Abstract
Context: Cancer genetics is fundamental for preventive medicine, in particular in pheochromocytoma-associated syndromes. Variants in two susceptibility genes, SDHC and RET, were found in a kindred with head and neck paraganglioma. This observation of coincident DNA variants, both reported as pathogenic, in two known susceptibility genes prompted the question of their pathogenic relevance.
Objective: Our objective was to elucidate the pathogenic role of the detected variants and study the prevalence of such variants.
Patients: Patients were registrants from the European-American Pheochromocytoma-Paraganglioma and German von Hippel-Lindau Disease Registries.
Design: Analysis of germline mutation screening results for all pheochromocytoma-paraganglioma susceptibility genes, including RET [multiple endocrine neoplasia type 2 (MEN 2)] and VHL [von Hippel-Lindau disease (VHL)]. Cases in which more than one DNA variant was found were clinically reevaluated, and cosegregation of the disease with the variant was analyzed within the registrants’ families. A total of 1000 controls were screened for the presence of detected variants, and in silico analyses were performed.
Results: Three variants were identified, RET p.Tyr791Phe and p.Ser649Leu and VHL p.Pro81Ser. The frequencies of RET p.Ser649Leu (0.07%) and p.Tyr791Phe (0.9%) compared with controls excluded the two variants’ role in the etiology of MEN 2 and VHL. None of the carriers of the RET variants who underwent prophylactic thyroidectomy showed medullary thyroid carcinoma. Clinical reinvestigation of 18 variant carriers excluded MEN 2. VHL variant p.Pro81Ser, also previously described as a mutation, did not segregate with the VHL in one family. In silico analyses for these variants predicted unmodified protein function.
Conclusions: RET p.Tyr791Phe and p.Ser649Leu and VHL p.Pro81Ser are definitely not pathogenic mutations for VHL and MEN 2. Misinterpretation results in irreversible clinical consequences.
RET p.Tyr791Phe and p.Ser649Leu, and VHL p.Pro81Ser are not pathogenic mutations for VHL and MEN 2.
Identification of heritable mutations in cancer susceptibility genes as reliable and actionable molecular diagnostic and predictive testing tools has ushered in the practice of clinical cancer genetics. The genes, which when mutated, predispose to heritable endocrine neoplasias, in particular, have been viewed as providing useful models for clinical cancer genetics practice, which includes genetic counseling. For example, multiple endocrine neoplasia type 2 (MEN 2), characterized by medullary thyroid carcinoma (MTC), pheochromocytoma, and hyperparathyroidism (HPT), and its susceptibility gene RET is considered the classic model. Current practice guidelines suggest RET testing in all cases of apparently sporadic MTC (1). When a specific RET mutation is identified, a molecular diagnosis of MEN 2 is made. Consequently, the individual can be managed for risks of pheochromocytoma and HPT. Furthermore, that individual’s as yet unaffected family members can be offered predictive testing for that specific RET mutation, and if positive, he/she can be offered relevant prophylactic surgeries and clinical surveillance (1). Although heritable MTC is due to one susceptibility gene, this is not the rule for heritable pheochromocytoma and/or paraganglioma (PGL), which has six known susceptibility genes, including RET (2). Germline mutations in VHL cause what is considered the classic pheochromocytoma syndrome, von Hippel-Lindau disease (VHL). In addition to pheochromocytoma, retinal and central nervous system angiomas/hemangioblastomas and clear-cell renal carcinoma are components.
The accuracy of molecular diagnosis and predictive testing followed by judicious clinical management based on gene test results is dependent on whether we can call a variant pathogenic, i.e. a mutation. In the field of endocrine neoplasia genetics, we had believed that we were exempt from variants of unknown significance (VUS); up until now, we believed that all described mutations were pathogenic. However, three key observation case reports caused us to rethink the issue of VUS and double germline mutations in endocrine neoplasias.
Key observations
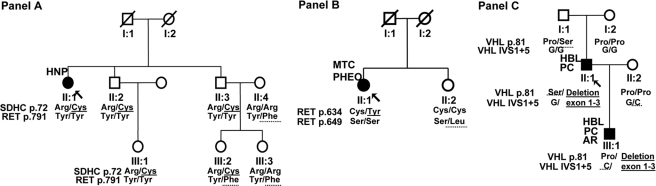
Key case 1 (Fig. 1A)
Figure 1.
