Abstract
Context: Medullary thyroid carcinoma (MTC) is a cancer of the parafollicular C cells commonly caused by an inherited or acquired RET proto-oncogene mutation. Therapeutic resistance and recurrence of the disease imply the presence of cancer stem cells in MTC.
Objective: In this study, we sought to identify and characterize cancer stem cell-like cells in MTC.
Main Outcome Measures: The characterization of stem cell properties was performed using immunostaining, flow cytometry, sphere formation assay, rederivation assay, Western blotting, and quantitative RT-PCR of defined markers of neural stem and progenitor cells. The role of ret proto-oncogene activation was assessed through RNA interference knockdown.
Results: CD133 positivity was identified by immunostaining patient MTC. Flow cytometry confirmed a subpopulation of CD133+ cells in two MTC cell lines. The CD133+ cells could be expanded by sphere formation assay, passaged multiple times, and expressed neural progenitor markers β-tubulin 3 and glial fibrillary acidic protein. The MZ-CRC-1 cell line, which harbors a M918T RET mutation, had greater CD133+ cell numbers and sphere-forming ability than the TT cell line, which harbors the less active C634W mutation. Sphere formation was more dependent on ret proto-oncogene activity than epidermal growth factor or fibroblast growth factor.
Conclusion: Our data support the existence of cancer stem-like cells in MTC, which exhibit the features of self-renewal and of multiple lineage differentiation that is dependent on ret proto-oncogene receptor activity. These findings may provide new insights to develop more promising therapy for MTC.
Self-renewing tumor cells exist as a subpopulation in medullary thyroid carcinoma and display tyrosine kinase receptor growth dependence.
There is growing evidence that many solid tumors contain a small subpopulation of cells capable of self-renewal and multilineage differentiation, termed cancer stem cells (CSC) (1,2,3). These slowly dividing CSC have also been proven to be resistant to irradiation and cytotoxic drugs, which may explain why tumors recur after conventional therapy even when a majority of the cancer cells have been eliminated (4). Although a universal marker for CSC has not been identified, human CD133, a membrane antigen, has been used in several types of cancers (5).
Medullary thyroid carcinoma (MTC) arises from the parafollicular C cells of the thyroid and accounts for approximately 5% of all thyroid cancer (6). Dominant-activating mutations of the RET tyrosine kinase receptor gene (RET) plays a fundamental role in the development of MTC. When MTC presents with local or distant metastasis, conventional therapy is ineffective, with 5-yr survival rates of about 50% (7). Given the current understanding that CSC may be responsible for resistance to therapy and cancer recurrence, identifying and characterizing the CSC within MTC is critical for development of new treatments.
Materials and Methods
Clinical samples
One normal thyroid (Newcomer Supply, Middleton, WI) and 10 MTC specimens were included. MTC samples were surgically obtained from patients with pathological confirmation of MTC under an approved institutional review board protocol (8).
Cell culture
The human MTC cell line MZ-CRC-1, was obtained from Dr. Massimo Santoro and grown in DMEM, supplemented with 10% fetal bovine serum. The human MTC cell line TT, was obtained from ATCC and maintained in Ham’s F-12K with 10% fetal bovine serum.
Flow cytometry
For CD133 positivity, cells were stained with anti-CD133-PE antibody (mouse monoclonal IgG1, 1:10; Miltenyi Biotec, Auburn, CA) or IgG1 isotype control antibody. For β-tubulin 3 (TUBB3) and glial fibrillary acidic protein (GFAP) positivity, cells were stained with mouse anti-TUBB3 (1:1000; Sigma Chemical Co., St. Louis, MO) or rabbit anti-GFAP (1:1000; Dako, Carpinteria, CA). The secondary antibodies were Alexa Fluor 488 goat antimouse or goat antirabbit (1:500; Molecular Probes, Eugene, OR). Flow cytometry was performed using a FACSCalibur (Becton Dickinson, San Jose, CA). At least 10,000 events were acquired for each sample.
Sphere culturing
Cells were plated at 2 × 103 cells/ml in serum-free DMEM-F12 (1:1), supplemented with 20 ng/ml basic fibroblast growth factor (bFGF; R&D Systems, Minneapolis, MN), 20 ng/ml epidermal growth factor (EGF; R&D Systems), and B-27 serum-free supplement (Invitrogen, Carlsbad, CA) onto 60-mm uncoated tissue culture plates (Cellstar; Greiner BioOne, Frickenhausen, Germany). Fresh medium (2.5 ml) was added every 4 d to replenish growth factors and nutrients. Spheres were passaged when they reached a size of 200–300 cells each. The sphere-formation and rederivation efficiency was evaluated by performing limiting dilution culture as described previously (3).
