Abstract
Context: Osteoporosis in men is becoming an increasingly important public health problem. One in five men over the age of 50 yr will suffer an osteoporotic fracture during their lifetime, and men who sustain fractures have an increased mortality risk.
Evidence Acquisition: Evidence was obtained by PubMed search and author’s knowledge of the field.
Evidence Synthesis: Studies using computed quantitative tomography and high-resolution peripheral computed quantitative tomography have provided new insights into the bone structural changes with aging in men, including the somewhat surprising demonstration of significant, ongoing trabecular bone loss starting in young adult life. In addition, there are now data demonstrating that serum estradiol levels are important predictors of fracture risk in men and that there is a threshold estradiol level below which not only bone loss but also fracture risk increases markedly. Criteria for diagnosing and managing osteoporosis in men are also evolving, including the application of the fracture risk assessment tool to derive 10-yr fracture risks in men. Three bisphosphonates (alendronate, risedronate, and zolendronic acid) and teriparatide are currently U.S. Food and Drug Administration approved for the treatment of osteoporosis in men, with a number of new compounds, including a monoclonal antibody against receptor activator of nuclear factor-κB ligand, selective estrogen receptor modulators, and selective androgen receptor modulators in varying stages of development.
Conclusions: Despite significant advances, there remain a number of key unresolved issues regarding the pathogenesis and management of male osteoporosis, not the least of which is increasing public awareness of this important cause of morbidity and mortality in men.
An update to current understanding of the epidemiology, pathogenesis, and management of male osteoporosis.
With the aging of the population, osteoporosis in men is becoming an increasingly important public health problem. Aging men lose bone mineral density (BMD) at a rate of approximately 1% per year (1), and one in five men over the age of 50 yr will suffer an osteoporotic fracture during their lifetime (2). Of all osteoporotic fractures, hip fractures contribute to the greatest morbidity and mortality, and almost 30% of all hip fractures occur in men (3). Although there is increasing recognition of the problem of male osteoporosis, there remain considerable gaps in knowledge regarding this disorder as well as in the care of these patients; for example, men are far less likely than women to receive a diagnosis of osteoporosis or treatment after a fracture (4). This brief update provides a summary of recent developments in the epidemiology, pathogenesis, and management of male osteoporosis, along with highlighting areas where additional work is needed to better understand and treat this disease.
Epidemiology
Johnell and Kanis (3) recently updated the worldwide prevalence of osteoporotic fractures using data from published sources. Of the annual incidence of 9 million fractures, 39% were in men. Thirty percent of hip, 20% of forearm, 42% of clinical vertebral, and 25% of humerus fractures occurred in men. As is the case in women, there appears to be geographic variation in the probability of hip fractures. Thus, for each 10° increment in latitude, there is a 0.3% increase in the 10-yr probability of hip fracture (5).
There are now clear data in men, as was previously reported in women (6), that the risk of subsequent fracture is markedly increased after a low-trauma fracture. In data from the Dubbo Osteoporosis Epidemiology Study of community-dwelling women and men aged 60 yr or older followed up for 16 yr, Center et al. (7) found that after a low-trauma fracture (caused by a fall from a standing height or less) at any skeletal site (excluding skull fractures and those due to underlying malignancy or Paget disease), the relative risk of subsequent fracture was 1.95 (95% confidence interval [CI], 1.70–2.25) in women and 3.47 (95% CI, 2.68–4.48) in men. As a result of the higher relative risk in men, the absolute risk of subsequent fracture was similar in women and men. These findings thus indicate that as for women, a careful fracture history and appropriate assessment for preexisting vertebral fractures, using either vertebral fracture assessment by dual energy x-ray absorptiometry (8) or spine x-rays, is essential in men.
Interestingly, although low-trauma fractures have been associated with BMD, Mackey et al. (9) demonstrated that in women and men, high-trauma fractures (e.g. due to motor vehicle crashes and falls from greater than standing height) are also associated with low BMD. In this study, each 1-sd reduction in total hip BMD in women was similarly associated with an increased risk of high-trauma fracture [multivariate relative hazard (RH), 1.45; 95% confidence interval (CI), 1.23–1.72] and low-trauma fracture (RH, 1.49; 95% CI, 1.42–1.57). Results were concordant in men (high-trauma fracture RH, 1.54; 95% CI, 1.20–1.96; low-trauma fracture RH, 1.69; 95% CI, 1.49–1.91). These findings have particular implications for osteoporosis clinical trials and observational studies, because it appears that high-trauma fractures should be included as outcomes in these studies. From a clinical perspective, the possibility of underlying skeletal fragility should also be considered in patients sustaining high-trauma fractures.
