Abstract
Context: Mitochondrial dysfunction has been proposed as an underlying mechanism in the pathogenesis of insulin resistance and type 2 diabetes mellitus.
Objective: To determine whether mitochondrial dysfunction plays a role in the free fatty acid (FFA)-induced impairment in insulin action in skeletal muscle of healthy subjects.
Design: Eleven lean normal glucose tolerant individuals received 8 h lipid and saline infusion on separate days with a euglycemic insulin clamp during the last 2 h. Vastus lateralis muscle biopsies were performed at baseline and after 6 h lipid or saline infusion. Inner mitochondrial membrane potential (Ψm) and mitochondrial mass were determined ex vivo by confocal microscopy.
Results: Compared with saline infusion, lipid infusion reduced whole-body glucose uptake by 22% (P < 0.05). Ψm decreased by 33% (P < 0.005) after lipid infusion and the decrement in Ψm correlated with change in plasma FFA after lipid infusion (r = 0.753; P < 0.005). Mitochondrial content and morphology did not change after lipid infusion. No significant changes in genes expression, citrate synthase activity, and total ATP content were observed after either lipid or saline infusion.
Conclusions: Short-term physiological increase in plasma FFA concentration in lean normal glucose tolerant subjects induces insulin resistance and impairs mitochondrial membrane potential but has no significant effects on mitochondrial content, gene expression, ATP content, or citrate synthase activity.
Short-term elevation in fatty free acid concentrations in healthy individuals decreases insulin sensitivity and mitochondrial inner membrane potential without significant effects on key mitochondrial gene expression.
Insulin resistance is a key metabolic defect in type 2 diabetes mellitus (T2DM) and precedes the onset of overt hyperglycemia by decades (1,2). Studies in humans and rodents consistently have demonstrated that experimental elevation in free fatty acids (FFA) in healthy subjects reduces insulin-stimulated glucose uptake in a dose-dependent manner (3). However, the mechanisms by which FFA induce insulin resistance are not fully understood. Intramyocellular accumulation of toxic FFA metabolites, i.e. fatty acyl-coenzyme A, diacylglycerol, and ceramides, has been shown to impair insulin signal transduction, glucose transport/phosphorylation, and glycogen synthesis (4,5,6).
Because skeletal muscle accounts for the majority (70–80%) of insulin-mediated glucose uptake in the postprandial state (7), skeletal muscle has been the focus of insulin resistance. Skeletal muscle relies on mitochondrial oxidative phosphorylation for ATP production. Mitochondrial dysfunction has been reported in skeletal muscle in patients with T2DM and in normal glucose tolerant (NGT) offspring of T2DM parents (8,9,10). Mitochondria from T2DM patients exhibit structural as well as functional abnormalities (11,12). Genes involved in oxidative phosphorylation are down-regulated in skeletal muscle in T2DM (13,14), and elevated plasma FFA levels have been linked to mitochondrial dysfunction in T2DM and obesity (15). Physiological elevation of plasma FFA for 48 h down-regulates multiple genes involved in mitochondrial biogenesis (16). Although prolonged 48-h lipid infusion has been shown to reduce mitochondrial gene expression, it is unclear whether short-term plasma FFA elevation induces mitochondrial dysfunction and inhibits mitochondrial gene expression. It also remains to be established whether the elevated plasma FFA concentration seen in T2DM is the cause or consequence of mitochondrial dysfunction.
A recent study demonstrated that short-term plasma FFA elevation impaired insulin-stimulated ATP synthesis in skeletal muscle of healthy subjects (17). Mitochondrial function, and thus ATP synthesis, is dependent on the proton motive force (Δp), which is determined by the voltage gradient across the inner mitochondrial membrane (ψm) and the proton gradient (ΔpH) generated by the respiratory chain (18). The inner mitochondrial membrane potential, which is related to the state of mitochondrial energization in muscle, can be measured with tetramethylrhodamine ethyl ester (TMRE) which accumulates in mitochondria in response to change in ψm (19). We hypothesized that FFA-induced insulin resistance is closely related to disruption of the inner mitochondrial membrane potential and reduced mitochondrial gene expression. In the present study, we demonstrate that short-term lipid infusion significantly reduces inner mitochondrial membrane potential in healthy NGT subjects.
