Abstract
Context: Little is known about the role of testosterone and estradiol on cognition in healthy older men.
Objective: The cognitive effects of increasing or lowering testosterone or estradiol were examined.
Design: Cognition was assessed before and after 6 wk of double-blind placebo-controlled hormone modification.
Setting: The study was conducted at an academic medical center.
Participants: Healthy older (ages 60–80 yr) and younger men (ages 25–35 yr) were recruited from the community.
Intervention: Men were randomized to one of four treatments: 1) maintain testosterone and estradiol at eugonadal levels for young men (GnRH agonist + testosterone gel); 2) block testosterone’s conversion to estradiol (GnRH agonist + testosterone gel + aromatase inhibitor); 3) induce hypogonadism (GnRH agonist alone); and 4) all placebo.
Main Outcome Measures: Measures of executive function, memory, and spatial cognition were obtained before and after treatment. Hormone levels were obtained 10 times over the course of the study.
Results: Counter to expectations, hormone treatment did not affect cognition (P > 0.10). Free testosterone was positively related to spatial cognition in older men after treatment and controlling for age and estradiol level or exclusion of the hypogonadal men (P = 0.02). Estradiol was negatively associated with working memory controlling for the same variables (P = 0.01). Blinding to treatment assignment was maintained, with the exception of the hypogonadal group.
Conclusions: A significant change in sex hormone status, including complete hypogonadism, does not modify cognition in men. These findings, along with studies that show a risk for neurodegenerative disease in those with low testosterone, suggest that sex hormone status may be important for neuroprotection in aging but not modulation of normal day-to-day cognitive function.
Contrary to expectations, radical loss of either testosterone or estradiol did not affect cognition, nor did a moderate increase in testosterone improve cognition in older men.
There is intense interest in whether the decline in testosterone in older men results in functional impairments in multiple organ systems including the brain (1). Even the effects of complete hypogonadism on mental abilities and brain health are unknown, and their impact on the aging brain is equally a mystery. This is despite the fact that testosterone loss in aging is common. On average, men in their 70s have 40% less testosterone than men in their 20s. Approximately 70% of men older than 70 have bioavailable testosterone levels less than 70 ng/dl, a level rarely found in younger men (2).
The potential consequences of hypogonadism in aging are serious. Epidemiological studies suggest that lower testosterone predisposes elderly men to Alzheimer’s disease (3,4), and bioavailable testosterone is protective against amnestic mild cognitive impairment, considered a precursor to Alzheimer’s disease (5). Such findings would be expected from neurobiological studies showing that androgens can regulate a β-amyloid (6), a neuropathological indicator of Alzheimer’s disease, and that castration increases β-amyloid accumulation in the brains of a mouse model of Alzheimer’s disease (7).
Studies of associations between native testosterone levels and cognition in older men have produced mixed results. For instance, higher bioavailable testosterone is related to better cognitive performance, particularly on memory measures (8), although a recent study suggested that higher bioavailable testosterone was associated with poorer memory performance (9) potentially by negatively affecting the speed of cognitive processing (10). There are a number of other contrasting examples that suggest any relation between endogenous testosterone and cognitive performance is dependent on the range of testosterone in the sample, the form of cognition examined, the age of the men, and potentially other health and demographic factors (11,12).
However, associations between native testosterone levels and cognitive status or risk for neurodegenerative disease are not informative as to whether testosterone can modulate cognition in older men. That is, do significant changes in testosterone (raising or lowering levels) affect the brain and cognition? A recent large, double-blind study found no improvements on cognition after 6 months of testosterone supplementation in healthy older men (13). This study was notable for including multiple measures that would assess specific cognitive domains above and beyond dementia screening. However, the study has generated significant controversy, in part because supplementation did not maintain higher testosterone levels throughout the study, and the men did not have particularly low testosterone at the initiation of the study (14,15). Some smaller studies suggest that cognitive functions negatively affected by aging, such as working and long-term memory or spatial cognition, are improved by testosterone supplementation in older men (16), at least those with initially low testosterone levels (17,18,19), but others find no consistent effects (20,21). Pharmacological induction of superphysiological testosterone levels in older men negatively impacts memory (22,23).
Our understanding of testosterone’s effects has been hampered by lack of control of hormone levels in the studies of older men. Therefore, we report the results of a blinded study in which we induced hypogonadism in healthy older men, normalized testosterone levels to those of younger men, or blocked testosterone’s conversion to estradiol and examined cognition. Unlike previous studies, our treatment regimen controlled for changes in endogenous production induced by supplementation of testosterone. In addition, a sample of younger men provided a “gold standard” for cognitive function. Thus, the current study assessed the cognitive effects of 6 wk of hypogonadism, higher testosterone, or lowered estradiol in men.
Subjects and Methods
Participants
Healthy younger (n = 26; 25–35 yr) and older (n = 62; 60–80 yr) men were recruited through direct mailing and advertisements. Participants provided health histories over the phone and in person. Inclusion criteria required that men understand English, have adequate hearing and vision to complete computer and paper-pencil tasks (with correction if necessary), and have no sign of dementia (Mini Mental State Exam score >25; Ref. 24) or depression (Geriatric Depression Scale <10; Ref. 25). Men completed the Wechsler Adult Intelligence Scale-Revised (WAIS-R) vocabulary test as a measure of functional intelligence (inclusion criterion of age-scaled score >8; Ref. 26). The older and younger men in this study were matched for years of education and WAIS-R scores (Table 1). Exclusion criteria included current medications affecting sex hormones or cognition (i.e. testosterone gel, antidepressants, etc.), tobacco or excessive alcohol use (more than three drinks per day), or a history of neurological disorders. Older men were also required to have a prostate specific antigen level of less than 4 ng/ml and a normal manual prostate exam. All men were in good health, with total testosterone and estradiol blood levels within 2 sd values of the mean for men of their age (example ranges for men: total testosterone, 241–827 ng/dl; free testosterone, 52–280 pg/ml) (27,28). Not included in the description of the participants or data analysis were two older men who were removed based on pharmacy dispensing errors and one older man who could not complete the posttreatment testing session due to personal issues.