In a subsequent study, the Dubbo group also demonstrated that all low-trauma fractures were associated with increased mortality risk for 5–10 yr (10) (Fig. 1). As is evident, for all ages, mortality after fracture was higher for men than for women, and this was most marked in the older age groups. Examination of death certificates suggested no difference between causes of death in the fracture group and general population, with cardiac, respiratory, cerebrovascular disease, and malignancy being the major causes. Thus, the precise cause of the excess mortality after fracture in men (or in women) remains unclear.
Figure 1.
Mortality rates for the general participants and fracture patients according to age from the Dubbo Osteoporosis Epidemiology Study in women (A) and men (B). In this population, there were 2245 women and 1760 men aged 60 yr and older. Of the fracture patients, 952 were women and 343 were men. Error bars indicate 95% CI. [Reproduced with permission from D. Bliuc et al.: JAMA 301:513, 2009 (10). ©American Medical Association.]
The past several years have seen a marked increase in studies on the genetics of BMD and osteoporosis (for review, see Ref. 11), a detailed discussion of which is beyond the scope of this brief update. However, two recent studies are worth highlighting. In the first of these, Styrkarsdottir and colleagues (12) performed a quantitative trait analysis of data from 5861 Icelandic subjects where they examined the association between 301,019 single-nucleotide polymorphisms and BMD of the hip and lumbar spine. They then tested for an association between 74 single-nucleotide polymorphisms from this initial discovery set in replication sets of Icelandic, Danish, and Australian subjects (4165, 2269, and 1491 subjects, respectively). As in previous studies, however, the majority of the subjects were women (87% in the Icelandic population and 61–100% in the test populations). This analysis identified sequence variants in five genomic regions that were significantly associated with BMD in both the discovery and replications sets. Three regions were close to or within genes known to be relevant for bone metabolism: receptor activator of nuclear factor-κB ligand (RANKL), osteoprotegerin (OPG), and estrogen receptor 1 (ESR1). The other two regions were close to the zinc finger and BTB domain-containing 40 gene (ZBT40) and the major histocompatibility complex region. Somewhat disappointing was the finding that although statistically highly significant, these genes combined accounted for only approximately 3% of the total variation in hip and spine BMD. Thus, they are unlikely to be clinically useful but do provide further insights into the biochemical pathways underlying osteoporosis. Because the vast majority of subjects in this, and other, genetic studies of osteoporosis have been women, Peacock et al. (13) measured spine and hip BMD in 515 pairs of brothers, aged 18–61 yr, and performed linkage analysis in this sample as compared with results in a previous sample of 774 sister pairs to identify possible sex-specific quantitative trait loci (QTL). In this analysis, the investigators identified significant QTL (LOD > 3.6) for BMD on chromosome 4q21 (hip), 7q34 (spine), 14q32 (hip), 19p13 (hip), 21q21 (hip), and 22q13 (hip). By comparison with the previous female data, the QTL on chromosomes 7, 14, and 21 were male specific, whereas the others were not sex specific. Collectively, these data highlight the complexity of the genetics of a trait such as BMD as well as the fact that the genes determining BMD are likely at least partly gender specific.
Pathogenesis
Male osteoporosis remains a heterogeneous entity, with multiple underlying causes. In addition, several different factors may contribute to bone loss in a given individual. Conceptually, however, it is useful to separate male osteoporosis into primary causes (age-related and idiopathic osteoporosis) and secondary causes (those due to clearly identifiable diseases or drugs).