Immunohistochemistry
Normal thyroid and MTC specimens were evaluated for CD133 positivity. Immunohistochemical staining was carried out on 5-mm sections of formalin-fixed, paraffin-embedded samples. After deparaffinization and rehydration, sections were incubated at 4 C overnight with anti-CD133 rabbit monoclonal antibody (1:100, clone C24B9; Cell Signaling Technology, Danvers, MA), followed by Dako EnVision+System (rabbit; Dako) for 30 min and developed with diaminobenzidine solution. Tumor spheres were fixed with 4% paraformaldehyde, embedded, and serially sectioned (5 μm) on a standard cryostat at −23 C. Blocking was performed in PBS containing 0.3% Triton X-100 and 5% normal goat serum for 1 h, followed by incubation with primary antibodies for 2 h at room temperature. Antibodies used included rabbit anti-CD133 (1:500; Abcam, Cambridge, MA), rabbit anti-GFAP (1:1000; Dako), and mouse anti-TUBB3 (1:1000; Sigma). Secondary antibodies were the same as described for flow cytometry. Sections were mounted using medium containing 4′,6-diamidino-2-phenylindole to identify cell nuclei.
RNA extraction and quantitative RT-PCR (qRT-PCR)
Total RNA was extracted and prepared with RNeasy Mini Kit (QIAGEN, Chatsworth, CA). Gene expression levels were analyzed using a pooled cDNA mixture by either SYBR Green assays or TaqMan assays on StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA). Primers for SYBR Green assays were CD133 forward, 5′-TTGTGGCAAATCACCAGGTA-3′, and reverse, 5′-TCAGATCTGTGAACGCCTTG-3′, and GAPDH forward, 5′-ACTTTGGTATCGTGGAAGGA-3′, and reverse 5′-CTCAGTGTAGCCCAGGATGC-3′. TaqMan assays for TUBB3 and GFAP were purchased from Applied Biosystems. The assays were done in triplicate with a 95% confidence level.
Western analysis
Protein blotting was performed as previously described (9). Primary antibodies included ret proto-oncogene (RET) and phospho-RET (Tyr1062) (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), which were incubated at 4 C overnight. Secondary antibodies used for visualization included: IR680-labeled goat antimouse (1:5000; Invitrogen) or IR680-labeled donkey antigoat and IR800-labeled goat antirabbit (1:5000; Li-Cor Biosciences, Lincoln, NE). All results were scanned using the Odyssey imaging system (Li-Cor Biosciences).
Small interference RNA (siRNA)
RNA knockdown was achieved through transient transfection of double-stranded RNA (dsRNA) specifically targeting RET (Sigma). Control treatments were performed using a randomized dsRNA. Cells at 40–50% confluency were transfected using 100 pmol siRNA and Lipofectamine 2000 (Invitrogen). At 48 h after transfection, the cells were harvested and replated using sphere formation conditions described above.
Results
Identification of a CD133+ population in human MTC specimens and cell lines
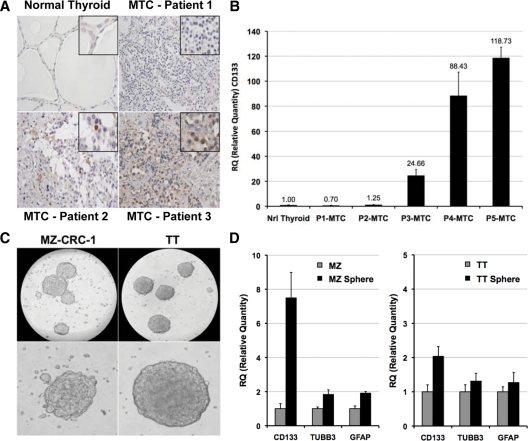
CD133 is a commonly used marker for identifying stem and precursor cell populations (5). Expression of the CD133 antigen was assessed by immunohistochemistry and qRT-PCR in MTC tumors. The proportion of CD133+ cells showed considerable variability among tumors ranging from complete lack of immunoreactivity to expression in single cells or staining of cell clusters (Fig. 1A). A similar result was observed at the mRNA level (Fig. 1B). These findings led us to examine human MTC cell lines for the presence of CD133+ cells with CSC properties. Neural stem cells were originally identified based on their capacity to proliferate and form free-floating spheres (neurospheres) in the serum-free medium with presence of the growth mitogens bFGF and EGF. More recently, the approach has been used to identify neural crest stem cells (the thyroid C-cell progenitor) (10). Therefore, we attempted to enrich for cancer stem-like cells using the neurosphere growth assay. Tumor cell-derived spheres resembling classical neurospheres (tumor spheres) formed within 2 wk for MZ-CRC-1 cells and 3 wk for TT cells (Fig. 1C). qRT-PCR analysis showed a significant increase in CD133 with tumor sphere culturing (Fig. 1D). Neural progenitor cell markers TUBB3 and GFAP showed slight increases.