Subjects and Methods
Research subjects and study design
Eleven healthy volunteers (eight males, three females; age = 32 ± 2 yr; body mass index = 24.4 ± 1 kg/m2; percent body fat > 21 ± 1.3) without family history of diabetes participated in the study. Fasting plasma glucose (92 ± 2 mg/dl), glycosylated hemoglobin (5.2 ± 0.1%), fasting plasma insulin (5 ± 1 μU/ml), and plasma total, low-density lipoprotein, and high-density lipoprotein cholesterol levels were normal in all subjects. All volunteers gave their written, informed consent before enrollment, and the study protocol was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio.
All subjects had a normal 75-g oral glucose tolerance test (2-h plasma glucose = 94 ± 7 mg/dl) according to current American Diabetes Association criteria. During the oral glucose tolerance test, blood was collected at −30, −15, 0, and every 15 min thereafter for measurement of plasma glucose and insulin concentrations.
Subjects were not taking any medications and were in general good health based upon medical history, physical examination, blood chemistries, urinalysis, lipid panel, complete blood count, and electrocardiogram. No subject participated in a heavy exercise program, and weight was stable (±3 lbs) over the 6 months before study. Fat-free mass (FFM) and percent body fat were measured by dual-energy x-ray absorptiometry (GE Lunar Corp., Madison, WI). Each subject received in random order at least 4–6 wk apart an 8-h saline and lipid infusion with a hyperinsulinemic euglycemic clamp to quantitate insulin-stimulated glucose disposal during h 6–8.
Lipid/saline infusion protocol and euglycemic insulin clamp
On the day of the study, subjects arrived at 0600 h after a 10- to 12-h overnight fast. Baseline blood samples were obtained for insulin, C peptide, and FFA measurements, and a percutaneous biopsy of the vastus lateralis muscle was performed under local anesthesia, as previously described (3). A 20% neutral triglyceride solution (Liposyn III; Hospira Inc., Lake Forest, IL) containing 54.5% linoleic, 22.4% oleic, 10.5% palmitic, 4.2% stearic, and 8.3% linolenic acid, or 0.9% NaCl solution was then started at a rate of 60 ml/h for 8 h, and blood samples were obtained at 2-h intervals for FFA measurement. A second muscle biopsy was performed after 6 h lipid/saline infusion. The 2-h euglycemic insulin clamp was performed during h 6–8. All subjects received a primed-continuous insulin infusion at 80 mU/m2 · min. During insulin administration, plasma glucose was maintained at each subject’s fasting glucose level with a coefficient of variation of less than 5%. Hepatic glucose production was not measured because at this insulin infusion rate, endogenous glucose production is suppressed by more than 95% in NGT individuals (20).
Carbohydrate and lipid oxidation were measured by continuous indirect calorimetry (Deltatrac; Sensormedics, Anaheim, CA) during the 40-min period before the start of the lipid/saline infusion and during the last 40 min of the insulin clamp. Upon completion of study, patients were fed and discharged from the unit.
Ex vivo measurement of inner mitochondrial membrane potential and mitochondrial mass
Mitochondria were labeled using mitochondria-specific dyes, TMRE (Invitrogen, Carlsbad, CA) for ex vivo quantitation of mitochondrial inner membrane potential (ψm) and MitoTracker Deep Red (Invitrogen) for measurement of mitochondrial mass, using confocal laser scanning microscopy. TMRE accumulates in cells in a Nernstian fashion in response to the inner mitochondrial membrane potential (19,21). This method provides a semiquantitative measurement of inner mitochondrial membrane potential and mitochondrial content. Muscle specimens (∼2 × 2 mm) were immediately incubated for 30 min at 37 C in Krebs-Ringer solution containing TMRE (150 nm) and MitoTracker Deep Red (150 nm) in separate vials. Specimens were scanned using an Olympus FluoView FV500 confocal laser scanning system (Olympus America, Center Valley, PA). For each experiment, an average of 10 images were recorded and digitally stored for further analysis. Fluorescence intensity (FI) measurements were performed using Image J version 1.37 image analysis software (National Institutes of Health, Bethesda, MD). Briefly, ψm was calculated as a function of the ratio between the FI of mitochondria and cytosol, adjusted for the background fluorescence (TMRE solution): ψm = log(FImitochondria − FIbackground/FIcytosol − FIbackground).
For mitochondrial content, MitoTracker Deep Red FI was measured in mitochondria, cytosol and calculated as the ratio between FImitochondria/FIcytosol, upon correction for background fluorescence.