Table 1.
Participant characteristics
| n | Age | Education | WAIS-R | MMSE | GDS | |
|---|---|---|---|---|---|---|
| Older men | ||||||
| 1. GnRH agonist, T-gel | 15 | 67.47 (5.50) | 16.60 (3.14) | 11.80 (1.90) | 28.47 (.99) | 1.53 (2.36) |
| 2. GnRH agonist, T-gel, Arimidex | 17 | 66.82 (4.48) | 15.82 (3.11) | 11.88 (2.55) | 28.35 (1.00) | 3.06 (3.05) |
| 3. GnRH agonist, placebo gel | 15 | 69.40 (6.14) | 14.33 (2.74) | 11.73 (2.37) | 28.47 (1.41) | 2.27 (2.40) |
| 4. Placebo | 15 | 67.33 (5.41) | 16.07 (1.83) | 12.13 (2.67) | 28.40 (.91) | 2.07 (2.58) |
| Young men | ||||||
| 1. GnRH agonist, T-gel | 8 | 28.63 (3.82) | 17.13 (2.17) | 11.25 (1.04) | ||
| 2. GnRH agonist, T-gel, Arimidex | 5 | 27.80 (2.59) | 17.80 (2.68) | 11.60 (2.30) | ||
| 3. GnRH agonist, placebo gel | 8 | 29.50 (3.74) | 16.63 (2.00) | 11.00 (2.20) | ||
| 4. Placebo | 5 | 30.40 (2.70) | 15.00 (2.00) | 10.40 (1.82) | ||
The protocol was approved by the Oregon Health & Science University Institutional Review Board, and all participants gave a written informed consent. Participants were paid $325 for their involvement in the study.
Experimental design
Men were randomized in a double-blind manner into one of four treatment groups that resulted in: group 1, eugonadal testosterone (i.e. testosterone levels equal to endogenous levels in young men); group 2, eugonadal testosterone but low estradiol; group 3, hypogonadism; and group 4, no change (placebo).
The treatments were used to examine direct effects on cognition of increasing testosterone in older men to levels similar in younger men, testosterone effects vs. testosterone’s conversion to estradiol, and the effects of hypogonadism. A GnRH agonist (Depot-Lupron, 7.5 mg; TAP Pharmaceuticals, Chicago, IL) was used to suppress gonadal function and stop natural production of testosterone in groups 1, 2, and 3. Lupron was administered im in two injections 4 wk apart to maintain cessation of endogenous testosterone production. Therefore, testosterone replacement was under the condition of no endogenous production. Testosterone was replaced with an initial daily dose of transdermal testosterone (T-gel, 75 mg for the older men and 100 mg for the younger men; Auxilium Pharmaceutical, Inc., Malvern, PA) in groups 1 and 2. This was initiated approximately 10 d after the first GnRH agonist injection so as not to augment the “flair” of testosterone caused by the GnRH agonist. Men in group 2 received a daily dose of the aromatase inhibitor anastrazole (Arimidex, 1 mg; AstraZenca Pharmaceuticals, Wilmington, DE), with the intent to reduce estradiol while maintaining normal testosterone levels. Men in group 3 received placebo T-gel (Auxilium Pharmaceutical) and thus were hypogonadal throughout the study. Group 4 received all placebo medications and served as a control group with unaltered hormone levels.
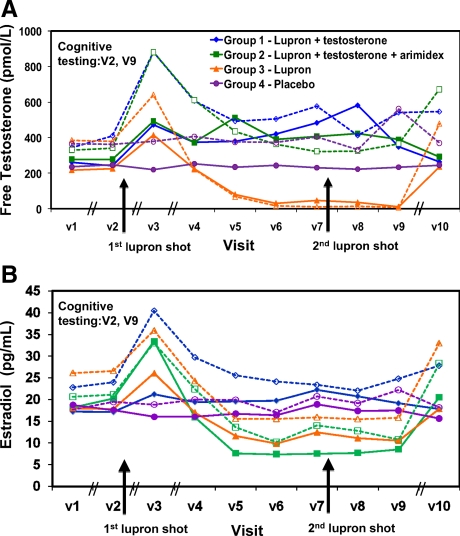
Men visited the laboratory 10 times during the course of the study. The first visit (V1) was to obtain screening information, and it preceded the next study visit by an average of 21 d. Men performed baseline cognitive measures before treatment (V2; see Fig. 2) and then initiated medications. The response to medication was monitored in a midweek study visit (V3), followed by weekly visits thereafter (V4–V9). The study staff was unaware of participant’s treatment assignment or hormone levels during the study. The unblinded study physician (M.H.S.), who was not involved in the day-to-day study procedures, monitored health status and possible adverse events and also monitored testosterone levels in groups 1 and 2 to initiate testosterone and alter T-gel doses if necessary. The doses were adjusted to obtain a target total testosterone level in all men in the normal range of 400–600 ng/dl permitting a comparison of young and old with equivalent levels. Dose adjustment was rare; two older men and two younger men had dose adjustments (one from each age from groups 1 and 2). Cognitive testing was repeated after 6 wk of treatment (V9; see Fig. 2), after which participants stopped all medications. A posttreatment blood draw verified that hormone and prostate specific antigen levels were back to pretreatment levels (V10). Older men who received active T-gel (groups 1 and 2) received another prostate exam to ensure that there were no changes in the prostate during the course of treatment.