Figure 1.
Identification of CD133+ cells in MTC. A, Representative immunohistochemical analysis of CD133+ cells in human MTC specimens. Micrographs show the variable immunoreactivity of CD133 in MTC tumors compared with normal thyroid. Insets highlight regions of interest. B, qRT-PCR analysis of CD133 expression in human MTC tumor samples. Values provided are relative to normal human thyroid and represent the relative quantity (RQ) ratio ± RQ maximum of three independent reactions. C, MTC sphere formation in DMEM/F12, supplemented with B27, bFGF, and EGF. Spheres derived from MZ-CRC-1 cells and TT cells are shown in representative micrographs. Scale bar, 0.1 mm. D, qRT-PCR analysis of CD133, TUBB3, and GFAP expression in human MTC cell lines and tumor spheres. Values provided are relative to parental cell lines and represent the RQ ratio ± RQ maximum of three independent reactions.
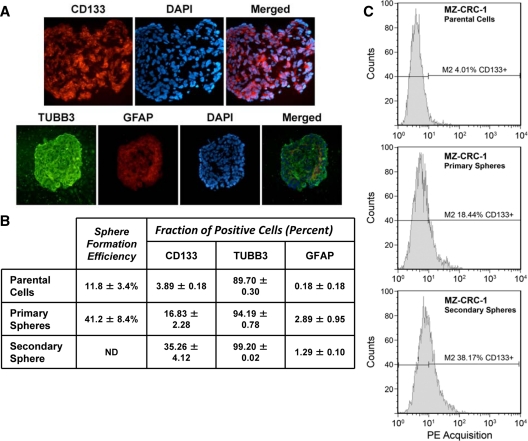
To confirm that MTC parental cells and tumor spheres indeed expressed these markers, we performed immunofluorescent staining and flow cytometry analysis. Immunofluorescent staining showed that CD133+ cells were greatly enriched in the MZ-CRC-1 spheres (Fig. 2A). Surprisingly, a large fraction cells within the spheres were positive for the neural progenitor cell markers TUBB3, whereas few cells were GFAP positive. The presence of both markers implies multiple lineage differentiation. To determine the true fraction of CSC, we performed rederivation assays. When individual spheres were isolated and plated as single cells, we saw a 3- to 4-fold increase in sphere formation compared with parental cells. From single cells, sphere formation was 41.2 ± 8.4% for MZ-CRC-1 cells (Fig. 2B) and 10.3 ± 5.5% for TT cells (not shown). In addition, we determined the fraction of CD133-, TUBB3-, and GFAP-positive MZ-CRC-1 cells by flow cytometry. CD133+ cells increased more than 4-fold in primary spheres and more than 9-fold in secondary spheres compared with parental cells (Fig. 2). TUBB3 and GFAP showed only modest increases indicating that sphere culturing was enriching a specific subpopulation (for representative sorting and qRT-PCR analysis, see supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
Figure 2.
MTC cells form CD133 immunoreactive tumor cell spheres. A, Immunofluorescent staining revealed expression of CD133, TUBB3, and GFAP in MZ-CRC-1-derived tumor spheres. Some cells in the tumor sphere expressed neural progenitor markers TUBB3 and GFAP. TUBB3-positive cells were highly abundant but did not appear to colocalize with GFAP-positive cells. Scale bar, 0.1 mm. B, MZ-CRC-1 tumor sphere formation is associated with an increased fraction of CD133+ cells. The sphere formation efficiency was determined by rederivation assay. Flow cytometry analysis was performed to determine the average fraction of CD133-, TUBB3-, and GFAP-positive cells. Numbers represent the average of three independent experiments ± sd. C, Representative flow cytometry analysis of CD133-bearing cells in MZ-CRC-1 parental cells and tumor spheres is shown in. A total of 10,000 events were counted in each panel. Sphere formation efficiency of secondary spheres was not determined (ND).