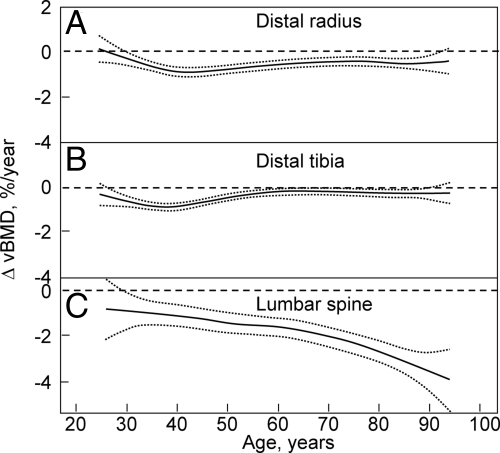
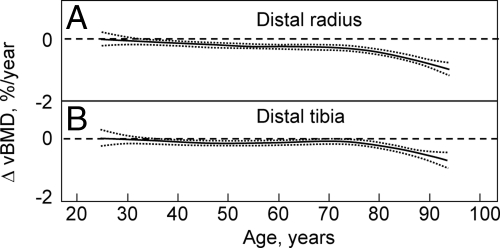
Recent studies using central and peripheral quantitative computed tomography (QCT), including high-resolution peripheral QCT (HRpQCT), have allowed for a clearer definition of age-associated changes in volumetric BMD (vBMD), geometry, and microstructure. In cross-sectional studies, Riggs et al. (14) found large decreases in vBMD at the spine (which consists principally of trabecular bone) over life, with a similar pattern of changes in trabecular bone at the femur neck, distal radius, and distal tibia, which seemed to begin in the third decade in both men and women. By contrast, cortical vBMD at the distal radius, distal tibia, and femur neck showed little change until midlife in either men or women, with linear decreases in both sexes thereafter. These cross-sectional findings were confirmed by longitudinal data (15), which showed significant trabecular bone loss at the spine, distal radius, and distal tibia before midlife in men, with an apparent attenuation in rates of trabecular bone loss at the distal radius and tibia, but not at the spine, in older men (Fig. 2, A–C). By contrast, cortical vBMD remained relatively stable until age 65–70 yr, with loss of cortical bone thereafter (Fig. 3, A and B). Essentially similar findings were present in women. These cross-sectional and longitudinal studies thus indicate that in both sexes, trabecular bone loss begins in young adult life, whereas cortical bone loss begins after midlife.
Figure 2.
Age-specific rates of change in vBMD at trabecular scanning sites in men at the distal radius (A), distal tibia (B), and lumbar spine (C). Data are shown with a smoothing spline and the 95% CI. [Reproduced with permission from B. L. Riggs et al.: J Bone Miner Res 23:205, 2008 (15). ©American Society for Bone and Mineral Research.]
Figure 3.
Age-specific changes in vBMD at cortical scanning sites at distal radius (A) and distal tibia (B) in men. Data are shown with a smoothing spline and the 95% CI. [Reproduced with permission from B. L. Riggs et al.: J Bone Miner Res 23:205, 2008 (15). ©American Society for Bone and Mineral Research.]
Studies using HRpQCT, which allows for in vivo assessment of bone microstructure, demonstrated that at the distal radius, women undergo loss of trabeculae with aging, whereas men primarily sustain trabecular thinning (16), findings that are similar to previous data from cadaveric transiliac bone biopsies (17). Because decreases in trabecular number have been shown by finite element modeling (18) to have a much greater impact on bone strength compared with decreases in trabecular thickness, these findings may help explain the lower lifelong risk of fractures in men, especially their virtual immunity to age-related increases in distal forearm fractures.
Recent studies have also further defined the relative contributions of estrogen and testosterone toward fracture risk in men. Previous work had demonstrated that estrogen played an important, and perhaps dominant, role in regulating bone density (19), bone resorption (20,21), and bone loss (22) in elderly men. In addition, there appeared to be a threshold serum estradiol level below which the male skeleton became estrogen deficient (22,23). Consistent with previous studies (19), Orwoll and colleagues (24) demonstrated that in men age 65 yr and older from the Osteoporotic Fractures in Men Study (MrOS), testosterone and estradiol levels, and their free fractions, declined significantly with age. Although free estradiol and free testosterone were modestly correlated (r = 0.20; P < 0.001), at any level of free testosterone, there was considerable variation in free estradiol levels. Amin and colleagues (25) related these declining sex steroid levels in aging men from the Framingham Study to the risk for hip fractures. In this study, 793 men (mean age, 71 yr) evaluated between 1981 and 1983 were followed until the end of 1999. The men were stratified into three groups, according to serum estradiol and testosterone levels. Based on 39 men who sustained a hip fracture during follow-up, incidence rates for hip fracture (per 1000 person-years) were 11.0, 3.4, and 3.9 for the low (2.0–18.1 pg/ml), middle (18.2–34.2 pg/ml), and high (≥34.3 pg/ml) estradiol groups, respectively. After adjustment for age, body mass index, height, and smoking status, the adjusted hazard ratios (HRs) for men in the low and middle estradiol groups, relative to the high estradiol group, were 3.1 (95% CI, 1.4–6.9) and 0.9 (95% CI, 0.4–2.0), respectively, consistent with the presence of a threshold estradiol level around 18 pg/ml below which fracture risk increased in the men. By contrast, in similar adjusted analyses evaluating men by their testosterone levels, the investigators found no significant increased risk for hip fracture associated with low testosterone levels.