Pedigrees of the three key case families. A, Pedigree of key case 1; B, pedigree of key case 2; C, pedigree of key case 3. The essential molecular genetic findings for mutations are indicated by underscoring by a solid line and for polymorphisms by a dotted line. The arrow indicates the index case of the family. AR, Angiomatosis retinae; HBL, hemangioblastoma of the central nervous system; HNP, head and neck PGL; PC, pancreas cysts; PHEO, pheochromocytoma; y, age at manifestation for affected cases (filled symbols) and current age for the unaffected (clear symbols). Genotypes are represented for each considered variant on the protein (p.) level. Del, Large deletions. Findings as Arg/Cys in A, subject II.1, show the results for allele 1 and allele 2. IVS1 is intervening sequence (intron) 1, +5 is the fifth intronic nucleotide. Note that in C, the protein81 and IVS1 + 5 findings are shown for only one allele, because the other allele is deleted.
A 62-yr-old female presenting with a glomus tympanicum PGL was found to have a germline SDHC c.214 C→T (p.Arg72Cys) mutation (II.1). By an in-laboratory mistake, her relatives were screened for mutations in the RET gene. Two of the probands’ nieces (III.2 and III.3) were identified with what had been believed to be a RET mutation, c.2372 A→T (p.Tyr791Phe), which was not present in their SDHC mutation-positive father (II.3), who is the proband’s brother, but found in their unaffected mother (II.4). As expected, the index case, her other brother, and his daughter did not carry the RET variant. Clinical screening of the proband’s niece’s mother (II.4) excluded the presence of MTC, pheochromocytoma, and HPT. In summary, this family shows a coincidence of a SDHC mutation and an accidentally detected RET variant with transmission from non-blood-related family members (the proband’s sister-in-law) whose oldest subject was found tumor free at age 54.
Key case 2 (Fig. 1B)
The index case (II.1) was a 57-yr-old female with bilateral pheochromocytoma and MTC carrying the RET c.1901 G→A (p.Cys634Tyr) mutation in exon 11. This proband did not harbor any other variants in RET or other genes. By screening exon 11 as a predictive test in the healthy 60-yr-old sister (II.2), the RET c.1946 C→T, p.Ser649Leu variant, but not the family-specific mutation, was found. She had normal results for basal and stimulated calcitonin and PTH and excretion of catecholamines.
Key case 3 (Fig. 1C)
A 50-yr-old male (II.1) presenting with hemangioblastomas of the central nervous system was found to have a hemizygous VHL c.241 C→T (p.Pro81Ser) variant and a concomitant large deletion encompassing all exons of the VHL gene. The screening of his relatives confirmed that the p.Pro81Ser has been inherited from his healthy father (I.1), whereas the large deletion occurred de novo. The proband’s son (III.1) inherited his father’s germline VHL large deletion, and not the VHL p. Pro81Ser variant. In addition, the son (III.1) was found to have a hemizygous VHL c.340 + 5 G→C (IVS1 + 5) polymorphic variant, present on the allele inherited from his healthy mother (II.2). The son (III.1) was, therefore, clinically screened and found to have retinal and cerebellar hemangioblastomas as well as pancreatic cysts, typical lesions of VHL demonstrating that VHL cosegregated with, and is therefore caused by, the VHL large deletion and not the p.Pro81Ser variant.
These case reports were, therefore, instrumental in raising two issues. First, could the long-presumed pathogenic RET and VHL mutations actually be harmless polymorphisms? Second, if the RET and VHL variants are pathogenic, then how commonly do patients with heritable PGL harbor two or more pathogenic mutations in the same or different genes?
Subjects and Methods
Research subjects
For purposes of this report, we checked the population-based registries the European-American Pheochromocytoma-Paraganglioma (EAPP) Registry and the German von Hippel-Lindau Disease Registry (VHL Registry).