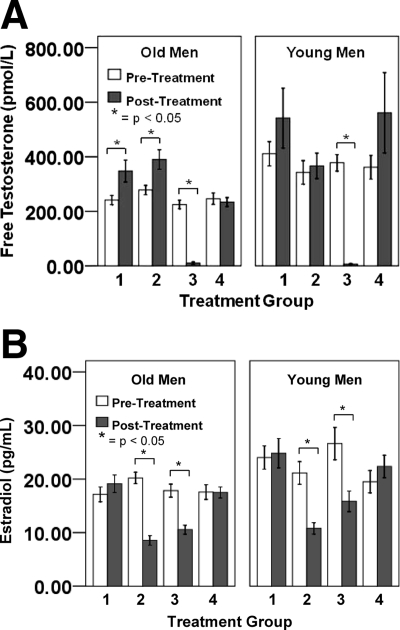
Figure 2.
Pre- and posttreatment free testosterone (A) and estradiol (B) levels for each group at each age. Error bars are sem.
Hormone assays
Blood samples were stored at −70 C at the Oregon Clinical and Translational Research Institute core laboratory for batched analysis to prevent the effects of interassay variability in participants’ hormone levels. Serum estradiol [Diagnostic Systems Laboratory, Webster, TX; sensitivity threshold, 2.2 pg/ml; mean interassay coefficient of variation (CV), 10.9%] and total testosterone (Siemens Healthcare Diagnostics, Deerfield, IL; sensitivity threshold, 4.0 ng/dl; mean interassay CV, 7.4%) levels were quantified using standard RIA procedures. Free testosterone levels were calculated using total testosterone levels and the SHBG levels (29). SHBG levels were quantified using a chemiluminescent enzyme assay (Siemens Healthcare Diagnostics; sensitivity threshold, 0.2 nmol/liter; mean interassay CV, 5.5%).
Cognitive measures
The measures chosen were those that decline particularly in aging and have shown sex differences and/or sex steroid effects in previous studies. The brain systems used in each cognitive domain have been established in previous studies. Thus, effects on cognitive performance before and after hormone treatment on these measures will also index testosterone effects on specific brain systems.
Working memory tasks
Working memory is the ability to hold information in mind and flexibly update it. It indexes the function of the prefrontal cortex (30) and is compromised in aging (31). Performance on the two tasks used here has been associated with testosterone levels in elderly men (8,17).
Trail-making task (Trails) (32,33).
The participant connects 25 randomly ordered, numbered dots in numerical order as quickly as possible (Trails A) and then connects dots alternating between letters and numbers (i.e. 1-A-2-B; Trails B). The difference in performance between these subtests (Trails B-A) represents cognitive flexibility and other components of working memory, adjusting for sensorimotor speed. Lower values indicate better (faster) performance.
Subject-ordered pointing (34).
On this task, the participant chooses drawings on a computer screen and uses working memory to choose a new design with each subsequent response. Participants complete each set containing eight and 10 drawings three times. The total number of errors across all trials was the outcome measure; higher scores indicate poorer performance.
Verbal fluency (35)
Verbal fluency relies on multiple cognitive processes including the ability to select and generate words from a range of possible responses. It requires an intact inferior prefrontal cortex (36). Participants verbally generate as many words as they can in 1 min starting with a given letter of the alphabet (e.g. F, A, S). Proper names, numbers, and words repeated with different endings are not permitted. The total number of valid words generated was the measure of interest.
Spatial cognition
Spatial cognition is composed of cognitive processes that permit assessment of where an object is in space as well as the objects’ perceptual features. Specific parietal cortex regions mediate mental rotation and figure discrimination. Mental rotation is sensitive to hormone status (37) and declines with aging (38). We contrast these tasks to isolate specific cognitive processes and examine the specificity of hormone effects.
In the Mental Rotation Task.
(Based on Ref. 39), participants view 112 pairs of two-dimensional shapes on a computer screen and indicate whether the pair would be a match if one shape was rotated in the plane of the screen (same), or if the shape has been mirror-reversed (different). The number of correct responses was the measure of interest.
In the Figure Discrimination Task.
Participants view 84 pairs of shapes similar to those used for mental rotation and indicate whether shapes are the same (matched) or different (unmatched). In unmatched pairs, a small detail differs in one shape. The total number of errors was the measure of interest. Two participants were excluded from analysis on this measure due to a computer malfunction.
Verbal memory
The Paragraph Recall Test from the Wechsler Memory Scale-Revised (40) was used to assess memory, which requires an intact hippocampus and temporal cortex (41). Verbal memory declines with aging (42), and memory is impaired on this measure in men with long-term complete hypogonadism (43). Participants recall the contents of paragraphs that have been read to them, both immediately and after a 30-min retention interval. The total number of elements recalled was the measure of interest. One participant was excluded from the analysis due to experimenter error in collecting recall data.
Motor functioning and measures of emotion were also assessed as part of this study. The effects on motor function (44) and emotion and mood (in preparation) are reported elsewhere.
Data analyses
To determine differences between groups before vs. after hormone treatment (or placebo), a repeated measures ANOVA was conducted with pre-/posttreatment as the repeated measure and age (older and younger men) and treatment group (group 1, 2, 3, and 4) as the between-subject measures. Bonferroni corrections were based on the number of post hoc comparisons needed to disambiguate main effects and interactions in each ANOVA.
To assess the direct relationships between estradiol, free testosterone, and cognitive performance, a multiple regression analysis was used in the older men only. In the presence of significant hormone effects, age at contact was added into the model as a predictor variable to assess whether the relationship was actually due to participant’s age rather than the co-occurrence of age effects and hormone levels. All statistical analyses were conducted with a two-tailed α level of 0.05.