Skeletal muscle ATP content and citrate synthase activity
ATP content was measured in homogenized skeletal muscle using a luciferase assay in a Synergy HT multidetection microplate reader (Bio-Tek Instruments Inc., Winooski, VT), as previously described (22). Citrate synthase was assayed in skeletal muscle whole-cell extracts in duplicate using a commercial assay (Sigma-Aldrich, St. Louis, MO) based on the modified method of Srere (23).
RNA extraction and quantitative RT-PCR
Frozen skeletal muscle samples were homogenized and total RNA was isolated using Trizol reagents (Invitrogen), purified with RNeasy (QIAGEN Sciences, Germantown, MD), quantified by absorbance at 250 nm and integrity determined by RNA 6000 Nano Chips (Agilent Technologies, Waldbronn, Germany). RNA expression of five mitochondrial encoded genes [MT-ND3 (NADH dehydrogenase subunit 3), MT-ND5 (NADH dehydrogenase subunit 5), MT-ATP8 (ATP synthase 8), Cytochrome b, and Cytochrome C oxidase subunit 2) (gene assays on demand Hs02596876_s1, Hs02596878_s1, Hs02596863_g1, Hs02596864_g1, Hs02596867, respectively) and eight nuclear encoded genes [COX 18 (Cytochrome C oxidase assembly homolog), CS (Citrate synthase), MFN (Mitofusin-1), IMMT (inner mitochondrial membrane protein-Mitofilin), UCP-2 (uncoupling protein-2), UCP-3 (uncoupling protein-3), PGC-1α (peroxisome proliferator-activated receptor γ, coactivator α), and PGC-1β (peroxisome proliferator-activated receptor γ, coactivator β)] (gene assays on demand Hs00403166_m1, Hs00830726_s1, Hs00250475_m1, Hs00272794_m1, Hs00163349_m1, Hs01106052_m1, Hs00173304_m1, Hs00991677_m1, respectively) were measured by TaqMan RT-PCR using an ABI PRISM 7900HT system (Applied Biosystems, Foster City, CA) and normalized to 18S rRNA. Each reaction was carried out in duplicate and the analysis performed by the 2−ΔΔCT method (24).
Electron microscopy
Immediately after the biopsy, skeletal muscle from each subject was divided in small sections (∼1 × 1 × 2 mm), fixed overnight, processed, and scoped in a JEOL JEM-1230 electron microscope (JEOL Ltd., Tokyo, Japan) at ×15,000 and ×30,000 magnification, as previously described (12).
Analytical procedures
Plasma glucose concentration was determined by the glucose oxidase reaction (glucose oxidase analyzer; Beckman, Fullerton, CA), and plasma insulin concentration was measured by RIA (Coat A Coat; Diagnostic Products Corp., Los Angeles, CA). Plasma samples were frozen immediately and stored at −80C until analyzed. Plasma FFA concentration was measured by standard colorimetric methods (Wako Chemicals, Neuss, Germany).
Calculations
Insulin sensitivity was calculated as the mean glucose infusion rate (milligrams per kilogram per minute) necessary to maintain euglycemia during the last 30 min of the euglycemic-hyperinsulinemic clamp. Net glucose and lipid oxidation rates were calculated from oxygen consumption (VO2) and carbon dioxide production (VCO2) using standard calorimetric equations (25). Nonoxidative glucose disposal, which primarily represents glycogen synthesis, was calculated by subtracting the rate of glucose oxidation (measured by indirect calorimetry) from the rate of total-body glucose disposal.
Statistical analysis
Unless otherwise stated, data represent the mean ± se. Differences between saline and lipids within a group were determined by the paired Student’s t test using SPSS for Windows (version 15.0; SPSS, Chicago, IL). Comparisons between lipid and saline interventions were performed with ANOVA with repeated measures. Correlation between variables of interest was performed using either Pearson’s or Spearman’s correlation where appropriate. A P value ≤ 0.05 was considered statistically significant.
