Abstract
Context: Despite having low visceral and sc fat depots, women with anorexia nervosa (AN) have elevated marrow fat mass, which is inversely associated with bone mineral density (BMD). Adipocytes and osteoblasts differentiate from a common progenitor cell, the human mesenchymal stem cell. Therefore, understanding factors that regulate this differentiation process may provide insight into bone loss in AN.
Objective: The objective of the study was to investigate the relationship between preadipocyte factor-1 (Pref-1), a member of the epidermal growth factor-like family of proteins and regulator of adipocyte and osteoblast differentiation, and fat depots and BMD in AN.
Design: This was a cross-sectional study.
Setting: The study was conducted at a clinical research center.
Patients: Patients included 20 women with AN (26.8 ± 1.5 yr) and 10 normal-weight controls (29.2 ± 1.7 yr).
Interventions: There were no interventions.
Main Outcomes Measure: Pref-1, leptin, IGF-I, IGF binding protein (IGF-BP)-2 and estradiol levels were measured. BMD of the spine and hip was measured by dual-energy x-ray absorptiometry. Marrow fat content of the L4 vertebra and femur was measured by 1H-magnetic resonance spectroscopy.
Results: Pref-1 levels were significantly higher in AN compared with controls (P = 0.01). There was a positive correlation between Pref-1 and marrow fat of the proximal femoral metaphysis (R = 0.50, P = 0.01) and an inverse association between leptin and L4 marrow fat (R = −0.45, P < 0.05). There was an inverse association between Pref-1 and BMD of both the anteroposterior spine and lateral spine (R = −0.54, P = 0.003; R = −0.44, P = 0.02, respectively).
Conclusions: Pref-1 is elevated in AN. Pref-1, IGF-I, IGF-BP2 and leptin are associated with marrow adiposity and BMD.
In women with anorexia nervosa, Pref-1 levels are elevated and Pref-1 and leptin are associated with marrow adiposity and bone mineral density.
Anorexia nervosa (AN) is a primary psychiatric disorder, characterized by extreme self-imposed starvation, affecting 0.5–1% of college-aged women in the United States (1,2). There are many significant medical complications and comorbidities associated with the disease, and bone loss is among the most common. An estimated 50% of women with AN have osteopenia, with an additional 35% having evidence of osteoporosis (3).
Low bone mass in adolescents and adults occurs in the setting of low sc and visceral fat depots (4), and loss of nutritionally dependent factors are important in the pathogenesis of bone loss. Recent advances in mesenchymal stem cell differentiation have demonstrated a possible inverse relationship between osteoblast differentiation and adipocyte differentiation (5,6). Because osteoblasts and adipocytes originate from a common progenitor, the human mesenchymal stem cell (hMSC), understanding the factors that potentially regulate the differentiation process of hMSCs into bone and fat may be of great importance in understanding clinical states of low bone mass.
We have previously shown that although peripheral and visceral fat stores are low in AN, bone marrow adiposity is increased and inversely associated with bone mineral density (BMD) (7). The clinical significance of bone marrow adiposity has been shown in a number of studies. For example, Schellinger et al. (8) demonstrated that individuals with radiographic evidence of vertebral bone weakness, including wedging of vertebrae or vertebral body compression fractures, had higher percentages of vertebral marrow fat content compared with those without such findings.