Results
Hormone concentrations
Hormone manipulation resulted in expected changes in free testosterone and estradiol levels, as per the study design [see time course in Fig. 1, pre-/posttreatment levels in Fig. 2 and Table 2; also see Supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org) for hormone levels obtained at each study visit]. The first Lupron injection caused the expected “flair” in testosterone and estradiol levels in groups 1, 2, and 3 (Fig. 1, A and B), which was followed by a significant decrease in testosterone levels for both young and older men and resulted in hypogonadal levels for group 3. Subsequently in older men, free testosterone was significantly increased in both the testosterone replacement groups (groups 1 and 2) compared with the placebo group (group 4), resulting in no difference in free testosterone levels between older and younger men after treatment and levels within the normal range. Estradiol decreased for groups 2 and 3, resulting in lower estradiol than the other groups after treatment (Fig. 2B). There was no change in hormone levels for men in the placebo group (group 4) or young men in the testosterone-supplemented groups, showing that the testosterone suppression and replacement in the young men maintained their native hormone levels. Therefore, the hormone manipulations were successful in altering hormone levels as intended.
Figure 1.
Time course of average free testosterone (A) and estradiol (B) levels. Older men are shown in solid lines and younger men in dotted lines. Black arrows depict the timing of the lupron shots. Cognitive testing occurred on the second (V2, pretreatment) and ninth (V9 posttreatment) study visits. The intervals among study visits varied based on the goal of the study visit, and the irregular intervals are delineated by slash marks on the x-axis. V1–V2 was the time between screening and the first study and averaged 20.7 d (sd 14.9), V3 was 4.0 (sd 1.1.) d later to monitor the effects of medication, V4 completed the first study week (4.2 d; 1.9 sd from V3) and was followed by weekly study visits to obtain hormone levels and monitor health thereafter (V5, 6, 7, 8, and 9). This was followed by a safety visit (V10) to ensure that testosterone levels had returned to prestudy, normal values, and the V10 visit was repeated if normal levels were not obtained. The time from V9 to V10 visit that yielded normal levels was 51.1 d (sd 18.6).
Table 2.
Hormone levels
| Treatment group | n | Free testosterone (pmol/liter)
|
Estradiol (pg/ml)
|
||
|---|---|---|---|---|---|
| V2, pre-Tx | V9, post-Tx | V2, pre-Tx | V9, post-Tx | ||
| Old | |||||
| 1. GnRH agonist, T-gel | 15 | 241.4ac (65.5) | 347.6a (155.2) | 17.1 (5.3) | 19.1a (6.4) |
| 2. GnRH agonist, T-gel, Arimidex | 17 | 278.1ac (70.8) | 390.1a (147.1) | 20.2b (4.4) | 8.6b (3.7) |
| 3. GnRH agonist, placebo gel | 15 | 224.9bb (60.4) | 10.2b (18.2) | 17.8b (4.7) | 10.6b (3.2) |
| 4. Placebo | 15 | 246.4a (80.9) | 233.9c (63.6) | 17.6 (5.3) | 17.5a (4.0) |
| Effect of treatment group within visit | P = 0.19 | P < 0.001 | P = 0.29 | P < 0.001 | |
| Young | |||||
| 1. GnRH agonist, T-gel | 8 | 411.0 (125.8) | 541.9a (310.2) | 24.0 (6.2) | 24.8a (7.8) |
| 2. GnRH agonist, T-gel, Arimidex | 5 | 342.8 (96.2) | 366.7a (104.3) | 21.1a (4.8) | 10.8b (2.4) |
| 3. GnRH agonist, placebo gel | 8 | 377.9b (85.7) | 6.6b (6.0) | 26.6b (8.5) | 15.8b,c (5.4) |
| 4. Placebo | 5 | 361.7 (97.4) | 561.3a (329.1) | 19.5 (4.6) | 22.3a,c (4.7) |
| Effect of treatment group within visit | P = 0.69 | P < 0.001 | P = 0.26 | P = 0.001 | |
Data represent mean (sd). T-gel, Testosterone gel; pre-Tx, pretreatment; post-Tx, posttreatment. Different letter subscripts indicate significant, between-group post hoc differences within age group at P < 0.05. All subscripts reflect uncorrected post hoc P values.
P < 0.05 for comparisons within age groups where pre-Tx > post-Tx.
P < 0.001 for comparisons within age groups where pre-Tx > post-Tx.
P < 0.05 for comparisons within age groups where pre-Tx < post-Tx.
Cognitive measures
There was a significant main effect of age, with young men performing better than old on all cognitive measures (Fs > 7.96; P < 0.01; Table 3). In addition, significant main effects for time (pre- < posttreatment) were found for the spatial cognitive tasks (Figure Discrimination and Mental Rotation) and the tasks of verbal memory (immediate and delayed Paragraph Recall), indicating a practice effect on those tasks (Fs > 9.07; ps < 0.01). Treatment group was not related to cognition, and there were no interactions between treatment group and age or time (Fs < 1.77, ps > 0.10 for main effect of treatment for all measures; Fs < 2.54, P > 0.10 for all interactions). Nor did hormone status affect cognition when the analyses compared men with testosterone (groups 1, 2, and 4 combined) vs. hypogonadal men (group 3).
Table 3.