Tumor sphere formation was independent of the presence of growth factors and dependent on RET activity
The growth of neural crest-derived stem cells is known to be dependent on RET activity and the addition of exogenous bFGF and EGF (10,11). As part of the transformation process, neural tumor spheres lose their requirement for exogenous bFGF and EGF (12). Therefore, we investigated the role of growth factors and RET activity on MZ-CRC-1 sphere formation. Similar to brain CSC, MZ-CRC-1 cells formed spheres regardless of the presence of exogenous bFGF and/or EGF (supplemental Fig. 2). No significant difference was found in the sphere numbers using four different culture conditions, but the average sphere size was larger in the absence of bFGF and EGF. These findings suggested that intrinsic pathways activated in MZ-CRC-1 contribute to CSC maintenance. For this reason, we examined the activation of oncogenic RET protein. The MZ-CRC-1 cells possess a M918T RET mutation, which results in ligand-independent receptor activation (13). RET activation (determined by the presence of phospho-RET) was unaffected by the presence or absence of bFGF and EGF during sphere culturing (supplemental Fig. 2). To test whether RET activity plays a role in tumor sphere formation, we performed knockdown experiments. Transient treatment of MZ-CRC-1 cells with RET targeting siRNA resulted in a sustained 50% reduction in RET compared with control siRNA-treated cells after 2 d culture in standard growth conditions (Fig. 3A). In parallel cultures propagated using neurosphere growth conditions (bFGF+, EGF+), there was a greater than 50% reduction in the number of tumor spheres formed by 7 d in RET knockdown cells (P = 0.01) (Fig. 3). Inhibition of tumor sphere formation was reproducible using an alternative knockdown approach (supplemental Fig. 3). Interestingly, removal of bFGF and EGF significantly enhanced the reduction of sphere formation after RET knockdown (90% reduction, P < 0.001).
Figure 3.
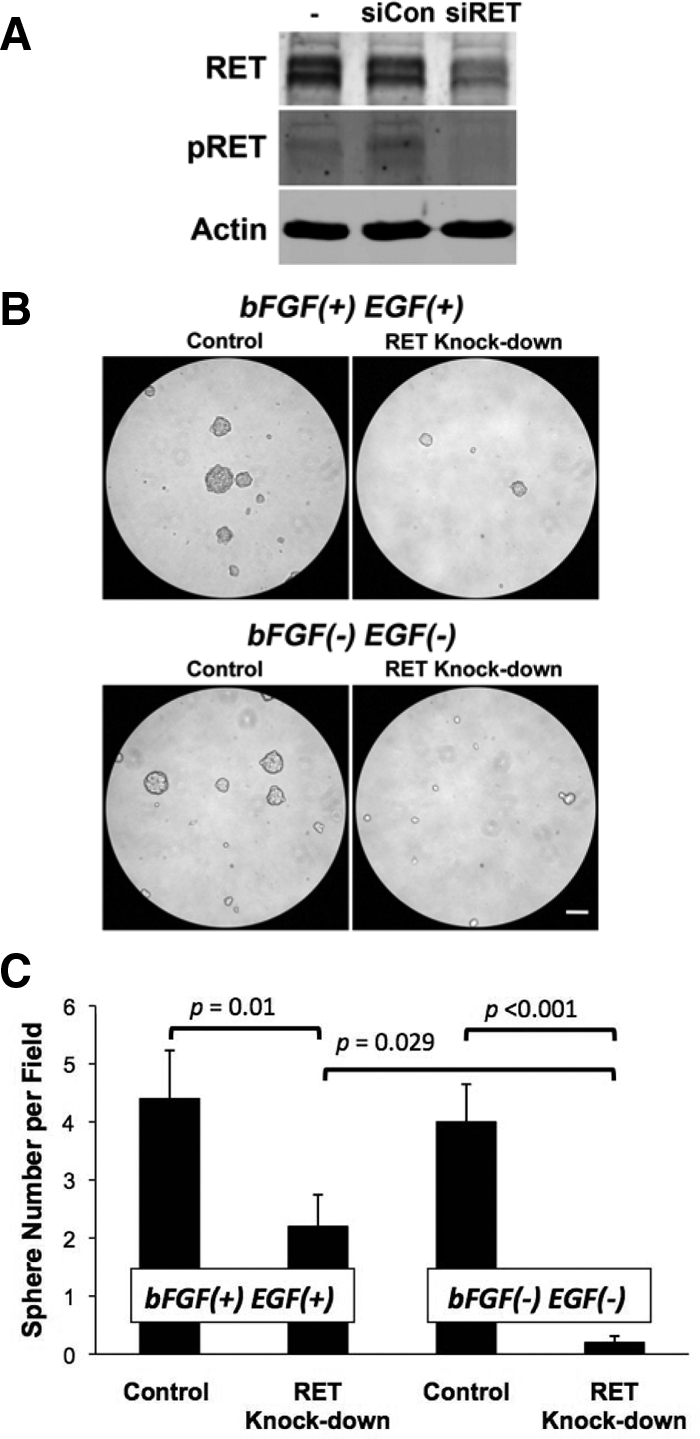
Sphere formation is dependent on RET activity. A, Western blot analysis shows the efficiency of RET knockdown and phospho-RET activity 48 h after transfection with control (siCon) or targeted (siRET) dsRNA. B, Representative micrographs show sphere formation of MZ-CRC-1 with and without RET knockdown. Neurosphere assay was performed after RET targeting with siRNA treatment in the presence or absence of bFGF and EGF as indicated. Scale bar, 0.1 mm. C, Sphere numbers were analyzed 7 d after plating. Values provided represent average determinations in 10 fields under ×10 magnification and are presented as the mean ± sd.
Discussion