Results
Plasma FFA and insulin concentrations
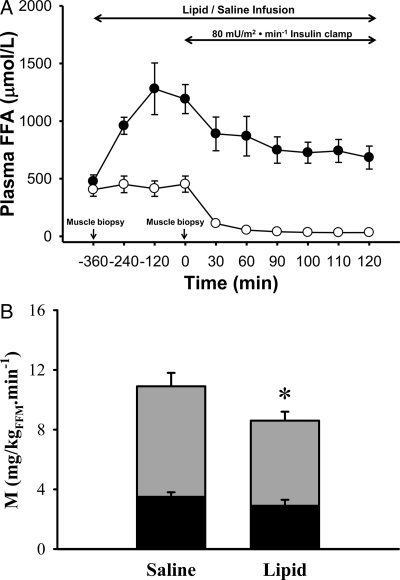
During lipid infusion, plasma FFA concentration rose from 476 ± 57 to 959 ± 74 at 2 h to 1280 ± 224 μmol/liter at 6 h (P < 0.005) and declined to 757 ± 55 μmol/liter 2 h after insulin infusion. Plasma FFA concentrations did not change significantly during saline infusion (405 ± 57 vs. 453 ± 70 μmol/liter at 6 h, P value not significant) and declined to 50 ± 6 μmol/liter after insulin infusion (Fig. 1A). Steady-state plasma insulin concentrations during the euglycemic clamp during lipid (91 ± 15 μU/ml) or saline (101 ± 20 μU/ml) infusions were similar.
Figure 1.
A, Plasma FFA concentration during lipid (•) and saline (○) infusion study visits; B, insulin-mediated total-body glucose disposal (total height of bars), glucose oxidation (black portion of bars), and nonoxidative glucose disposal, which primarily represents muscle glycogen synthesis (gray portion of bars). *, P < 0.05–0.01, lipid vs. saline infusion.
Whole-body insulin-stimulated rate of glucose disposal
Whole-body insulin-stimulated rate of glucose disposal (M), which primarily reflects muscle, decreased after lipid infusion (10.9 ± 0.9 vs. 8.7 ± 0.6 mg/kg FFM · min (P < 0.01, Fig. 1B). The decline in insulin sensitivity correlated with the increment in plasma FFA concentration (r = 0.72; P < 0.05). Basal glucose oxidation (0.84 ± 0.04 and 0.87 ± 0.06 mg/kg FFM · min) was similar during saline and lipid studies. Glucose oxidation was significantly reduced during the insulin clamp preceded by lipid infusion (0.96 ± 0.03 vs. 2.25 ± 0.5 mg/kg FFM · min, P < 0.01). As expected, lipid oxidation was higher (2.2 ± 0.1 vs. 1.3 ± 0.3 mg/kg FFM · min, P = 0.01) during the insulin clamp preceded by lipid infusion vs. insulin clamp after saline infusion. During the insulin clamp preceded by lipid infusion, the decrease in insulin-mediated glucose disposal primarily was accounted by decreased nonoxidative (7.5 ± 0.7 vs. 5.9 ± 0.7 mg/kg FFM · min, P < 0.05) glucose disposal (Fig. 1B).
Inner mitochondrial membrane potential and mitochondrial content
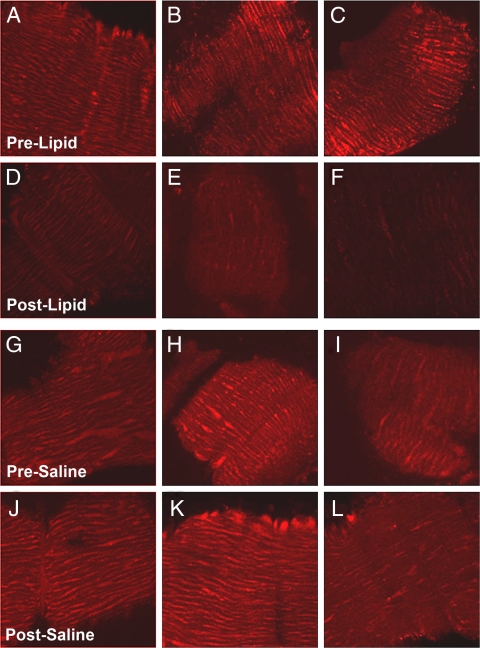
Mitochondria membrane potential (ψm), measured as TMRE FI, decreased by 33% from 0.442 ± 0.03 to 0.299 ± 0.02 arbitrary units (AU) (P < 0.005, Fig. 2) after 6 h lipid infusion, whereas no change in TMRE fluorescence was noted after saline infusion (0.352 ± 0.01 vs. 0.364 ± 0.01, P value not significant). The decrement in ψm correlated with change in plasma FFA concentration (ΔFFA) during lipid infusion (r = 0.753; P < 0.005). The decrement in ψm also correlated with change in glucose oxidation rate after lipid/saline infusion (r = 0.479; P = 0.02). TMRE fluorescence after lipid infusion also correlated with the decrement in insulin-stimulated glucose disposal after lipid infusion (r = 0.751; P < 0.05). Total mitochondrial content, quantitated with Mitotracker Deep Red, did not change after lipid infusion (0.527 ± 0.2 to 0.449 ± 0.07 AU, P value not significant).