Somewhat contrasting findings were reported from men in the Dubbo cohort by Meier and colleagues (26), who found that although serum testosterone was not related to lumbar spine or femoral neck BMD in 609 men over the age of 60 yr, lower testosterone levels were stronger predictors of low-trauma fractures than were estradiol levels, despite the fact that estradiol was significantly associated with spine and hip BMD in these men. These findings suggested that the effect of testosterone on fracture risk in elderly men may be mediated via nonskeletal factors, such as muscle strength and/or fall risk.
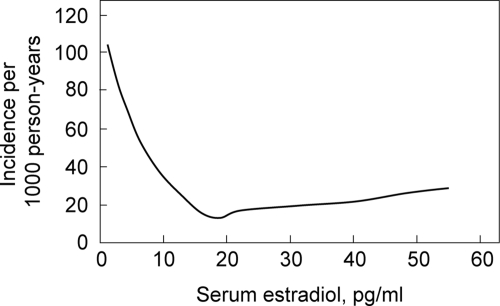
The most definitive data addressing this issue have come from the MrOS cohort. In the first of these studies, Mellström et al. (27) analyzed sex steroids using gas chromatography-mass spectroscopy in 2639 elderly men (mean age, 75 yr) from the Swedish arm of MrOS and evaluated fractures over a mean follow-up period of 3.3 yr. In multivariable proportional hazards regression models, free estradiol and SHBG, but not free testosterone, were independently associated with fracture risk. In further subanalyses, free estradiol was inversely associated with clinical vertebral fractures (HR per sd decrease, 1.57; 95% CI, 1.36–1.80), nonvertebral osteoporotic fractures (HR per sd decrease, 1.42; 95% CI, 1.23–1.65), and hip fractures (HR per sd decrease, 1.44; 95% CI, 1.18–1.76). Furthermore, consistent again with a threshold effect, the inverse relation between serum estradiol and fracture risk was nonlinear (Fig. 4). Specifically, the yearly incidence of fractures was inversely associated with serum estradiol levels at estradiol levels less than 16 pg/ml; above this level, there was no relationship between fracture incidence and estradiol levels.
Figure 4.
Yearly incidence of fractures as a function of serum estradiol levels in subjects from MrOS Sweden. Poisson regression models were used to determine the relation between serum estradiol levels and fracture risk. [Reproduced with permission from Mellström et al.: J Bone Miner Res 23:1552, 2008 (27). ©American Society for Bone and Mineral Research.]
LeBlanc et al. (28) have subsequently expanded these findings to include the larger U.S. cohort of MrOS involving 5995 men age 65 yr and older, of which they randomly selected a subcohort of 1436 white men and all 446 minority participants, plus all subjects with incident hip and other nonvertebral fractures. Consistent with the previous data and with the notion of a threshold, they found that men in the lowest bioavailable (non-SHBG bound) estradiol quartile (<11.4 pg/ml) or highest SHBG quartile (>59.1 nm) had greater risk of all nonvertebral fractures; by contrast, men with the lowest bioavailable testosterone level had no increased fracture risk after adjustment for bioavailable estradiol. However, these investigators did observe an interesting interaction (P = 0.03) between SHBG and bioavailable testosterone: men with low bioavailable testosterone and high SHBG did have an increase in fracture risk, with the highest risk of fracture occurring in men with low bioavailable estradiol, low bioavailable testosterone, and high SHBG.
Taken together, these studies using fracture as outcomes have provided further support for a key role for estradiol in determining fracture risk in aging men as well as the presence of a threshold estradiol level (which may vary, depending on the particular assay used) below which fracture risk increases in men. Testosterone may also contribute to fracture risk, particularly in the setting of high SHBG levels. Moreover, it is probable that a significant component of the testosterone effect on risk of fracture is mediated by nonskeletal effects, such as on muscle mass, balance, or risk of falls, although further studies directly addressing this issue are needed.