Systematic mutation analysis
All unrelated registered index cases of the EAPP Registry underwent germline mutation analyses for exons 10–11 and 13–16 of RET (RefSeq:NM_020975.4) and all exons of the VHL (RefSeq:NM_000551.2), SDHB (RefSeq:NM_003000.2), SDHC (RefSeq:NM_003001), and SDHD (RefSeq:NM_003002.2) (SDHx) genes. The mutation scanning included analysis for large deletions/duplications of the VHL and SDHx genes.
We checked the mutation results and noted those individuals with the presence of two germline variants, which may represent two pathogenic mutations, two polymorphisms, or one mutation and one polymorphism. In addition to the three variants from the key reports (RET p.Ser649Leu and p.Tyr791Phe and VHL p.Pro81Ser), a fourth variant of the VHL gene (p.Leu188Val) found concomitantly with VHL p.Pro81Ser was added to our study. For the specific variants considered in this manuscript, we studied 1000 control samples (supplemental material, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
In silico analyses
For each VUS, we checked the affected codons for evolutionary conservation among species using the EPO multiple alignment analysis provided by ENSEMBL Browser (www.ensembl.org). Furthermore, we conducted in silico analysis based on sequence similarity analyses, the physical properties of amino acids, and the structure and function of human proteins using the SIFT predictor software (Fred Hutchinson Cancer Research Center, Seattle, WA).
Clinical investigation
Index cases and relatives carrying the RET variants were reevaluated for MEN 2A-associated tumors. Ultrasonography of the thyroid and parathyroid glands, basal and pentagastrin-stimulated serum calcitonin and PTH levels as well as 24 h-urine catecholamines and abdominal magnetic resonance imaging were analyzed. Index cases and relatives carrying VHL variants were investigated for VHL-associated tumors by ophthalmoscopy, by magnetic resonance imaging of the central nervous system and abdomen, and by 24-h catecholamines.
Ethics committee
All subjects gave written consent for the study, and the project was approved by the ethical committee of the University Medical Center in Freiburg and the respective human subjects’ protection committee of the respective collaborating centers.
Results
Patients and relatives with RET variants
From the EAPP Registry, 1475 index cases were analyzed for RET gene mutations.
Thirteen of 1475 (0.9%) registrants carried the RET p.Tyr791Phe variant, compared with eight of 1000 (0.8%) population-based controls. The RET p.Ser649Leu variant was identified in one of 1475 (<0.1%) registered cases, compared with six of 1000 (0.6%) population-based controls. Screenings of respective relatives identified another four carriers. None of them carried another variant or a mutation in another gene.
The conservation through evolution analysis showed both amino acids are conserved, although the nucleotide c.2372 A (codon 791) is only moderately conserved. SIFT analysis confirmed that the tyrosine→phenylalanine change at position 791 and the serine→leucine change at position 649 of the RET gene were predicted to be tolerated substitutions.
Clinical reevaluation for MEN 2
Clinical reevaluation was performed in 17 of the 22 cases found to have the RET variants p.Tyr791Phe (15 cases) and p.Ser649Leu (two cases) (Table 1). The cases who did not agree to further clinical investigation, two with head and neck PGL and one with pheochromocytoma, were seemingly otherwise healthy, and family history was negative for MEN 2.
Table 1.