Figure 2.
Representative images from skeletal muscle sections stained with TMRE and laser scanned using confocal microscopy before (A–C) and after (D–F) lipid infusion and before (G–I) and after (J–L) saline infusion.
Real-time quantitative RT-PCR for RNA and mitochondrial DNA (mtDNA)
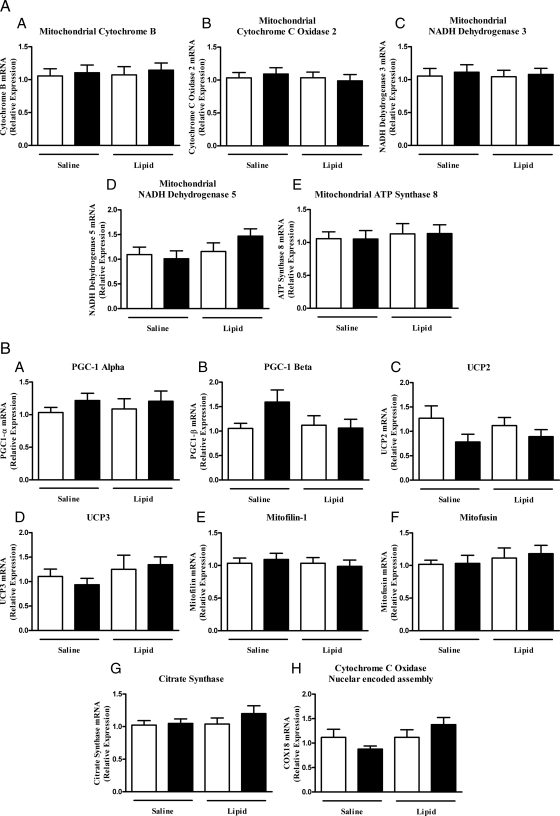
Because coregulated sets of nuclear and mitochondrial encoded genes are usually altered in T2DM, we quantified the mRNA expression by quantitative RT-PCR for mitochondrial-encoded (Fig. 3A) and nuclear-encoded (Fig. 3B) genes before and after lipid and saline infusion. There were no significant changes in any of the mitochondrial-encoded or nuclear-encoded genes after lipid or saline infusion. However, there was a trend toward increase in PGC-1β gene expression after saline infusion (P = 0.07 vs. saline baseline), and lipid infusion tends to blunt this effect. We also examined whether the mtDNA content (expressed as mtDNA copies relative to nuclear DNA copies) was affected by lipid infusion, and no significant changes in mtDNA were found after lipid infusion (2297 ± 439 vs. 2508 ± 414; before and after intervention, respectively).
Figure 3.
Relative mRNA expression of mitochondrial (A) and nuclear (B) encoded genes related to mitochondrial function at baseline (white bars) and after either lipid or saline infusion (black bars).
ATP content and citrate synthase activity
Total ATP content in whole extracts did not differ at baseline between the saline and the lipid infusion (274 ± 50 vs. 240 ± 71 AU, P value not significant), and no significant difference in muscle ATP content was observed after saline or lipid infusion (159 ± 35 vs. 192 ± 23 AU, P value not significant). There was a trend for total ATP content to decline after both lipid and saline infusion, with a slightly greater decline after saline vs. lipid infusion. Citrate synthase activity was similar at baseline in both lipid and saline experiments (data not shown). After lipid infusion, citrate synthase activity trended to increase. No change in citrate synthase activity was observed after saline infusion.
Electron microscopy
Since mitochondrial content and morphology have been reported to be altered with short-term interventions, including high-fat diet and intensive exercise (24), we examined mitochondrial content and morphology by electron microscopy in skeletal muscle before and after short-term elevation of FFA concentrations. The total area occupied by mitochondria, number of mitochondria in the subsarcolemmal and intermyofibrillar regions, and average area per mitochondria did not change significantly after either lipid infusion or saline. No changes in subsarcolemmal or intramyofibrillar mitochondrial morphology and content were observed after both interventions.
