bone turnover were not significant.
The relationship between leptin and bone is complex, with both negative and positive correlations reported between the hormone and bone mineral density in humans (recently reviewed in Refs. 28 and 29). In simple regression analysis of our data, the increase in serum NTX was significantly correlated with the decrease in leptin at both 6 and 18 months. We found positive but weak and nonsignificant correlations between reduction in leptin and increase in BAP and osteocalcin, perhaps due to the smaller magnitude of the increase in these markers of bone turnover compared with that for NTX. The reduction in leptin from presurgery levels at 18 months (47.5 ± 22.7 ng/ml) was similar to that observed at the 6-month time point (46.5 ± 18.0 ng/ml), indicating that caloric restriction had a greater effect on serum leptin than weight loss. We hypothesize that the rapid fall in serum leptin postsurgery provided a signal of caloric restriction to the skeleton, resulting in increased bone turnover. As leptin levels continued to be suppressed at 18 months, the signal for increased bone turnover was maintained.
In multiple regression analysis, the reduction in leptin was a significant predictor of the increase in NTX. The reduction in BMI was not significantly correlated with the increase in NTX in subjects at 6 months or in the multiple regression analysis. These data suggest that a leptin signal of caloric restriction may take precedence for the skeleton over the reduction in leptin due to weight change, and that mechanical unloading may have only a small effect on bone metabolism in post-bariatric surgery patients. However, it should be noted that the multiple regression analysis explained only 26% of the variance in serum NTX; thus, factors other than leptin may significantly influence the increase in serum NTX after bariatric surgery.
Karsenty (17) and colleagues have shown that leptin regulates skeletal metabolism via binding to its receptor in the brain and activation of both sympathetic- and cocaine amphetamine regulated transcript (CART)-responsive neurons. Leptin promotes bone resorption by inhibiting osteoblast proliferation and promoting Receptor activator of nuclear factor-kappaB ligand (RANKL) expression via the sympathetic nervous system, whereas it inhibits resorption through activation of CART neurons and inhibition of osteoblast RANKL expression. If the dominant effect of leptin on bone was to promote resorption via sympathetic signaling to osteoblasts, one might expect that bone density would be decreased in obese subjects with high leptin levels compared with bone density in lean individuals with low leptin and that weight loss would promote bone accretion. However, resistance to leptin signaling in the central nervous system develops in obesity, and this resistance can be specific to certain neurons and signaling pathways (30,31). Thus, it is possible that resistance to the central effect of leptin to promote bone resorption develops in obese subjects and that leptin sensitivity is restored when serum leptin falls below a certain threshold, thus allowing leptin to promote bone resorption in postsurgery subjects despite lower levels of the hormone. Alternatively, if leptin inhibition of bone resorption through CART-responsive neurons was predominant in the obese subject, resulting in greater bone density, the reduction in serum leptin after surgery could relieve this inhibition and allow bone resorption to occur.
Recent observations suggest that serotonin synthesized in duodenal enterochromaffin cells regulates bone formation. Yadav et al. (32) found that LDL-receptor related protein 5 (Lrp5) inhibits expression of tryptophan hydroxylase 1, the rate-limiting enzyme in serotonin synthesis. Inactivating mutations in Lrp5, studied because they result in low bone mass, result in overproduction of serotonin by enterochromaffin cells, inhibiting bone formation. Blocking serotonin synthesis in the gut restores a normal bone phenotype in Lrp5 knockout mice. These findings establish that gastrointestinal tract hormones regulate skeletal metabolism. Roux-en-Y surgery significantly changes gastrointestinal tract anatomy, resulting in altered release of incretins and other hormones (33,34). Thus, altered hormone release from the surgically rearranged gut likely also influences skeletal metabolism after bariatric procedures, a possibility for future investigation.