Cases carrying the RET p.Tyr791Phe and p.Ser649Leu variants
| Family/case ID | Identified variants | Prophylactic thyroidectomy | MTC/CCH (age, yr) | PGL/PHEO (age, yr) | HPT | Other tumors | Family history |
|---|---|---|---|---|---|---|---|
| Incidentally identified cases | |||||||
| Fig. 1 III.2 | RET p.Tyr791Phe | No | Fig. 1 | ||||
| Fig. 1 III.3 | RET p.Tyr791Phe | No | |||||
| Fig. 1 II.4 | RET p.Tyr791Phe | No | No (53) | No (53) | No | No | |
| Fig. 2 II.2 | RET p.Ser649Leu | No | No (59) | No (59) | No | No | Fig. 2 |
| EAPP Registry cases | |||||||
| 1/1 | RET p.Ser649Leu | No | No (57) | A, left (41) | No | No | Negative |
| 2/1 | RET p.Tyr791Phe | No | Glomus tympancium, left (77) | No | Negative | ||
| 3/1 | RET p.Tyr791Phe | Noa | No (23) | EA (72) | Nob | No | Negative |
| 4/1 | RET p.Tyr791Phe | No | No (43) | A, left (36); A, right (40) | No | No | Negative |
| 5/1 | RET p.Tyr791Phe | No | No (51) | A, right (39) | No | No | Negative |
| 6/1 | RET p.Tyr791Phe | No | No (57) | EA (39) | No | No | Relative with PHEO at age 30 |
| 6/2 | RET p.Tyr791Phe | No | No (79) | Glomus caroticum, left (57) | No | No | |
| 7/1 | RET p.Tyr791Phe | No | No (35) | A, left (25); A, left (30) | No | No | Negative |
| 8/1 | RET p.Tyr791Phe | No | No (47) | Multiple malignant PHEO (30) | Nob | No | No |
| 8/2 | RET p.Tyr791Phe | No | No (11) | No | No | No | Daughter of 8/1 |
| 9/1 | RET p.Tyr791Phe | No | No (62) | No (62) | No | Pituitary adenoma and small-cell lung cancer | Negative |
| 10/1 | RET p.Tyr791Phe | No | No (61) | A, right (40) | Nob | No | Negative |
| 11/1 | RET p.Tyr791Phe | No | No (78) | Glomus jugulare, right (74) | No | No | Negative |
| 12/1 | RET p.Tyr791Phe | Yes | No (31) | A, left (29) | No | No | Negative |
| 12/2 | RET p.Tyr791Phe | Yes | No (73) | No (74) | No | No | Grandmother of 12/1 |
| 12/3 | RET p.Tyr791Phe | Yes | No (53) | No (53) | No | No | Mother of 12/1 |
| 13/1 | RET p.Tyr791Phe | No | Glomus jugulare, left (51) | Negative | |||
| 14/1 | RET p.Tyr791Phe | No | A, left (25) | Negative |
PTH levels were normal in 14 cases. Three cases had slightly elevated levels of PTH but normal serum calcium and phosphate levels. Four p.Tyr791Phe carriers underwent prophylactic thyroidectomy. Histology excluded MTC in all cases. In one case, a single CCH focus was identified. Investigation of the remaining 13 cases with pentagastrin-stimulated calcitonin testing and ultrasonography of the thyroid gland excluded the presence of MTC. No indicates no clinical evidence. A, Adrenal; EA, extraadrenal pheochromocytoma; PHEO, pheochromocytoma.
Thyroidectomy was performed due to goiter before genetic testing.
Slightly elevated PTH; Ca and PO4normal; 25-OH vitamin D not tested.
In summary, there was no family history or additional MEN 2 features in 18 carriers primarily diagnosed with pheochromocytomas or PGLs.
Patients and relatives with VHL variants
Analyses of the 1475 index cases of the EAPP Registry revealed the coincidence of two different VHL variants in three (0.2%) apparently unrelated index cases. Each of these three cases carried both the VHL p.Pro81Ser and the VHL p.Leu188Val variants. Both variants were lying on the same allele. Haplotyping analyses of the three families suggested the presence of a common ancestor (data not shown).
The key case number 3 (see above) was the other p.Pro81Ser carrier found in the German VHL Registry. Analyses of 1000 controls did not show any of these two variants.
The conservation through evolution analysis showed less than 50% conservation of the VHL c.241 C nucleotide comparing the human sequence with 29 eutherian mammals. In contrast, the VHL c.340 C nucleotide turned out to be highly conserved. SIFT analysis showed that the proline→serine at position 81 of the VHL gene was predicted to be a tolerated substitution, whereas the leucine at position 188 of the VHL gene was deleterious. Additionally, functional assays indicated that certain VHL type 2C mutants (including p.Leu188Val) have defects in fibronectin matrix (3,4).
Clinical reevaluation for VHL
In total, 10 of 13 carriers of the concomitant VHL p.Pro81Ser and p.Leu188Val have been clinically reevaluated. Pheochromocytoma alone was present in four cases (one single and three multiple). Retinal and spinal hemangioblastoma were diagnosed concomitantly in three cases, of which one also had renal cysts, whereas none of them had pheochromocytoma. Pheochromocytoma and spinal hemangioblastoma have been identified in two cases. One case of age 36 did not present with any VHL manifestation.