Little is known about the hormonal determinants of marrow fat. We therefore investigated preadipocyte factor-1 (Pref-1), an important factor in mesenchymal stem cell differentiation. We also investigated other hormonal mediators of low bone mass in AN including leptin, an adipokine linked to bone mass and decreased in AN, IGF-I, IGF binding protein (IGF-BP)-2 and estradiol. Pref-1 is a member of the epidermal growth factor (EGF)-like-family of proteins and is expressed in several progenitor cell types including hMSCs and preadipocytes (9). Pref-1 is present on the extracellular membrane and is cleaved before it is released into the extracellular space to exert suppressive effects in an autocrine and paracrine manner on adipocyte and osteoblast differentiation (10,11). Interestingly, Pref-1 circulates in relatively high concentrations, although it is unclear how the circulating levels relate to target tissues. IGF-I is a nutritionally dependent hormone that is known to stimulate bone formation through effects on osteoblastic function (12,13,14). IGF-BP2 is one of the six IGF-BPs that binds IGF-I in the circulation and has been previously shown to be abnormally elevated in adult women with AN (15,16,17) and has been shown to be inversely associated with markers of bone formation in AN (16). Although estrogen therapy has not been shown to increase BMD in women with AN (18,19), estrogen therapy in postmenopausal women has been shown to decrease marrow adipocyte volume and prevent increases in marrow adipocyte number (20). Leptin has been shown to increase trabecular bone volume and trabecular number in ovariectomized rats when administered peripherally (21). In humans with hypothalamic amenorrhea, a state characterized by low leptin levels, treatment with leptin has been shown to increase markers of bone formation including osteocalcin and bone-specific alkaline phosphatase (22). Given the phenotypic nature of AN with very little peripheral adipose tissue, low leptin, and reduced bone formation, we hypothesized that Pref-1, leptin, IGF-I, IGF-BP2 and estradiol would be associated with marrow adiposity in AN.
Subjects and Methods
Subjects
Thirty women were studied: 20 women with AN (aged 19–41 yr) and 10 normal-weight controls of comparable age (aged 25–42 yr). The 20 women with AN were recruited through referrals from local eating disorder providers and on-line advertisements, and the 10 normal-weight controls were recruited through on-line advertisements. Subjects met Diagnostic and Statistical Manual of Mental Disorders, fourth edition, weight and psychiatric criteria for AN. None of the subjects had received estrogen within 3 months of the study. All control subjects had a normal body mass index (BMI), a history of regular menstrual cycles and were receiving no medications known to affect bone mass. Control subjects did not have a past or present history of an eating disorder. Subjects with abnormal TSH, elevated FSH, chronic diseases known to affect BMD (other than AN), or diabetes mellitus were excluded from participation.
All subjects were examined and blood was drawn for laboratory studies at a single study visit at our Clinical Translational Science Center. Height was measured as the average of three readings on a single stadiometer, and subjects were weighed on an electronic scale while wearing a hospital gown. BMI was calculated using the formula [weight (kilograms)/height (meters)2].
The study was approved by the Partners Institutional Review Board and complied with the Health Insurance Portability and Accountability Act guidelines. Written informed consent was obtained from all subjects. The clinical characteristics and magnetic resonance imaging and dual-energy x-ray absorptiometry (DXA) data of nine subjects with AN and all of the control subjects have been previously reported (7).
Biochemical assessment
Pref-1 was measured with the Quantikine human Pref-1 immunoassay (ELISA) (R&D Systems, Minneapolis, MN) with a mean minimum detectable level of 0.012 ng/ml and intraassay coefficient of variation (CV) of 3.1–4.3%. RIA was used to measure serum leptin (Linco Diagnostics, Inc., St. Louis, MO; sensitivity 0.5 ng/ml, CV 3.4–8.3%). Estradiol was measured by automated immunoassay (ARCHITECT; Abbott Diagnostics, Chicago, IL) with a minimum reportable concentration of 10 pg/ml with interassay CV of 2–9.6%. IGF-I and IGF-BP2 were measured by RIA in the laboratory of David Clemmons (Chapel Hill, NC) as previously described (23). The intraassay CV for IGF-I was 3.3% and the interassay CV was 5.4% (23). For IGF-BP2, the intraassay CV was 5.6% and the interassay CV was 5.9% (23).