There are a number of strengths to the study. The same subjects were studied before and after bariatric surgery, and both women and men were included. The weight loss was substantial. Several limitations also deserve mention. We did not measure bone mineral density and do not know the extent to which it changed in our subjects. A second limitation is that our longitudinal study included two different cohorts at 6 and 18 months. A future study of serum bone markers and bone density in a larger cohort with extended follow-up will be needed to fully understand the relationship between changes in leptin and bone metabolism. Third, the baseline and follow-up samples were not obtained under identical fasting conditions, which could have influenced measures of glucose and lipid metabolism. Serum markers of bone turnover have been reported (35) to be reduced by prolonging the overnight fast; however, these markers do not exhibit a postprandial increase and were likely unaffected by the length of fast.
In summary, Roux-en-Y bariatric surgery resulted in substantial weight loss and significant improvements in glucose and lipid homeostasis. Surgery-induced weight loss was also associated with a significant long-term increase in serum markers of bone turnover in both men and women, even after they had achieved body weight in the normal/overweight range. The increase in serum NTX was highly correlated with the decrease in leptin. Because studies from others suggest that leptin regulates bone metabolism, our findings here support a role for leptin as a signal to the bone of caloric restriction and body weight.
Acknowledgments
The investigators thank the subjects for their participation in this study. We also thank the staff of St. Vincent’s Bariatric Center, Marta Bennett and Katie McGovern (Carmel Surgical Specialists), and Lauren Bell (Department of Integrative Physiology) for assistance with sample collection.
Footnotes
This work was supported in part by the National Institutes of Health (M01RR000750), and has been presented in abstract form at the 66th and 68th Scientific Sessions of the American Diabetes Association.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 26, 2009
Abbreviations: BAP, Bone specific alkaline phosphatase; BMI, body mass index; CART, cocaine amphetamine regulated transcript; CV, coefficient of variation; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lrp5, LDL-receptor related protein 5; NTX, N-telopeptide of type 1 collagen; 25-OH vitamin D, 25-hydroxy vitamin D.
References
- Brolin RE 1996 Update: NIH consensus conference. Gastrointestinal surgery for severe obesity. Nutrition 12:403–404 [DOI] [PubMed] [Google Scholar]
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K 2004 Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737 [DOI] [PubMed] [Google Scholar]
- Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM, Swedish Obese Subjects Study 2007 Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357:741–752 [DOI] [PubMed] [Google Scholar]
- Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC 2007 Long-term mortality after gastric bypass surgery. N Engl J Med 357:753–761 [DOI] [PubMed] [Google Scholar]
- Davis MM, Slish K, Chao C, Cabana MD 2006 National trends in bariatric surgery, 1996–2002. Arch Surg 141:71–74; discussion 75 [DOI] [PubMed] [Google Scholar]
- Schilling PL, Davis MM, Albanese CT, Dutta S, Morton J 2008 National trends in adolescent bariatric surgical procedures and implications for surgical centers of excellence. J Am Coll Surg 206:1–12 [DOI] [PubMed] [Google Scholar]
- Elder KA, Wolfe BM 2007 Bariatric surgery: a review of procedures and outcomes. Gastroenterology 132:2253–2271 [DOI] [PubMed] [Google Scholar]
- DeMaria EJ 2007 Bariatric surgery for morbid obesity. N Engl J Med 356:2176–2183 [DOI] [PubMed] [Google Scholar]
- Crookes PF 2006 Surgical treatment of morbid obesity. Annu Rev Med 57:243–264 [DOI] [PubMed] [Google Scholar]
- Johnson JM, Maher JW, Samuel I, Heitshusen D, Doherty C, Downs RW 2005 Effects of gastric bypass procedures on bone mineral density, calcium, parathyroid hormone, and vitamin D. J Gastrointest Surg 9:1106–1110; discussion 1110–1111 [DOI] [PubMed] [Google Scholar]