Discussion
By-chance observations initiated our reconsideration of current approaches of identification of germline mutations in the autosomal dominant cancer syndromes MEN 2 and VHL. Our key case observations questioned the pathogenicity of VUS RET p.Tyr791Phe, and p.Ser649Leu and VHL p.Pro81Ser, previously published and considered by clinical labs as pathogenic mutations (supplemental material). There is, moreover, one report by Vierhapper et al. (5) who analyzed RET in 75 cases with elevated calcitonin levels and 70 with normal calcitonin levels, with two individuals in each group with the p.Tyr791Phe variant. This is the first report of RET p.Tyr791Phe mutation in normal controls. Interestingly, all three variants were initially reported after molecular screening of a series of apparently sporadic (nonfamilial, nonsyndromic) disease manifestations: MTC (RET p.Ser649Leu), Hirschsprung disease (RET p.Tyr791Phe), and cerebellar hemangioblastoma (VHL p.Pro81Ser) (6,7,8). These studies share several commonalities including absence of clinical evidence for familial disease in the variant carriers and small sample size or absence of healthy controls tested for these variants. Furthermore, in silico tools predicted a tolerated effect of the amino acid changes on the protein. In contrast to the published reports, we tested 1000 control samples (2000 chromosomes) and confirmed that these variants are rare and likely to be missed by smaller sample sizes of controls tested. The frequencies that we observed in our registries are similar to the frequencies of these variants found in the published series and are equally represented between controls and cases.
Whether a variant is pathogenic, polymorphic, or a VUS is vital for molecular diagnosis, predictive testing, and prophylactic procedures. Unfortunately, although RET p.Tyr791Phe and p.Ser649Leu were previously considered pathogenic, individuals carrying what we show to be polymorphisms, at best VUS, had already undergone prophylactic thyroidectomy (1,9,10,11,12,13), including in four subjects from our study group carrying the RET p.Tyr791Phe. None of these had MTC in the thyroidectomy specimen. Three had normal histological findings, and one had a C-cell hyperplasia (CCH). CCH is considered the precursor lesion of MTC (14,15). But CCH can also be found in other thyroid diseases and even in the normal population and is not at all pathognomonic for MEN 2 (16,17,18,19,20,21). Furthermore, in MEN 2 patients, CCH is monoclonal and multifocal, diffuse with a nodular growth pattern (14). The case from our study had a single CCH focus. In previous reports of carriers of this RET variant, MTC has never been identified and reported CCH not validated by independent thyroid pathologists (9,10,11,12,13).
Together with reexamination of previous reports of these RET and VHL variants, our current study demonstrates that none of these variants are pathogenic germline mutations responsible for MEN 2 and VHL, respectively. We have shown that these variants do not cosegregate with the disease in question, are inherited from unrelated family members who are healthy, and are equally represented between cases and controls. Although this provides multiple lines of proof that RET p.Tyr791Phe and p.Ser649Leu and VHL p.Pro81Ser are not pathogenic mutations, we cannot exclude their function as modifiers of known germline high-penetrance pathogenic mutations. As more genetic knowledge accrues over time, clinical practice must be quickly and critically updated. Premature declaration of DNA variants as pathogenic mutations, when they are not, put an unjustified burden on the patient and the given family by the message of an inherited disorder and can even result in irreversible treatment procedures.
Supplementary Material
Acknowledgments
We thank Aurelia Winter (study nurse), Gani Berisha, Tobias Bluem, Mary Buchta, and Andrea Schwentek (laboratory technicians) from the University Hospital Freiburg for their support.
Footnotes
Disclosure Summary: The authors have nothing to disclose.
H.P.H.N. is supported by grants from the Deutsche Krebshilfe (107995), the Deutsche Forschungsgemeinschaft (NE 571/5-3), and the European Union (LSHC-CT-2005-518200). C.E. is the Sondra J. and Stephen R. Hardis Endowed Chair of Cancer Genomic Medicine at the Cleveland Clinic, is the recipient of a Doris Duke Distinguished Clinical Scientist Award, is an American Cancer Society Clinical Research Professor, and is an National Institutes of Health-funded investigator. M.R. is supported by a grant from Fondo de Ivestigaciones Sanitarias (FIS) (FIS 080883) and CIBERER.