These findings also have potential clinical implications. First, from a diagnostic standpoint, measurement of serum sex steroid levels, particularly estradiol levels, in men with osteoporosis may be useful. However, this has to await further evaluation after standardization of sex steroid assays, likely using mass spectroscopy. Second, these data strengthen the rationale for assessing the use of selective estrogen receptor modulators (SERMs) in preventing bone loss in aging men. Third, these findings raise potential concerns about the utility of nonaromatizable selective androgen receptor modulators (SARMs) in preventing bone loss in men, because to the extent that the major sex steroid effect on bone is mediated by estrogen, these nonaromatizable compounds may have minimal skeletal effects, at least in humans. Nonetheless, SARMs may be useful in enhancing muscle strength and reducing fall risk and have antifracture effects through these nonskeletal actions.
Although there are a large number of possible secondary causes of osteoporosis in men, one has received considerable attention recently, the use of selective serotonin reuptake inhibitors. In a cross-sectional analysis of MrOS data, Haney et al. (29) found that mean BMD among men who used selective serotonin reuptake inhibitors (n = 160) was 3.9% lower at the total hip and 5.9% lower at the lumbar spine compared with BMD in men not on antidepressants (n = 5708; P = 0.002 for total hip; P < 0.001 for spine). By contrast, other antidepressants (trazodone hydrochloride and tricyclic antidepressants) were not associated with decreased BMD. These clinical findings are of particular interest given recent provocative data by Yadav and colleagues (30) showing that duodenum-derived serotonin inhibits bone formation, unveiling perhaps an entirely novel enteroskeletal regulatory system. In women, serum serotonin levels have recently been found to be inversely associated with bone mass and structural parameters at various skeletal sites (31); whether similar associations are present in men and the precise role for serotonin in regulating bone turnover in humans remains an area of active investigation.
Management
The original World Health Organization criteria for diagnosing osteoporosis were developed for women (32), and since then, there has been a vigorous debate regarding whether male- or female-referent T-scores should be used to diagnose osteoporosis in men. There are data indicating that the relationship between the absolute level of BMD and fracture risk is the same in men and women (33), arguing for the use of female-specific T-scores, even in men. By contrast, other studies have noted sex differences in the relationship between BMD and fracture risk, which are most apparent at younger ages and become less evident in older men (34). Currently, most densitometer reports provide gender-specific T-scores for men. However, the new World Health Organization fracture risk assessment tool (FRAX) uses the absolute femoral neck BMD (independent of sex) or, if T-scores are used, the calculator uses NHANES reference values for women aged 20–29 yr (i.e. a female-referent T-score) (35). The FRAX tool for predicting absolute 10-yr fracture risk can be used with or without BMD and includes key risk factors identified from nine prospective, population-based cohorts: a prior fragility fracture, parental history of hip fracture, current tobacco smoking, ever long-term use of oral glucocorticoids, rheumatoid arthritis, other causes of secondary osteoporosis, and daily alcohol consumption of three or more units daily. For women and men, the National Osteoporosis Foundation (NOF) has recently performed a U.S.-specific cost-effectiveness analysis, which incorporates the cost and health consequences of hip, spine, forearm, shoulder, rib, pelvis, and lower leg fractures, and identifies a 10-yr hip fracture probability of 3% or greater [or major osteoporotic (spine, forearm, hip, or shoulder) fracture of 20% or greater] as being sufficient to justify treatment (36). In contrast to this approach, the United Kingdom National Osteoporosis Guideline Group has adopted a variable threshold for treatment that at each age is set at a risk equivalent to that associated with a prior fracture and, therefore, rises with age (37). Both groups, however, clearly recommend pharmacological treatment for men or women with a prior history of fragility fracture.
The FRAX algorithm for the United States has just recently been revised (38), and just how FRAX and the new NOF guidelines for the treatment of osteoporosis in men (or women) will impact clinical practice remains to be determined. For example, there is concern that a substantial number of men (or women) with femoral neck BMD in the osteopenic range may be recommended for treatment based on the NOF guidelines. However, evidence that these men will have a reduction in fractures after therapy (as opposed to those subjects with T-scores below −2.5) is lacking. At the least, however, the ability of physicians to provide information to patients on absolute risks of fracture (rather than simply the somewhat abstract concept of a T-score) should lead to more informed decisions by the physician and patient regarding the initiation of pharmacological therapy.