Cognitive performance
| Treatment group | n | Trails A and B-Aa,c (sec)
|
Subject-ordered Pointinga,c (total errors)
|
Verbal Fluencya (total words)
|
|||
|---|---|---|---|---|---|---|---|
| V2, pre-Tx | V9, post-Tx | V2, pre-Tx | V9, post-Tx | V2, pre-Tx | V9, post-Tx | ||
| Old | |||||||
| 1. GnRH agonist, T-gel | 15 | A 38.0a (9.7), B-A 42.2 (20.8) | A 34.1a (9.5), B-A 51.3 (23.3) | 9.47 (3.6) | 9.60 (4.8) | 12.0 (2.1) | 11.4 (2.5) |
| 2. GnRH agonist, T-gel, Arimidex | 17 | A 31.2b (8.8), B-A 43.2 (17.7) | A 27.9b (8.2), B-A 39.2 (19.0) | 9.71 (3.1) | 10.1 (3.9) | 13.3 (3.9) | 12.4 (3.7) |
| 3. GnRH agonist, placebo gel | 15 | A 30.8b (8.7), B-A 54.8 (21.4) | A 30.6a,b (8.8), B-A 50.8 (28.2) | 8.86 (3.9) | 8.36 (2.5) | 12.9 (4.7) | 12.4 (4.1) |
| 4. Placebo | 15 | A 34.2a,b (11.3), B-A 45.3 (16.1) | A 32.5a,b (6.5), B-A 53.0 (23.0) | 9.67 (3.6) | 11.3 (6.3) | 11.7 (3.0) | 11.7 (3.0) |
| Effect of treatment group within visit | A P = 0.15, B-A P = 0.26 | A P = 0.19, B-A P = 0.32 | P = 0.91 | P = 0.38 | P = 0.56 | P = 0.78 | |
| Young | |||||||
| 1. GnRH agonist, T-gel | 8 | A 24.2 (4.7), B-A 29.5 (20.0) | A 19.7 (1.3), B-A 39.5a (25.5) | 4.75 (2.7) | 4.25 (2.8) | 17.0 (4.9) | 16.7 (5.5) |
| 2. GnRH agonist, T-gel, Arimidex | 5 | A 20.2 (8.0), B-A 24.1 (5.0) | A 17.8 (5.2), B-A 17.7b (3.0) | 5.00 (2.1) | 4.60 (3.0) | 14.4 (4.0) | 13.9 (4.7) |
| 3. GnRH agonist, placebo gel | 8 | A 21.1 (5.1), B-A 35.0 (17.2) | A 19.8 (6.3), B-A 30.8a,b (9.8) | 3.88 (2.8) | 4.50 (3.8) | 14.3 (4.6) | 16.7 (4.2) |
| 4. Placebo | 5 | A 21.3 (4.9), B-A 38.1 (14.9) | A 19.2 (4.7), B-A 38.4a,b (19.5) | 5.80 (3.6) | 4.80 (3.0) | 15.2 (4.2) | 15.8 (4.4) |
| Effect of treatment group within visit | A P = 0.58, B-A P = 0.52 | A P = 0.91, B-A P = 0.17 | P = 0.69 | P = 0.99 | P = 0.59 | P = 0.61 | |
| Paragraph Recalla,b (total score; 50 possible)
|
Mental Rotationa,b (no. correct; 112 possible)
|
Figure Discriminationa,b,c (no. errors; 84 possible)
|
|||||
|---|---|---|---|---|---|---|---|
| V2, pre-Tx | V9, post-Tx | V2, pre-Tx | V9, post-Tx | V2, pre-Tx | V9, post-Tx | ||
| Old | |||||||
| 1. GnRH agonist, T-gel | 15 | Imm 23.2 (7.0), Delay 19.0 (8.8) | Imm 26.4 (7.1), Delay 24.1 (6.3) | 75.2a (10.8) | 78.0a (11.6) | 10.1a (3.2) | 7.47 (3.3) |
| 2. GnRH agonist, T-gel, Arimidex | 17 | Imm 25.5 (5.1), Delay 20.9 (4.7) | Imm 26.2 (6.3), Delay 23.8 (6.8) | 84.2b (6.3) | 86.5b (4.9) | 7.06b,c (2.5) | 5.71 (4.0) |
| 3. GnRH agonist, placebo gel | 15 | Imm 26.6 (6.8), Delay 21.9 (6.7) | Imm 29.5 (4.8), Delay 25.9 (4.5) | 76.4a (13.9) | 78.9a (13.5) | 9.87a,b (6.3) | 8.40 (6.7) |
| 4. Placebo | 15 | Imm 25.6 (5.1), Delay 19.9 (4.2) | Imm 29.0 (5.6), Delay 26.0 (6.3) | 80.3a,b (8.4) | 81.3a,b (9.4) | 6.80c (3.4) | 5.80 (4.6) |
| Effect of treatment group within visit | Imm P = 0.49, Delay P = 0.64 | Imm P = 0.29, Delay P = 0.63 | P = 0.06 | P = 0.09 | P = 0.04 | P = 0.33 | |
| Young | |||||||
| 1. GnRH agonist, T-gel | 8 | Imm 29.4 (5.4), Delay 24.1 (4.1) | Imm 31.5 (3.9), Delay 31.4 (3.3) | 91.3 (2.5) | 93.9 (3.0) | 3.00 (.93) | 2.00 (1.3) |
| 2. GnRH agonist, T-gel, Arimidex | 5 | Imm 28.0 (8.5), Delay 23.0 (7.6) | Imm 34.6 (4.7), Delay 31.4 (3.8) | 88.2 (4.5) | 94.6 (2.3) | 4.00 (4.5) | 3.80 (5.2) |
| 3. GnRH agonist, placebo gel | 8 | Imm 29.8 (7.5), Delay 26.3 (8.9) | Imm 33.9 (5.5), Delay 30.6 (6.7) | 88.6 (5.0) | 91.1 (4.6) | 2.86 (2.7) | 2.43 (1.7) |
| 4. Placebo | 5 | Imm 25.8 (8.5), Delay 20.2 (7.6) | Imm 29.0 (8.7), Delay 26.6 (8.3) | 85.4 (11.0) | 89.8 (7.4) | 4.00 (1.4) | 2.00 (2.3) |
| Effect of treatment group within visit | Imm P = 0.79, Delay P = 0.53 | Imm P = 0.38, Delay P = 0.48 | P = 0.42 | P = 0.27 | P = 0.79 | P = 0.68 | |
Values represent means (sd). Imm, Immediate recall; T-gel, testosterone gel; pre-Tx, pretreatment; post-Tx, posttreatment. Different letter subscripts indicate significant, between-group differences within age group at P < 0.05. All subscripts reflect uncorrected post hoc P values. See Subjects and Methods for description of missing data for each cognitive task; numbers may vary.