First Published Online November 11, 2009
Abbreviations: CCH, C-cell hyperplasia; EAPP, European-American Pheochromocytoma-Paraganglioma; HPT, hyperparathyroidism; MEN 2, multiple endocrine neoplasia type 2; MTC, medullary thyroid carcinoma; PGL, paraganglioma; VHL, von Hippel-Lindau disease; VUS, variants of unknown significance.
Department of Nephrology (Z.E., M.Su., G.F., H.P.H.N.), Section of Preventive Medicine, and Department of Laboratory Medicine (M.M.H.), Albert-Ludwigs-University, Freiburg, D-79106 Freiburg, Germany; Department of Hypertension (M.P., A.J.), Institute of Cardiology, 04-628Warsaw, Poland; Department of Endocrinology (I.H.), University Medical Clinic, D-91054 Erlangen, Germany; Departments of Endocrinology, Diabetology, and Rheumatology (M.Sc.) and Pathology (H.E.G.), Heinrich-Heine University of Duesseldorf, D-40225 Duesseldorf, Germany; Division of Endocrinology (M.V.), Helsinki University Central Hospital, FIN-00290 Helsinki, Finland; Department of Otorhinolaryngology (S.F.P.), University of Köln, D-50931 Köln, Germany; Nuclear Medicine and Endocrine Oncology Department (K.H.-L.), Cancer Center Maria Sklodowska-Curie Memorial Institute, 44-100 Gliwice, Poland; Department of Endocrinology and Nuclear Medicine (D.W.), Holycross Cancer Center, 25-406 Kielce, Poland; Hereditary Endocrine Cancer Group (M.R.), Spanish National Cancer Center, Madrid, and Instituto de Salud Carlos III Center for Biomedical Research on Rare Diseases (CIBERER), E-28029 Madrid, Spain; and Genomic Medicine Institute, Lerner Research Institute, Taussig Cancer Institute (C.E.), Cleveland Clinic, Cleveland, Ohio 44195
References
- Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells Jr SA 2009 Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19:565–612 [DOI] [PubMed] [Google Scholar]
- Erlic Z, Neumann HP 2009 Familial pheochromocytoma. Hormones 8:29–38 [DOI] [PubMed] [Google Scholar]
- Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin Jr WG 2001 von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum Mol Genet 10:1019–1027 [DOI] [PubMed] [Google Scholar]
- Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ, Maher ER 2001 Contrasting effects on HIF-1α regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum Mol Genet 10:1029–1038 [DOI] [PubMed] [Google Scholar]
- Vierhapper H, Bieglmayer C, Heinze G, Baumgartner-Parzer S 2004 Frequency of RET proto-oncogene mutations in patients with normal and with moderately elevated pentagastrin-stimulated serum concentrations of calcitonin. Thyroid 14:580–583 [DOI] [PubMed] [Google Scholar]
- Wiench M, Wygoda Z, Gubala E, Wloch J, Lisowska K, Krassowski J, Scieglinska D, Fiszer-Kierzkowska A, Lange D, Kula D, Zeman M, Roskosz J, Kukulska A, Krawczyk Z, Jarzab B 2001 Estimation of risk of inherited medullary thyroid carcinoma in apparent sporadic patients. J Clin Oncol 19:1374–1380 [DOI] [PubMed] [Google Scholar]
- Seri M, Yin L, Barone V, Bolino A, Celli I, Bocciardi R, Pasini B, Ceccherini I, Lerone M, Kristoffersson U, Larsson LT, Casasa JM, Cass DT, Abramowicz MJ, Vanderwinden JM, Kravcenkiene I, Baric I, Silengo M, Martucciello G, Romeo G 1997 Frequency of RET mutations in long- and short-segment Hirschsprung disease. Hum Mutat 9:243–249 [DOI] [PubMed] [Google Scholar]