Radiological imaging
All control subjects and a subset of 10 women with AN underwent 1H-magnetic resonance spectroscopy of bone marrow of the L4 vertebral body, the proximal femoral epiphysis, metaphysis, and diaphysis to determine lipid content using a 3.0T magnetic resonance imaging system (Siemens Trio, Siemens Medical Systems, Erlangen, Germany); fitting of the 1H-magnetic resonance spectroscopy data were performed using LCModel software (version 6.1–4A; Stephen Provencher, Oakville, Ontario, Canada) as previously described (7). A single axial magnetic resonance imaging slice through the abdomen at the level of L4 and a single slice through the midthigh were obtained (Siemens Trio, 3T; Siemens Medical Systems) to determine abdominal sc adipose tissue (SAT), visceral adipose tissue (VAT), and total adipose tissue (TAT) as well as SAT of the thigh.
All subjects (20 with AN and 10 healthy controls) underwent DXA to measure BMD of the anteroposterior (AP) lumbar spine (L1-L4), lateral spine (L2-L4), distal radius, total hip, femoral neck, and total body and body composition including fat mass (kilograms), lean mass (kilograms), and percent body fat using a Discovery A densitometer (Hologic Inc., Waltham, MA). CVs of DXA have been reported as less than 1% for bone (24), 1.1% for lean body mass, and 2.7% for fat mass (25).
Statistical analysis
Statistical analysis was performed using JMP software (SAS Institute, Carry, NC). The means and sem measurements were calculated for AN and the control group, and the means were compared using the Student’s t test. Correlations are for the group as a whole unless otherwise noted. Where data were not normally distributed, we either performed a transformation to approximate a normal distribution or used nonparametric tests. Log transformations were performed for leptin, abdominal SAT, VAT, and TAT.
Results
Clinical characteristics
Clinical characteristics of the study subjects are presented in Table 1. Subjects with AN had lower weight, BMI, percent ideal body weight and percent body fat compared with controls.
Table 1.
Clinical characteristics of subjects with AN and healthy controls
| AN (n = 20) | Healthy controls (n = 10) | P value | |
|---|---|---|---|
| Age | 26.8 ± 1.5 | 29.2 ± 1.7 | 0.13 |
| Weight (kg) | 48.2 ± 1.0 | 61.7 ± 2.2 | <0.0001 |
| BMI | 17.6 ± 0.2 | 21.9 ± 0.5 | <0.0001 |
| Percent IBW | 78.3 ± 1.0 | 99.5 ± 1.8 | <0.0001 |
| AP spine BMD (g/cm2) | 0.84 ± 0.02 | 1.03 ± 0.03 | <0.0001 |
| Lateral spine BMD | 0.69 ± 0.02 | 0.80 ± 0.02 | 0.004 |
| Total hip BMD (g/cm2) | 0.80 ± 0.03 | 0.94 ± 0.03 | 0.004 |
| Total body BMD (g/cm2) | 1.03 ± 0.02 | 1.14 ± 0.04 | 0.04a |
| Percent body fat by DXA | 18.7 ± 1.1 | 27.6 ± 1.9 | 0.0002 |
| VAT of abdomenb | 23 ± 1.9 | 47.8 ± 6.2 | 0.0006a |
| SAT of abdomenb | 81.6 ± 6.8 | 170.2 ± 24.8 | 0.007 |
| TAT of abdomenb | 104.6 ± 8.1 | 218 ± 29.8 | 0.005 |
| SAT of thighb | 46.7 ± 5.2 | 83.6 ± 9.0 | 0.002 |
Wilcoxon test.
Data reported are for subset of 10 ANs.
Hormonal parameters
Pref-1, leptin, and estradiol
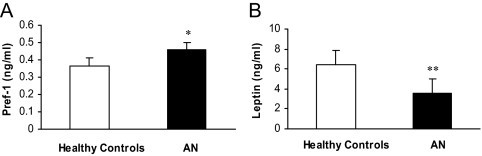
Pref-1 was significantly higher in AN compared with controls [AN: 0.46 ± 0.03 ng/ml (mean ± 1 sem) vs. control: 0.37 ± 0.02 ng/ml, P = 0.01] (Fig. 1A). Leptin was significantly lower in AN compared with controls (AN: 3.6 ± 0.5 ng/ml vs. control: 6.4 ± 1.3 ng/ml, P = 0.01) (Fig. 1B). Estradiol levels were significantly lower in AN than healthy controls (median estradiol level in AN ± interquartile range: 34.5 ± 58.5 pg/ml vs. controls: 152 ± 245 pg/ml, P = 0.02).