Discussion
In the present study we show, for the first time, that a short-term, physiological increase in plasma FFA concentration decreases the inner mitochondrial membrane potential, and that the decrease in membrane potential is related to increment in plasma FFA concentration. There was, however, no effect of lipid infusion on mitochondrial morphology, representative genes involved in mitochondrial function, ATP content, or citrate synthase activity.
Mitochondrial ATP synthesis is dependent on the voltage potential gradient across the inner mitochondrial membrane, which provides the electromotive driving force for ATP synthesis. The inner mitochondrial membrane potential is dependent upon normal function of the electron transport chain (18). Thus, inhibition of electron transport chain could represent an important mechanism via which elevated plasma FFA concentration decreases mitochondrial membrane potential and impairs ATP synthesis.
Although we demonstrated that elevation of the plasma FFA concentration is associated with a decrease in the estimated inner mitochondrial membrane potential, the mechanisms via which increased plasma FFA affect the inner mitochondrial membrane remain to be defined. In addition to producing energy, mitochondria are a major site of reactive oxygen species (ROS) generation (26). ROS have a very short half-life and react rapidly with proteins, DNA, and lipids, leading to oxidative damage (27). Fatty acids are particularly prone to oxidative damage and lead to the formation of peroxide products that are toxic and can reduce the inner mitochondrial membrane (28). Recent in vitro studies have shown that incubation of L-6 skeletal muscles with palmitate can lead to increased ROS generation and myocyte apoptosis leading to reduced mtDNA copy number (29). Increased plasma FFA concentrations also have been shown to increase ROS generation in mononuclear cells in humans (30). FFA also act as uncouplers and cause up-regulation of UCP-3 in the skeletal muscle in healthy subjects (31). Up-regulation of UCP-3 may lead to a decrease in inner mitochondrial membrane potential (32). However, UCP-2 and UCP-3 mRNA were not up regulated in the current study and cannot explain the decrease in inner mitochondrial membrane potential that we observed.
A strength of the current study is that we examined the direct effect of lipid infusion on mitochondrial function as opposed to all previous studies in humans in which the effect of lipid infusion on mitochondrial function was examined in the insulin-stimulated state (17).
The lack of effect of lipid infusion on mitochondrial morphology (electron microscopy) and mitochondrial content (Mitotracker Deep Red assay) indicates that the short-term effect of plasma elevation is a functional defect that is unrelated to gene expression and should be reversible after the lipid challenge is withdrawn. Recently, it has been demonstrated that skeletal muscle mitochondria respond in a biphasic manner to increasing FFA concentrations ex vivo (33). Although increased ATP synthesis is seen with lower FFA concentrations (0–2 μm), higher FFA concentrations (75 μm) inhibited ATP synthesis. Thus, it is possible that either a longer duration of lipid infusion or a higher plasma FFA concentration might lead to impairment in ATP synthesis. It should be noted that we did not measure ATP synthesis rate but rather the total ATP content in the skeletal muscle biopsies. During saline infusion, there was a trend to a greater decline in ATP content compared with lipid infusion. This could be explained, in part, by the fact that during saline infusion, mitochondria are in the resting state associated with a decline in ATP content, whereas after lipid infusion, increased substrate availability for ATP synthesis resulted in a slightly higher ATP content. It will be of interest to examine the effect of insulin on ATP synthesis rate after lipid infusion to see whether under insulin-stimulated conditions ATP generation is inhibited as was observed by Brehm et al. (17).
The present study demonstrates discordance between the change in mitochondrial membrane potential and mitochondrial mRNA gene expression and mitochondrial function. In a previous study, we demonstrated that prolonged (48 h) lipid infusion to elevate the plasma FFA concentration reduced the expression of genes involved in oxidative phosphorylation (16). A high-fat diet in healthy volunteers also has been shown to decrease the expression of multiple genes involved in oxidative phosphorylation (24). It is likely, therefore, that the short duration of lipid infusion in the current study may not have allowed sufficient time to detect a change in mRNA expression. However, in support of our observations, several recent studies have noted discordance between mitochondrial content/function and insulin sensitivity (34,35). Furthermore, when PGC-1 was overexpressed in mice, it led to increased expression of genes regulating mitochondrial function, yet the mice were more insulin resistant (35). Of interest, there was a clear trend toward an increased expression of PGC-1β after saline infusion and a slight decrease after lipids (Fig. 3B). This compensatory increase in PGC-1β suggests that PGC-1β plays an important role in regulation of glucose homeostasis during prolonged fasting. Elevation of FFA levels during lipid infusion appears to inhibit the rise in PGC-1β, thereby leading to mitochondrial dysfunction. This finding is consistent with previous reports that highlight the key role of PGC-1β in mitochondrial biogenesis, function, and thermogenesis (36,37).