In terms of therapy, given the critical role discussed above for estrogen in bone metabolism in men, a logical hypothesis is whether low doses of estrogen or SERMs may be effective as treatment modalities for osteoporosis in men. Although neither is currently approved for use in men, raloxifene does reduce bone turnover in men with low endogenous estradiol levels (39,40) and has been found to have positive effects on BMD at multiple sites in men undergoing gonadal suppression therapy for prostate cancer (41). A more recent study of 1389 men with prostate cancer on androgen deprivation therapy found that another SERM, toremifene, significantly reduced vertebral fractures (42). Thus, SERMs do hold promise in the treatment of hypogonadal bone loss in men with prostate cancer, but further studies are needed to assess their possible efficacy in aging men not undergoing gonadal suppression.
The question of whether SARMs might also represent a useful option for the treatment of osteoporosis in men has received considerable attention in recent years. Although there is preclinical animal data on a number of compounds in this class that appear to have beneficial effects on bone and muscle without adverse effects on the prostate (43), there are currently no published human data on the skeletal or nonskeletal effects (particularly effects on muscle mass and function) or safety of these compounds.
The issue of testosterone and/or the adrenal androgen dehydroepiandrosterone (DHEA), treatment of elderly men for preservation of bone and muscle mass as well as overall quality of life remains an active area of debate and discussion. In a recent study, Nair and colleagues (44) performed a 2-yr placebo-controlled, randomized, double-blind study involving 87 elderly men with low levels of DHEA-sulfate and bioavailable testosterone (defined as below the 15th percentile for young normal men). Over 2 yr of treatment, DHEA had no effect on body composition, and neither hormone altered the peak volume of oxygen consumed per minute, muscle strength, or insulin sensitivity. Men who received testosterone transdermally had a slight increase in fat-free mass, and men in both treatment groups had a modest (∼2%) increase in BMD at the femur neck but not at the spine, total hip, or radius. Neither treatment had an effect on the quality-of-life measures tested or had major adverse effects, including on prostate-specific antigen levels. These findings using transdermal testosterone at doses that had only modest effects on serum testosterone levels contrast with previous studies that used im testosterone administration and achieved higher circulating testosterone levels (45). In the latter, testosterone therapy was associated with more clinically significant increases in bone mass and muscle strength and improved body composition. Based on the current state of uncertainty regarding testosterone treatment of aging men, the Institute of Medicine has recommended that a series of clinical trials be done to help determine the efficacy of testosterone for several important outcomes, including bone (46).
As is the case in women, the mainstays of therapy for osteoporosis in men are the bisphosphonates. Currently, alendronate, risedronate, and zolendronic acid are U.S. Food and Drug Administration (FDA) approved for use in men. In addition, for patients at high risk of fracture, use of teriparatide in men has also been approved by the FDA. Because the general approach to the use of these drugs is similar to that in women, this is not discussed in detail here, and a more detailed description of bisphosphonate use in men has recently been presented elsewhere (47). The most recent option that is currently pending review by the FDA is denosumab, a fully monoclonal antibody against receptor activator of nuclear factor-κB ligand (RANKL). In a double-blind, multicenter study, Smith et al. (48) randomly assigned men receiving androgen deprivation therapy for prostate cancer to receive denosumab at a dose of 60 mg sc every 6 months or placebo (734 patients in each group). At 24 months, lumbar spine BMD increased by 5.6% in the denosumab group, as compared with a 1.0% loss in the placebo group (P < 0.001); denosumab was also associated with significant increases in BMD at the total hip, femoral neck, and distal radius. At 36 months, patients on denosumab had a 62% decreased incidence of new vertebral fractures (1.5 vs. 3.9% in the placebo group, P = 0.006). Thus, as for women, therapeutic options for treating osteoporosis in men are continuing to expand.
Key Unresolved Issues
Although there have been considerable advances in our understanding and management options for male osteoporosis, there are a number of important gaps in knowledge. Thus, although the FRAX algorithm has adopted the use of absolute femoral neck BMD and female-referent T-scores, the issue of whether male or female reference ranges should be used to define osteoporosis in men remains an active area of debate. This obviously impacts the question of the true prevalence of male osteoporosis. In terms of pathogenesis of bone loss, a better understanding is needed of the hormonal and nonhormonal factors causing bone loss in men, particularly the underlying mechanisms for the significant, ongoing trabecular bone loss in men (and women) in young adult life. Further studies are also needed regarding the potential utility of measuring serum estradiol levels, in addition to serum testosterone levels, using standardized mass spectroscopy assays in the evaluation of men with low bone mass. Additional studies on the use of SERMs, SARMs, and the skeletal, as well as nonskeletal, benefits vs. risks of testosterone treatment of aging men with declining total and bioavailable testosterone levels also need to be done. Finally, given that osteoporosis in men remains an underdiagnosed and undertreated disorder (4), increasing public awareness of this important cause of morbidity and mortality in men is key to ensure that as men age, they are spared the consequences of this disabling but now eminently preventable and treatable disease.