Young men performed significantly better than old at P < 0.01.
Men in both age groups performed significantly better posttreatment compared to pretreatment at P < 0.01.
Lower score reflects better performance.
Correlations of hormone levels and cognition
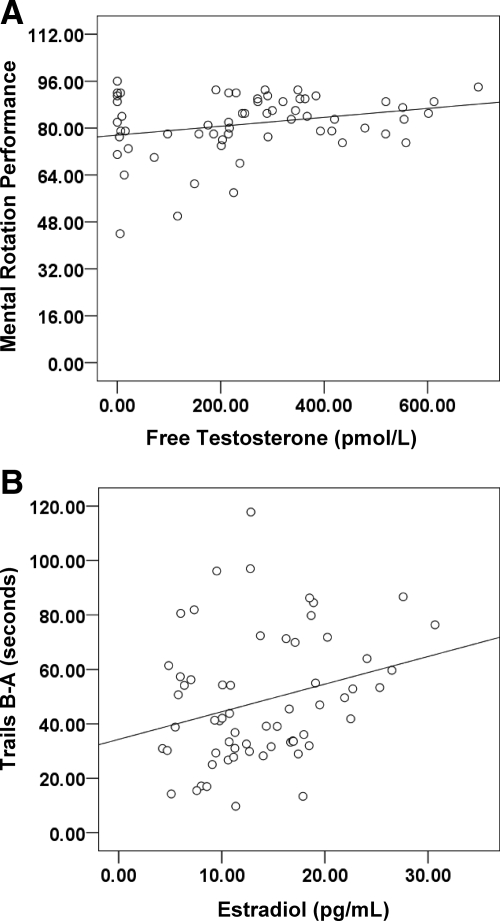
Although we had distinct treatment groups, the range of hormone levels among treatment groups overlapped. This overlap may obscure hormone effects in the group analysis. Thus, we used correlational analyses to assess the relation between hormone levels and cognitive performance. We calculated area under the curve (AUC) to examine total hormone exposure over the course of treatment (V2–V9). AUC was highly correlated with hormone level at the posttreatment test session (r = 0.76; P < 0.001), and in general the same significant relationships between performance and hormone level were found when AUC or posttreatment hormone level was used as the outcome measure. Therefore, hormone level on the day of cognitive testing was used in this correlational analysis of the older men. Testosterone was not related to cognition pretreatment, but higher estradiol was related to better Trails A and Figure Discrimination performance (r > 0.20; ps = 0.05). Higher free testosterone was positively related to Mental Rotation performance (r = 0.27; P = 0.04; Fig. 3A), and higher estradiol was associated with worse performance on Trails B-A after treatment (r = 0.27; P = 0.03; Fig. 3B). These correlations remained significant when the hypogonadal men (group 3) were omitted (Mental Rotation, r = 0.34, P = 0.02; Trails B-A, r = 0.36, P = 0.01), indicating that the correlation was not due to restricted range in hormone levels within the hypogonadal group. No other significant relationships were found.
Figure 3.
A, Higher free testosterone is associated with better mental rotation performance in older men (P < 0.05). B, Lower estradiol is associated with better cognitive flexibility (Trails B-A) performance in older men (P < 0.05). Lower score denotes better (faster) performance.
Hormone levels remained a significant predictor of performance on these tasks when we controlled for age within older men (mental rotation, r2 = 0.04, P = 0.05; Trails B-A, r2 = 0.07, P = 0.02) in a multiple regression analysis. Multiple regression also showed that free testosterone predicted mental rotation performance when controlling for estradiol (r2 = 0.08; P = 0.02), and estradiol predicted Trails B-A performance when controlling for free testosterone (r2 = 0.05; P = 0.02) after treatment. These significant correlations were not found at the pretreatment testing session. Thus, relations between free testosterone and spatial cognition, and estradiol and working memory, exist in older men, after hormone manipulation only. No significant correlations were observed in any of the remaining tasks after treatment or among young men.
Discussion
To our knowledge, this is the first study to examine cognition in men whose testosterone levels were pharmacologically controlled. Contrary to expectations, radical loss of either testosterone or estradiol did not affect cognition, nor did a moderate increase in testosterone (to midnormal levels of young men) improve cognition in older men. However, across the entire sample of older men, higher testosterone was associated with better mental rotation performance, and higher estradiol was associated with worse cognitive flexibility as indexed by Trails B-A performance. With regard to the latter finding, this is our third independent finding that suggests that higher estradiol in older men is associated with worse cognitive flexibility (17,43).
Although some studies find improvements in memory and spatial cognition in healthy older men after a few weeks of supplementation (16), others do not (20,21). Our study is clearly consistent with the latter set of findings. We do not have an obvious explanation for the differences among studies of healthy men. One possibility is that the studies showing improvement included men with initially lower testosterone levels and supplementation corrected their chronically low levels. Other possible explanations are the age of the men studied, the method of testosterone supplementation (injection vs. gel), or dose. Finally, it may also be that supplementing the native levels of testosterone in men induces a series of compensatory endocrine responses that could not occur in our situation where endogenous regulation of testosterone was blocked by the GnRH agonist.