A limitation of the present study is that we did not examine the effect of lipid infusion on insulin-stimulated mitochondrial function. Stump et al. (38) have shown that insulin infusion for 8 h up-regulates mitochondrial-encoded genes and increases the ATP production rate. It is possible that lipid-induced mitochondrial dysfunction could be more apparent if muscle were examined in the insulin-stimulated state. On the other hand, an advantage of our study design is that it examines the effect of lipid infusion only, because insulin infusion per se has been shown to up-regulate multiple genes and, thus, is likely to confound the effect of elevated plasma FFA (39). Because insulin sensitivity was measured around 1500–1700 h, it is possible that the circadian rhythm of hormones (i.e. GH and cortisol) could influence insulin sensitivity. However, each subject acted as his/her own control (saline infusion); thus, potential effects of the hormonal changes on insulin sensitivity are expected to be the same during each intervention.
Considerable controversy exists as to whether the decreased lipid oxidation seen in T2DM is a consequence or cause of mitochondrial dysfunction/deficiency (40). Our study clearly demonstrates that short-term lipid infusion can lead to early changes in mitochondrial membrane potential but has no effect on other parameters of mitochondrial function. It is likely, therefore, that insulin resistance and decreased inner mitochondrial membrane potential seen after short-term elevated plasma FFA concentrations are transient and reversible and at least partly can be explained by mechanisms independent of mitochondrial dysfunction.
In conclusion, short-term elevations of plasma FFA concentrations induce early changes in mitochondrial membrane potential, without altering gene expression, mitochondrial morphology, or content. These effects provide a potential link between elevated plasma FFA levels and insulin resistance.
Acknowledgments
We thank the nurses on the General Clinical Research Center (GCRC) for their diligent care of our patients, especially James King, R.N.; John Kincade, R.N.; Rose Kaminski, R.N.; and Norma Diaz, R.N., for carrying out the insulin clamp studies. Jimmy Wewer provided skilled technical support with the confocal microscopy experiments. Lorrie Albarado and Kimberly Delgado provided skilled secretarial support in the preparation of this manuscript.
Footnotes
This study was funded in part by a GCRC CREF grant (D.T.) and National Institutes of Health Grant DK-24092 (R.A.D.). Images were generated in the Core Optical Imaging Facility, which is supported by University of Texas Health Science Center at San Antonio, NIH-NCI P30 CA54174 (San Antonio Cancer Institute), NIH-NIA P30 AG013319 (Nathan Shock Center), and (NIH-NIA P01AG19316). A.O.C. was supported by a Mentor-Based Postdoctoral Fellowship from the American Diabetes Association awarded to R.A.D.
Disclosure Summary: Authors declare no conflict of interest.
First Published Online October 28, 2009
Abbreviations: AU, Arbitrary units; FFA, free fatty acids; FFM, fat-free mass; FI, fluorescence intensity; mtDNA, mitochondrial DNA; NGT, normal glucose tolerant; ROS, reactive oxygen species; T2DM, type diabetes mellitus; TMRE, tetramethylrhodamine ethyl ester.