Footnotes
This work was supported by National Institutes of Health Grants AG004875 and AR027065.
Disclosure Summary: The author has nothing to disclose.
Abbreviations: BMD, Bone mineral density; CI, confidence interval; DHEA, dehydroepiandrosterone; HR, hazard ratio; HRpQCT, high-resolution peripheral quantitative computed tomography; MrOS, Osteoporotic Fractures in Men Study; QTL, quantitative trait loci; RH, relative hazard; SARM, selective androgen receptor modulator; SERM, selective estrogen receptor modulator; vBMD, volumetric BMD.
References
- Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP 2000 Risk factors for longitudinal bone loss in elderly men and women: the Framingham osteoporosis study. J Bone Miner Res 15:710–720 [DOI] [PubMed] [Google Scholar]
- Melton 3rd LJ, Chrischilles EA, Cooper C, Lane AW, Riggs BL 1992 How many women have osteoporosis. J Bone Miner Res 7:1005–1010 [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA 2006 An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733 [DOI] [PubMed] [Google Scholar]
- Curtis JR, Adachi JD, Saag KG 2009 Bridging the osteoporosis quality chasm. J Bone Miner Res 24:3–7 [DOI] [PubMed] [Google Scholar]
- Johnell O, Borgstrom F, Jonsson B, Kanis J 2007 Latitude, socioeconomic prosperity, mobile phones and hip fracture risk. Osteoporos Int 18:333–337 [DOI] [PubMed] [Google Scholar]
- Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A 2004 A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382 [DOI] [PubMed] [Google Scholar]
- Center JR, Bliuc D, Nguyen TV, Eisman JA 2007 Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 297:387–394 [DOI] [PubMed] [Google Scholar]
- Lewiecki EM, Laster AJ 2006 Clinical applications of vertebral fracture assessment by dual-energy x-ray absorptiometry. J Clin Endocrinol Metab 91:4215–4222 [DOI] [PubMed] [Google Scholar]
- Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barret-Connor E, Cummings SR; Study of Osteoporotic Fractures (SOF) and Osteoporotic Fractures in Men (MrOS) Research Groups 2007 High-trauma fractures and low bone mineral density in older women and men. JAMA 298:2381–2388 [DOI] [PubMed] [Google Scholar]
- Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR 2009 Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 301:513–521 [DOI] [PubMed] [Google Scholar]
- Lei SF, Jiang H, Deng FY, Deng HW 2007 Searching for genes underlying susceptibility to osteoporotic fracture: current progress and future prospect. Osteoporos Int 19:1157–1175 [DOI] [PubMed] [Google Scholar]
- Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Center JR, Nguyen TV, Bagger Y, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K 2008 Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358:2355–2365 [DOI] [PubMed] [Google Scholar]
- Peacock M, Koller DL, Lai D, Hui S, Foroud T, Econs MJ 2009 Bone mineral density variation in men is influenced by sex-specific and non sex-specific quantitative trait loci. Bone 45:443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Melton III LJ, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S 2004 Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 19:1945–1954 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S 2008 A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton 3rd LJ 2006 Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res 21:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaron JE, Makins NB, Sagreiya K 1987 The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop 215:260–271 [PubMed] [Google Scholar]
- Silva MJ, Gibson LJ 1997 Modeling the mechanical behavior of vertebral trabecular bone: effects of age-related changes in microstructure. Bone 21:191–199 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton 3rd LJ, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL 1998 Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83:2266–2274 [DOI] [PubMed] [Google Scholar]
- Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S 2000 Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS 2003 Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab 88:204–210 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ, Atkinson EJ, O'Fallon WM 2001 Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab 86:3555–3561 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton 3rd LJ, Riggs BL 2002 Estrogen and the male skeleton. J Clin Endocrinol Metab 87:1443–1450 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, Cauley J, Ensrud K, Cummings S 2006 Testosterone and estradiol among older men. J Clin Endocrinol Metab 91:1336–1344 [DOI] [PubMed] [Google Scholar]