Figure 1.
Pref-1 was significantly higher in women with AN compared with healthy controls (*, P = 0.01) (A). Leptin was significantly lower in women with AN compared with healthy controls (**, P = 0.01 with logarithmic transformation) (B).
IGF-I and IGF-BP2
IGF-I was significantly lower in AN compared with controls (AN: 146.4 ± 11.1 ng/ml vs. control: 203.5 ± 17 ng/ml, P = 0.01). IGF-BP2 levels were significantly higher in AN compared with controls (AN: 188.3 ± 19.1 ng/ml vs. control: 131.4 ± 14.2 ng/ml, P = 0.03).
Hormonal parameters associated with body composition
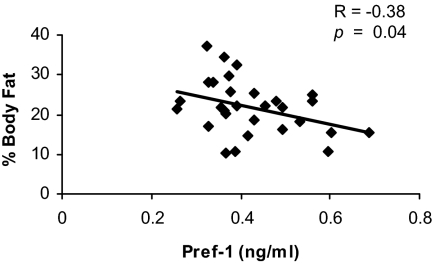
Percent body fat as measured by DXA, VAT and SAT were significantly lower in AN compared with the controls (Table 1). TAT as well as SAT of the thigh were also significantly lower in AN compared with controls (Table 1). There was an inverse correlation between Pref-1 and percent body fat (Fig. 2) and a positive association between leptin and percent body fat (R = 0.84, P < 0.0001), SAT of the abdomen (R = 0.63, P < 0.005), VAT of the abdomen (R = 0.52, P < 0.03), TAT of the abdomen (R = 0.63, P = 0.005), and SAT of the thigh (R = 0.76, P = 0.0002). There was a significant positive association between estradiol and percent body fat (Spearman’s rho: 0.49, P = 0.007). There was also a significant positive correlation between IGF-I and percent body fat (R = 0.51, P = 0.02). There were no correlations between IGF-I and VAT, SAT, or TAT of the abdomen or SAT of the thigh, and there were no significant correlations between IGF-BP2 and percent body fat or VAT, SAT, or TAT of the abdomen or SAT of the thigh.
Figure 2.
Pref-1 was inversely associated with percent body fat as measured by DXA.
Hormonal parameters associated with bone marrow fat content
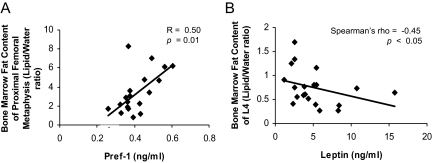
There was a positive correlation between Pref-1 and marrow fat of the proximal femoral metaphysis (R = 0.50, P = 0.01) (Fig. 3A). There was a negative correlation between leptin and marrow fat of L4 (Spearman’s rho = −0.45, P < 0.05) (Fig. 3B). There was a significant inverse association between IGF-I and marrow fat of L4 in AN (R = −0.70, P = 0.02) and a positive correlation between IGF-I and L4 marrow adiposity in the controls (R = 0.76, P = 0.01). There was a significant positive association between IGF-BP2 levels and marrow adiposity of L4 in the group as a whole (R = 0.46, P = 0.04). In healthy controls there was a significant inverse association between IGF-BP2 levels and marrow adiposity of L4 (R = −0.74, P = 0.01). There were no associations between estradiol levels and bone marrow fat content.
Figure 3.
Pref-1 was positively associated with marrow fat content of the proximal femoral metaphysis (A). Leptin was inversely associated with marrow fat content of L4 (B).