References
- DeFronzo RA 2004 Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 88:787–835, ix [DOI] [PubMed] [Google Scholar]
- Tripathy D, Eriksson KF, Orho-Melander M, Fredriksson J, Ahlqvist G, Groop L 2004 Parallel manifestation of insulin resistance and β-cell decompensation is compatible with a common defect in type 2 diabetes. Diabetologia 47:782–793 [DOI] [PubMed] [Google Scholar]
- Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, Defronzo RA, Cusi K 2005 Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes 54:1640–1648 [DOI] [PubMed] [Google Scholar]
- Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI 1999 Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI 1999 Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42:113–116 [DOI] [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI 1996 Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA 1988 Lilly lecture 1987. The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37:667–687 [DOI] [PubMed] [Google Scholar]
- Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, Bray GA, Smith SR 2007 Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes 56:720–727 [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB 2002 Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI 2004 Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS 2006 Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 55:3309–3319 [DOI] [PubMed] [Google Scholar]
- Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE 2005 Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54:8–14 [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC 2003 PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature genetics 34:267–273 [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ 2003 Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M 2007 Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 4:e154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DK, Kashyap S, Bajaj M, Cusi K, Mandarino SJ, Finlayson J, DeFronzo RA, Jenkinson CP, Mandarino LJ 2005 Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem 280:10290–10297 [DOI] [PubMed] [Google Scholar]
- Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhäusl W, Roden M 2006 Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 55:136–140 [PubMed] [Google Scholar]
- Brand MD, Reynafarje B, Lehninger AL 1976 Stoichiometric relationship between energy-dependent proton ejection and electron transport in mitochondria. Proc Natl Acad Sci USA 73:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaduto Jr RC, Grotyohann LW 1999 Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 76:469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA 1989 Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ, Chacon E, Ohata H, Harper IS, Nieminen AL, Tesfai SA, Herman B 1995 Measurement of electrical potential, pH, and free calcium ion concentration in mitochondria of living cells by laser scanning confocal microscopy. Methods Enzymol 260:428–444 [DOI] [PubMed] [Google Scholar]
- Manfredi G, Spinazzola A, Checcarelli N, Naini A 2001 Assay of mitochondrial ATP synthesis in animal cells. Methods Cell Biol 65:133–145 [DOI] [PubMed] [Google Scholar]
- Srere PA 1968 Studies on purified citrate-enzymes: metabolic interpretations. Biochem Soc Symp 27:11–21 [PubMed] [Google Scholar]
- Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR 2005 A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54:1926–1933 [DOI] [PubMed] [Google Scholar]
- Simonson DC, DeFronzo RA 1990 Indirect calorimetry: methodological and interpretative problems. Am J Physiol 258:E399–E412 [DOI] [PubMed] [Google Scholar]
- Halliwell B 1999 Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic Res 31:261–272 [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J 2003 Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17:1195–1214 [DOI] [PubMed] [Google Scholar]
- Gadelha FR, Thomson L, Fagian MM, Costa AD, Radi R, Vercesi AE 1997 Ca2+-independent permeabilization of the inner mitochondrial membrane by peroxynitrite is mediated by membrane protein thiol cross-linking and lipid peroxidation. Arch Biochem Biophys 345:243–250 [DOI] [PubMed] [Google Scholar]
- Rachek LI, Musiyenko SI, LeDoux SP, Wilson GL 2007 Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in l6 rat skeletal muscle cells. Endocrinology 148:293–299 [DOI] [PubMed] [Google Scholar]
- Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P 2003 Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 52:2882–2887 [DOI] [PubMed] [Google Scholar]
- Khalfallah Y, Fages S, Laville M, Langin D, Vidal H 2000 Regulation of uncoupling protein-2 and uncoupling protein-3 mRNA expression during lipid infusion in human skeletal muscle and subcutaneous adipose tissue. Diabetes 49:25–31 [DOI] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD 2002 Superoxide activates mitochondrial uncoupling proteins. Nature 415:96–99 [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, Chang Z, Tripathy D, Jani R, Molina-Carrion M, Monroy A, Folli F, Van Remmen H, DeFronzo RA 2008 Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab 295:E678–E685 [DOI] [PubMed] [Google Scholar]
- Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, Esterbauer H, Kozlov A, Kahn CR, Kroemer G, Rustin P, Burcelin R, Penninger JM 2007 Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 131:476–491 [DOI] [PubMed] [Google Scholar]
- Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, Zhang D, Cline GW, Handschin C, Lin J, Petersen KF, Spiegelman BM, Shulman GI 2008 Paradoxical effects of increased expression of PGC-1α on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA 105:19926–19931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly-Y M, Storlien L, Strömstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig A 2006 Ablation of PGC-1β results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4:e369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna CR, Huntgeburth M, Coppari R, Choi CS, Lin J, Krauss S, Barbatelli G, Tzameli I, Kim YB, Cinti S, Shulman GI, Spiegelman BM, Lowell BB 2006 Hypomorphic mutation of PGC-1β causes mitochondrial dysfunction and liver insulin resistance. Cell Metab 4:453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS 2003 Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100:7996–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta DK, Balas B, Chavez AO, Baig M, Abdul-Ghani M, Kashyap SR, Folli F, Tripathy D, Mandarino LJ, Cornell JE, Defronzo RA, Jenkinson CP 2008 Effect of acute physiological hyperinsulinemia on gene expression in human skeletal muscle in vivo. Am J Physiol Endocrinol Metab 294:E910–E917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO 2009 Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr 89:463S–466S [DOI] [PubMed] [Google Scholar]