Although MTC accounts for only 5–10% of all thyroid cancers, it has a disproportionate incidence of thyroid cancer death. Tumor recurrence after the surgery and the therapy-resistant nature of this disease may imply the existence of CSC in MTC. The discovery of CD133+ staining in patient tumor samples provided the impetus to study two well-characterized MTC cell lines, MZ-CRC-1 and TT, for the presence of cells possessing CSC characteristics. We provide evidence for the first time that cancer stem-like cells are present in these two cell lines, thereby supporting the existence of CSC in MTC tumors. The MTC cancer stem-like cells were CD133+, formed spheres when grown under standard neural stem cell culture conditions, were capable of rederivation, and expressed additional neuronal lineage-specific markers consistent with their neural progenitor cell origin. The use of CD133 epitopes as markers for neural crest-derived stem cells and CSC has been actively investigated (10,14). The proportion of CD133+ cells observed in our studies is consistent with other tumor types (5).
The substantial role played by the tyrosine kinase receptor RET has been well characterized in MTC. Activation of RET triggers a complex network of signal transduction pathways that contribute to cellular transformation (7). A specific role for RET in stem cell function has also been recognized in two developmental systems. RET is highly expressed in gut-derived neural crest stem cells (15). RET signaling has been suggested to control survival, proliferation of precursor cells, migration, and differentiation of neurons in the enteric nervous system (11). RET also plays a critical role in stem cell maintenance required for spermatogenesis (16,17). Loss of any of the components of the RET signaling pathway (GDNF, GFRA1, or RET) results in loss of spermatogonial stem cells (16,17). Our data demonstrated a requirement of RET activity in MZ-CRC-1 cell sphere formation. However, the presence of calcitonin-positive cells in the thyroids of RET knockout mice, albeit at a substantially reduced number, suggests that RET activation is not the only critical event for neural crest stem cell survival (18). Lee et al. (10) have found that neural crest cell sphere formation is highly dependent on extrinsic addition of bFGF and EGF. This may result from the absence of exogenously added glial cell derived neurotrophic factor in their culture conditions, which would be expected to be associated with lower RET activity. Indeed, we observed significantly fewer spheres formed in the absence of RET, bFGF, and EGF. These findings suggest that RET plays a major role in cancer stem-like cell maintenance and that in its absence, the bFGF and EGF pathways are able to partially contribute to CSC maintenance.
Our data demonstrate for the first time the existence of cancer stem-like cells in MTC and define a crucial role for RET, bFGF, and EGF in CSC self-renewal. They imply that in addition to RET, the bFGF and EGF signaling pathways contribute to MTC progression through facilitation of CSC renewal. Several emerging therapies for the treatment of MTC involve the inhibition of tyrosine kinase receptors (19). In vitro studies suggest that the simultaneous targeting of RET and FGF receptor provides greater efficacy than inhibiting either alone (20). Our results suggest that targeting all three receptors affords that best opportunity to reduce CSC populations in patients with MTC.