- Amin S, Zhang Y, Felson DT, Sawin CT, Hannan MT, Wilson PW, Kiel DP 2006 Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. Am J Med 119:426–433 [DOI] [PubMed] [Google Scholar]
- Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, Rockwood AL, Meikle AW, Center JR, Eisman JA, Seibel MJ 2008 Endogenous sex hormones and incident fracture risk in older men. Arch Intern Med 168:47–54 [DOI] [PubMed] [Google Scholar]
- Mellström D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, Odén A, Johansson H, Orwoll ES, Labrie F, Karlsson MK, Ljunggren O, Ohlsson C 2008 Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res 23:1552–1560 [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE, Hoffman AR, Laughlin G, Ohlsson C, Orwoll ES 2009 The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab 94:3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney EM, Chan BK, Diem SJ, Ensrud KE, Cauley JA, Barrett-Connor E, Orwoll E, Bliziotes MM 2007 Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch Intern Med 167:1246–1251 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G 2008 Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135:825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mödder UI, Achenbach SJ, Amin S, Riggs BL, Melton III LJ, Khosla S 13 July 2009 Relation of serum serotonin to bone density and structural parameters in women. J Bone Miner Res 10.1359/jbmr.090721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis JA 1994 Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4:368–381 [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton 3rd LJ, O'Neill T, Pols H, Reeve J, Silman AJ, Tenenhouse A 2005 Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Cawthon PM, Ensrud KE, Cauley JA, Fink HA, Orwoll ES, Groups ftOFiMaSoOFR 2006 BMD and risk of hip and nonvertebral fractures in older men: a prospective study and comparison with older women. J Bone Miner Res 21:1550–1556 [DOI] [PubMed] [Google Scholar]
- Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N 2005 Assessment of fracture risk. Osteoporos Int 16:581–589 [DOI] [PubMed] [Google Scholar]
- Tosteson AN, Melton 3rd LJ, Dawson-Hughes B, Baim S, Favus MJ, Khosla S, Lindsay RL 2008 Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int 19:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston J, Cooper A, Cooper C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P, Wilkins M; National Osteoporosis Guideline Group (NOGG) 2009 Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas 62:105–108 [DOI] [PubMed] [Google Scholar]
- Kanis JA, Johansson H, Anders O, Dawson-Hughes B, Melton LJI, McCloskey EV August 28, 2008 The effects of a FRAX revision for the US. Osteoporos Int 10.1007/s00198-009-1033-8 [DOI] [PubMed] [Google Scholar]
- Doran PM, Riggs BL, Atkinson EJ, Khosla S 2001 Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men. J Bone Miner Res 16:2118–2125 [DOI] [PubMed] [Google Scholar]
- Uebelhart B, Herrmann F, Pavo I, Draper MW, Rizzoli R 2004 Raloxifene treatment is associated with increased serum estradiol and decreased bone remodeling in healthy middle-aged men with low sex hormone levels. J Bone Miner Res 19:1518–1524 [DOI] [PubMed] [Google Scholar]
- Smith MR, Fallon MA, Lee H, Finkelstein JS 2004 Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab 89:3841–3846 [DOI] [PubMed] [Google Scholar]
- Lin DW, Marks LS, Morton RA, Rodriguez D 2009 Positive fracture reduction trial of toremifene 80 mg in men on ADT demonstrates significant fracture risk in untreated placebo group. Journal of Urology 181:229 (Abstract) [Google Scholar]
- Kilbourne EJ, Moore WJ, Freedman LP, Nagpal S 2007 Selective androgen receptor modulators for frailty and osteoporosis. Curr Opin Investig Drugs 8:821–829 [PubMed] [Google Scholar]
- Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton 3rd LJ, Smith GE, Khosla S, Jensen MD 2006 DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]
- Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL 2004 Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 89:503–510 [DOI] [PubMed] [Google Scholar]
- Committee on Assessing the Need for Clinical Trials of Testosterone Replacement Therapy 2004 Executive summary. In: Liverman CT, Blazer DG, eds. Testosterone and aging: clinical research directions. Washington, DC: The National Academic Press; 1–10 [Google Scholar]
- Khosla S, Amin S, Orwoll E 2008 Osteoporosis in men. Endocr Rev 29:441–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic A, Leder BZ, Goessl C 2009 Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 361:745–755 [Google Scholar]