- Neumann HP, Eng C, Mulligan LM, Glavac D, Zäuner I, Ponder BA, Crossey PA, Maher ER, Brauch H 1995 Consequences of direct genetic testing for germline mutations in the clinical management of families with multiple endocrine neoplasia, type II. JAMA 274: 1149–1151 [PubMed] [Google Scholar]
- Frank-Raue K, Heimbach C, Rondot S, Usadel KH, Meng W, Varma C, Fuchs-Hammoser R, Höppner W, Schulze E, Raue F 2003 [Hereditary medullary thyroid carcinoma: genotype-phenotype characterization]. Dtsch Med Wochenschr 128:1998–2002 (German) [DOI] [PubMed] [Google Scholar]
- Gimm O, Niederle BE, Weber T, Bockhorn M, Ukkat J, Brauckhoff M, Thanh PN, Frilling A, Klar E, Niederle B, Dralle H 2002 RET proto-oncogene mutations affecting codon 790/791: a mild form of multiple endocrine neoplasia type 2A syndrome? Surgery 132:952–959; discussion 959 [DOI] [PubMed] [Google Scholar]
- Brauckhoff M, Gimm O, Hinze R, Ukkat J, Brauckhoff K, Dralle H 2002 Papillary thyroid carcinoma in patients with RET proto-oncogene germline mutation. Thyroid 12:557–561 [DOI] [PubMed] [Google Scholar]
- Frank-Raue K, Buhr H, Dralle H, Klar E, Senninger N, Weber T, Rondot S, Höppner W, Raue F 2006 Long-term outcome in 46 gene carriers of hereditary medullary thyroid carcinoma after prophylactic thyroidectomy: impact of individual RET genotype. Eur J Endocrinol 155:229–236 [DOI] [PubMed] [Google Scholar]
- Colombo-Benkmann M, Brämswig J, Höppner W, Gellner R, Hengst K, Böcker W, Senninger N 2002 Surgical strategy in a kindred with a rare RET protooncogene mutation of variable penetrance with regard to multiple endocrine neoplasia. World J Surg 26:1286–1290 [DOI] [PubMed] [Google Scholar]
- Diaz-Cano SJ, de Miguel M, Blanes A, Tashjian R, Wolfe HJ 2001 Germline RET 634 mutation positive MEN 2A-related C-cell hyperplasias have genetic features consistent with intraepithelial neoplasia. J Clin Endocrinol Metab 86:3948–3957 [DOI] [PubMed] [Google Scholar]
- Machens A, Niccoli-Sire P, Hoegel J, Frank-Raue K, van Vroonhoven TJ, Roeher HD, Wahl RA, Lamesch P, Raue F, Conte-Devolx B, Dralle H 2003 Early malignant progression of hereditary medullary thyroid cancer. N Engl J Med 349:1517–1525 [DOI] [PubMed] [Google Scholar]
- Gibson WC, Peng TC, Croker BP 1981 C-cell nodules in adult human thyroid. A common autopsy finding. Am J Clin Pathol 75:347–350 [DOI] [PubMed] [Google Scholar]
- O'Toole K, Fenoglio-Preiser C, Pushparaj N 1985 Endocrine changes associated with the human aging process. III. Effect of age on the number of calcitonin immunoreactive cells in the thyroid gland. Hum Pathol 16:991–1000 [DOI] [PubMed] [Google Scholar]
- Scopsi L, Di Palma S, Ferrari C, Holst JJ, Rehfeld JF, Rilke F 1991 C-cell hyperplasia accompanying thyroid diseases other than medullary carcinoma: an immunocytochemical study by means of antibodies to calcitonin and somatostatin. Mod Pathol 4:297–304 [PubMed] [Google Scholar]
- de Krijger RR 2007 Early and precursor lesions in endocrine pathology: innocent lambs or wolves in sheep’s clothing? Pathobiology 74:277–278 [DOI] [PubMed] [Google Scholar]
- Libbey NP, Nowakowski KJ, Tucci JR 1989 C-cell hyperplasia of the thyroid in a patient with goitrous hypothyroidism and Hashimoto’s thyroiditis. Am J Surg Pathol 13:71–77 [DOI] [PubMed] [Google Scholar]
- Albores-Saavedra J, Monforte H, Nadji M, Morales AR 1988 C-cell hyperplasia in thyroid tissue adjacent to follicular cell tumors. Hum Pathol 19:795–799 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.