- Carrasco F, Ruz M, Rojas P, Csendes A, Rebolledo A, Codoceo J, Inostroza J, Basfi-Fer K, Papapietro K, Rojas J, Pizarro F, Olivares M 2009 Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg 19:41–46 [DOI] [PubMed] [Google Scholar]
- Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL 2004 Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab 89:1061–1065 [DOI] [PubMed] [Google Scholar]
- Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ 2008 The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab 93:3735–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT, Zhang Y, Hannan MT, Anderson JJ 1993 Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 8:567–573 [DOI] [PubMed] [Google Scholar]
- De Prisco C, Levine SN 2005 Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci 329:57–61 [DOI] [PubMed] [Google Scholar]
- El-Kadre LJ, Rocha PR, de Almeida Tinoco AC, Tinoco RC 2004 Calcium metabolism in pre- and postmenopausal morbidly obese women at baseline and after laparoscopic Roux-en-Y gastric bypass. Obes Surg 14:1062–1066 [DOI] [PubMed] [Google Scholar]
- Karsenty G 2006 Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 4:341–348 [DOI] [PubMed] [Google Scholar]
- von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U 2004 Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism 53:918–921 [DOI] [PubMed] [Google Scholar]
- Compher CW, Badellino KO, Boullata JI 2008 Vitamin D and the bariatric surgical patient: a review. Obes Surg 18:220–224 [DOI] [PubMed] [Google Scholar]
- Andersen T, McNair P, Hyldstrup L, Fogh-Andersen N, Nielsen TT, Astrup A, Transbøl I 1988 Secondary hyperparathyroidism of morbid obesity regresses during weight reduction. Metabolism 37:425–428 [DOI] [PubMed] [Google Scholar]
- Hamoui N, Kim K, Anthone G, Crookes PF 2003 The significance of elevated levels of parathyroid hormone in patients with morbid obesity before and after bariatric surgery. Arch Surg 138:891–897 [DOI] [PubMed] [Google Scholar]
- Hamoui N, Anthone G, Crookes PF 2004 Calcium metabolism in the morbidly obese. Obes Surg 14:9–12 [DOI] [PubMed] [Google Scholar]
- Ybarra J, Sánchez-Hernández J, Pérez A 2007 Hypovitaminosis D and morbid obesity. Nurs Clin North Am 42:19–27, v [DOI] [PubMed] [Google Scholar]
- Goldner WS, Stoner JA, Thompson J, Taylor K, Larson L, Erickson J, McBride C 2008 Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg 18:145–150 [DOI] [PubMed] [Google Scholar]
- Goldner WS, Stoner JA, Lyden E, Thompson J, Taylor K, Larson L, Erickson J, McBride C 2009 Finding the optimal dose of vitamin D following Roux-en-Y gastric bypass: Prospective, Randomized Pilot Clinical Trial. Obes Surg 19:173–179 [DOI] [PubMed] [Google Scholar]
- Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, Wu XP, Liao EY 2005 Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res 309:99–109 [DOI] [PubMed] [Google Scholar]
- Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I 2005 Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 331:520–526 [DOI] [PubMed] [Google Scholar]
- Takeda S 2008 Central control of bone remodelling. J Neuroendocrinol 20:802–807 [DOI] [PubMed] [Google Scholar]
- Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW 2008 Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res 23:17–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JK, Flier JS 2006 Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab 17:365–371 [DOI] [PubMed] [Google Scholar]
- Mark AL, Correia ML, Rahmouni K, Haynes WG 2002 Selective leptin resistance: a new concept in leptin physiology with cardiovascular implications. J Hypertens 20:1245–1250 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G 2008 Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135:825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetner R, McGinty J, Russell C, Pi-Sunyer FX, Laferrère B 2005 Incretins, diabetes, and bariatric surgery: a review. Surg Obes Relat Dis 1:589–597; discussion 597–598 [DOI] [PubMed] [Google Scholar]
- le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR 2006 Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen DB, Alexandersen P, Bjarnason NH, Vilsbøll T, Hartmann B, Henriksen EE, Byrjalsen I, Krarup T, Holst JJ, Christiansen C 2003 Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res 18:2180–2189 [DOI] [PubMed] [Google Scholar]