Supplementary Material
Acknowledgments
We thank the Rolanette and Berdon Lawrence Bone Disease Program of Texas and the Flow Cytometry and Cellular Imaging Core for use of their facilities.
Footnotes
This work was supported in part by National Cancer Institute Grant CA67946, The John K. Funk Endowment, and the Saranne and J. Livingston Kosberg Foundation, with additional core grant support provided by National Cancer Institute Grant CA16672.
Disclosure Summary: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
First Published Online November 6, 2009
Abbreviations: bFGF, Basic fibroblast growth factor; CSC, cancer stem cell; dsRNA, double-stranded RNA; EGF, epidermal growth factor; GFAP, glial fibrillary acidic protein; MTC, medullary thyroid carcinoma; qRT-PCR, quantitative RT-PCR; RET, ret proto-oncogene; siRNA, small interference RNA; TUBB3, β-tubulin 3.
References
- Dick JE 2003 Breast cancer stem cells revealed. Proc Natl Acad Sci USA 100:3547–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passegue E, Jamieson CH, Ailles LE, Weissman IL 2003 Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci USA 100(Suppl 1):11842–11849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB 2003 Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828 [PubMed] [Google Scholar]
- Dietrich J, Imitola J, Kesari S 2008 Mechanisms of disease: the role of stem cells in the biology and treatment of gliomas. Nat Clin Pract Oncol 5:393–404 [DOI] [PubMed] [Google Scholar]
- Wu Y, Wu PY 2 May 2009 CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev 10.1089/scd.2008.0338 [DOI] [PubMed] [Google Scholar]
- Schlumberger M, Carlomagno F, Baudin E, Bidart JM, Santoro M 2008 New therapeutic approaches to treat medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab 4:22–32 [DOI] [PubMed] [Google Scholar]
- Messina M, Robinson BG 2007 Technology insight: gene therapy and its potential role in the treatment of medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab 3:290–301 [DOI] [PubMed] [Google Scholar]
- Wohllk N, Cote GJ, Bugalho MM, Ordonez N, Evans DB, Goepfert H, Khorana S, Schultz P, Richards CS, Gagel RF 1996 Relevance of RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab 81:3740–3745 [DOI] [PubMed] [Google Scholar]
- Bruno IG, Jin W, Cote GJ 2004 Correction of aberrant FGFR1 alternative RNA splicing through targeting of intronic regulatory elements. Hum Mol Genet 13:2409–2420 [DOI] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L 2007 Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol 25:1468–1475 [DOI] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V 2007 Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci 8:466–479 [DOI] [PubMed] [Google Scholar]
- Hoelzinger DB, Demuth T, Berens ME 2007 Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst 99:1583–1593 [DOI] [PubMed] [Google Scholar]
- Santoro M, Carlomagno F, Romano A, Bottaro DP, Dathan NA, Grieco M, Fusco A, Vecchio G, Matoskova B, Kraus MH, Di Fiore PP 1995 Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science 267:381–383 [DOI] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R 2008 Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 15:504–514 [DOI] [PubMed] [Google Scholar]
- Iwashita T, Kruger GM, Pardal R, Kiel MJ, Morrison SJ 2003 Hirschsprung disease is linked to defects in neural crest stem cell function. Science 301:972–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H 2000 Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287:1489–1493 [DOI] [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J 2006 Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod 74:314–321 [DOI] [PubMed] [Google Scholar]
- Lindahl M, Timmusk T, Rossi J, Saarma M, Airaksinen MS 2000 Expression and alternative splicing of mouse Gfra4 suggest roles in endocrine cell development. Mol Cell Neurosci 15:522–533 [DOI] [PubMed] [Google Scholar]
- Santarpia L, Ye L, Gagel RF 2009 Beyond RET: potential therapeutic approaches for advanced and metastatic medullary thyroid carcinoma. J Intern Med 266:99–113 [DOI] [PubMed] [Google Scholar]
- Ezzat S, Huang P, Dackiw A, Asa SL 2005 Dual inhibition of RET and FGFR4 restrains medullary thyroid cancer cell growth. Clin Cancer Res 11:1336–1341 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.