These data add to an emerging picture of the role of testosterone on the brain and cognition in adulthood. Prior studies have categorized sex steroid effects as organizational when they exert developmental effects on the brain and cognitive development, resulting in permanent sex differences. Sex steroid effects are considered activational when they act transiently to maintain, initiate, or modulate a cognitive or neurophysiological process. Finally, sex steroid effects are neuroprotective if they reduce age-related cognitive decline and neurodegeneration. The lack of effects in this study, particularly in men made hypogonadal or with very low estradiol, suggest that neither testosterone nor estradiol is required to activationally maintain cognition in either young or elderly men. Nor does raising testosterone improve performance in the elderly. The lack of group differences suggests that any modulatory effects of testosterone or estradiol on the brain and cognition in men are subtle at best. However, the positive relation found between testosterone and mental rotation and the negative relation found between estradiol and cognitive flexibility only in the elderly and only after controlled treatment suggest that these hormones can modify cognition in the situation where it has become fragile, such as in aging. Both mental rotation and all aspects of working memory decline significantly even in the healthy elderly, and metabolic and atrophic changes in the brain regions mediating these cognitive processes have been well documented.
Particularly surprising was the lack of treatment effects or associations between verbal memory performance and hormone levels. Verbal memory also declines with aging but was not affected by significant changes in either testosterone or estradiol. Memory loss is a harbinger of neurodegenerative disease (particularly Alzheimer’s disease) much more so than either loss of spatial cognition or working memory, and it has a different brain basis than either of these other cognitive domains. However, long-term testosterone deprivation in men leads to a significant verbal memory loss (43), and both human and animal models suggest that this is a potential risk factor for age-related neurodegenerative disease (4,7). This suggests that testosterone has a neuroprotective role against neurodegenerative disease, at least for the brain system critical for memory. We suggest that the biological mechanisms are likely to be different for activational effects on cognition made fragile by aging, vs. neuroprotection from neurodegenerative disease. This model of sex steroid actions with potentially different underlying mechanisms and the data from this study where sex steroid levels were pharmacologically induced and controlled may help explain the disparate results among both clinical trials of testosterone effects and observational epidemiological studies. This model also suggests specific target cognitive functions and situations where novel treatments based on specific sex steroid neurobiological actions might be useful in aging.
Several limitations are worth noting. First, relatively small samples of younger men were studied. This was in part due to the difficulty of recruitment when 6 wk of complete hypogonadism was a potential outcome. However, estimates from the data obtained suggested that even doubling the sample size would be unlikely to result in significant treatment effects. Thus, in a cognitively high performing and intact younger brain, neither testosterone nor estradiol is necessary to maintain cognitive performance. Blinding was maintained, except for the groups with induced hypogonadism who were often aware of their treatment due to hot flashes or loss of sexual function. If anything, we might have expected a lack of blinding to increase group differences; however, no group differences were found. Sex steroid levels varied among and within subjects despite the pharmacological control used in this study. This could mask some subtle effects but would not explain the lack of effects of complete hypogonadism. However, these findings reinforce the difficulty clinical trials face when trying to find effects of increasing or decreasing sex steroids. Finally, it could be that cognitive effects would only be found with very long-term increases in testosterone or in the situation of correcting testosterone levels in men with chronically low testosterone, similar to our suggestion that long-term deprivation would be required to induce memory failures. However, it isn’t clear what neurobiological mechanism would take significant amounts of time other than one that acts on neurodegenerative processes. Certainly in prior studies of activational effects, such as variations in cognition across the menstrual cycle in younger women, changes occur over a few weeks—a much shorter period than the change in hormones induced in this study.
In summary, neither induction of hypogonadism in healthy young and older men nor increasing testosterone in older men to the level of younger men modifies cognition. The methods used here and the outcomes may help guide future clinical studies of sex steroid-derived cognitive enhancers in aging.
Supplementary Material
Acknowledgments
We thank Auxilium Pharmaceutical for initially providing the testosterone gel and for providing all of the placebo gel for this study. We thank Dr. Erin Leblanc for her help as a medical monitor for some aspects of this study.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant R01 AG18843 and Department of Defense Grant PC073093 (to J.S.J.) and the Oregon Clinical and Translational Research Institute (Grant NCRR UL1 RR024140), a component of the NIH Roadmap for Medical Research.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 30, 2009
Abbreviations: AUC, Area under the curve; CV, coefficient of variation; V, visit; WAIS-R, Wechsler Adult Intelligence Scale-Revised.
References
- Liverman CT, Blazer DG 2004 Testosterone and aging: clinical research directions. Washington, DC: National Academies Press [PubMed] [Google Scholar]
- Hijazi RA, Cunningham GR 2005 Andropause: is androgen replacement therapy indicated for the aging male? Ann Rev Med 56:117–137 [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM 2002 Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab 87:5001–5007 [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM 2004 Free testosterone and risk for Alzheimer disease in older men. Neurology 62:188–193 [DOI] [PubMed] [Google Scholar]
- Chu LW, Tam S, Lee PW, Wong RL, Yik PY, Tsui W, Song Y, Cheung BM, Morley JE, Lam KS 2008 Bioavailable testosterone is associated with a reduced risk of amnestic mild cognitive impairment in older men. Clin Endocrinol (Oxf) 68:589–598 [DOI] [PubMed] [Google Scholar]
- Yao M, Nguyen TV, Rosario ER, Ramsden M, Pike CJ 2008 Androgens regulate neprilysin expression: role in reducing β-amyloid levels. J Neurochem 105:2477–2488 [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ 2006 Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer’s disease. J Neurosci 26:13384–13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D, Patay B 1999 Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab 84:3681–3685 [DOI] [PubMed] [Google Scholar]
- Martin DM, Wittert G, Burns NR, Haren MT, Sugarman R 2007 Testosterone and cognitive function in ageing men: data from the Florey Adelaide Male Ageing Study (FAMAS). Maturitas 57:182–194 [DOI] [PubMed] [Google Scholar]
- Martin DM, Wittert G, Burns NR, McPherson J 2008 Endogenous testosterone levels, mental rotation performance, and constituent abilities in middle-to-older aged men. Horm Behav 53:431–441 [DOI] [PubMed] [Google Scholar]
- Tan RS, Fletcher JR, Carrejo MH 2007 Concerning paradoxical association of higher endogenous testosterone and poorer cognitive functioning in elderly men. Maturitas 58:325–326 [DOI] [PubMed] [Google Scholar]
- Beauchet O 2006 Testosterone and cognitive function: current clinical evidence of a relationship. Eur J Endocrinol 155:773–781 [DOI] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT 2008 Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 299:39–52 [DOI] [PubMed] [Google Scholar]
- Morgentaler A 2008 Re: effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. Eur Urol 53:1084 [DOI] [PubMed] [Google Scholar]
- Page S, Matsumoto AM 2008 Effects of testosterone therapy in older men. JAMA 299:1900–1901 [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E, Raskind MA, Brodkin K, Bremner W, Petrova A, LaTendresse S, Craft S 2001 Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 57:80–88 [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Orwoll E 2000 Sex steroids modify working memory. J Cogn Neurosci 12:407–414 [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES 1994 Testosterone influences spatial cognition in older men. Behav Neurosci 108:325–332 [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Craft S, Matsumoto AH 2003 Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: a preliminary report. J Androl 24:568–576 [DOI] [PubMed] [Google Scholar]
- Vaughan C, Goldstein FC, Tenover JL 2007 Exogenous testosterone alone or with finasteride does not improve measurements of cognition in healthy older men with low serum testosterone. J Androl 28:875–882 [DOI] [PubMed] [Google Scholar]
- Gray PB, Singh AB, Woodhouse LJ, Storer TW, Casaburi R, Dzekov J, Dzekov C, Sinha-Hikim I, Bhasin S 2005 Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. J Clin Endocrinol Metab 90:3838–3846 [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Johnson M, Craft S, Peskind ER, Raskind MA 2007 Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology 32:72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Ernst M, London ED, Mordecai KL, Perschler P, Durso SC, Brandt J, Dobs A, Resnick SM 2007 Intramuscular testosterone treatment in elderly men: evidence of memory decline and altered brain function. J Clin Endocrinol Metab 92:4107–4114 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR 1975 Mini-mental state: a practical method for grading the cognitive status of patients for the clinician. J Psychiat Res 12:189–198 [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO 1982 Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiat Res 17:37–49 [DOI] [PubMed] [Google Scholar]
- Wechsler D 1981 Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: Psychological Corp., Harcourt Brace Jovanovich [Google Scholar]
- Bagatell CJ, Heiman JR, Rivier JE, Bremner WJ 1994 Effects of endogenous testosterone and estradiol on sexual behavior in normal young men. J Clin Endocrinol Metab 78:711–716 [DOI] [PubMed] [Google Scholar]
- Mohr BA, Guay AT, O'Donnell AB, McKinlay JB 2005 Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 62:64–73 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P, Friedman H 1991 The circuitry of working memory revealed by anatomy and metabolic imaging. In: Levin H, Eisenberg H, Benton A, eds. Frontal lobe function and dysfunction. New York: Oxford University Press [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS 2002 Greater orbital prefrontal volume selectively predicts worse working memory performance in older adults. Cereb Cortex 12:494–505 [DOI] [PubMed] [Google Scholar]
- 1944 Army Individual Test Battery, Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office [Google Scholar]
- Tombaugh TN 2004 Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 19:203–214 [DOI] [PubMed] [Google Scholar]
- Petrides M, Milner B 1982 Deficits in subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia 20:249–262 [DOI] [PubMed] [Google Scholar]
- Lezak M, Howieson DB, Loring DW 2004 Neuropsychological assessment. 4th ed. New York: Oxford University Press [Google Scholar]
- Gabrieli J, Desmond J, Demb J, Wagner A, Stone M, Vaidya C, Glover G 1996 Functional magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychol Sci 7:278–283 [Google Scholar]
- Hampson E, Kimura D 1992 Sex differences and hormonal influences on cognitive function in humans. In: Becker J, Breedlove S, Crews D, eds. Behavioral endocrinology. Cambridge, MA: MIT Press (Bradford); 357–398 [Google Scholar]
- Dror IE, Kosslyn SM 1994 Mental imagery and aging. Psychol Aging 9:90–102 [DOI] [PubMed] [Google Scholar]
- Thurstone TG 1958 Manual for the primary mental abilities. Chicago: Science Research Associates [Google Scholar]
- Wechsler D 1987 Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corp., Harcourt Brace Jovanovich [Google Scholar]
- Cave CB, Squire LR 1991 Equivalent impairment of spatial and nonspatial memory following damage to the human hippocampus. Hippocampus 1:329–340 [DOI] [PubMed] [Google Scholar]
- Howieson DB, Holm LA, Kaye JA, Oken BS, Howieson J 1993 Neurologic function in the optimally healthy oldest old. Neuropsychological evaluation. Neurology 43:1882–1886 [DOI] [PubMed] [Google Scholar]
- Beer TM, Bland LB, Bussiere JR, Neiss MB, Wersinger EM, Garzotto M, Ryan CW, Janowsky JS 2006 Testosterone loss and estradiol administration modify memory in men. J Urol 175:130–135 [DOI] [PubMed] [Google Scholar]
- Siegel JA, Young LA, Neiss MB, Samuels MH, Roselli CE, Janowsky JS 2008 Estrogen, testosterone, and sequential movement in men. Behav Neurosci 122:955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.