Hormonal parameters associated with BMD
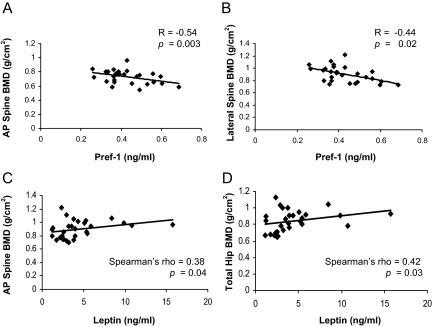
Subjects with AN had lower BMD of the total hip, AP spine, lateral spine, and total body compared with the controls (Table 1). There was an inverse correlation between Pref-1 and BMD of the AP spine (R = −0.54, P = 0.003) (Fig. 4A) and lateral spine (R = −0.44, P = 0.02) (Fig. 4B). Significant correlations were not found between Pref-1 and BMD of the hip, distal radius, or total body. Leptin was positively correlated with BMD of the AP spine (Spearman’s rho = 0.38, P = 0.04) (Fig. 4C) and hip (Spearman’s rho = 0.42, P = 0.03) (Fig. 4D). Significant correlations were not found between leptin and BMD of the lateral spine, distal radius, or total body. In AN, Pref-1 was inversely associated with BMD of the AP spine (R = −0.51, P = 0.02), and leptin was positively correlated with hip BMD (R = 0.50, P = 0.02). Estradiol levels were positively associated with BMD of the lumbar spine (Spearman’s rho: 0.39, P = 0.04). IGF-I was positively correlated with BMD of the AP spine (R = 0.46, P = 0.048) and BMD of the hip (R = 0.49, P = 0.03) in the group as a whole and with hip BMD in AN (R = 0.65, P = 0.04). IGF-BP2 was inversely associated with BMD of the lateral spine (R = −0.48, P = 0.04) and hip (R = −0.59, P = 0.008) in the group as a whole and with BMD of the hip in AN (R = −0.64, P = 0.048).
Figure 4.
Pref-1 was inversely associated with BMD of the AP spine (A) and lateral spine (B). Leptin was positively associated with BMD of the AP spine (C) and total hip (D).
Discussion
We have shown that women with AN have elevated levels of Pref-1. In addition, our data support the role of Pref-1 as a regulator of adipocyte and osteoblast differentiation. We have also shown that IGF-I is negatively associated with L4 marrow adiposity in AN in contrast to IGF-BP2, which is positively associated with L4 marrow fat. Our data, demonstrating an inverse association between leptin and L4 marrow adiposity, support the hypothesis that the role of marrow fat is distinct from that of sc and visceral fat depots.
AN is a psychiatric disorder characterized by extreme low body weight and is associated with multiple medical comorbidities including significant bone loss. Fracture risk is also significant in this population. A prospective study of 27 women with AN demonstrated a 7-fold increased risk of nonvertebral fracture during a mean of 2 yr of follow-up (26). A retrospective population-based study demonstrated a 3-fold increased risk of fracture many years after the initial diagnosis of AN, with the long-term cumulative incidence of any fracture being 57% (27). Thus, understanding the mechanisms of bone loss and the factors that regulate low bone mass in this population is of particular importance.
We have recently shown that women with AN have increased marrow fat content in the lumbar spine, femoral metaphysis, and diaphysis and that there is an inverse relationship between marrow adiposity and BMD at multiple skeletal sites (7). The clinical importance of bone marrow fat content has been demonstrated in a number of studies. Schellinger et al. (8) demonstrated that subjects with morphological evidence of bone weakness, such as Schmorl’s nodes, end plate depression, wedging of vertebrae, and/or compression fractures had elevated levels of vertebral marrow fat content. A recent study also demonstrated that marrow adiposity, unlike visceral and sc fat, is not associated with increased risk of cardiovascular disease, suggesting that the genesis of marrow fat is clinically distinct from that of visceral and sc fat (28).
Several studies have demonstrated an inverse relationship between marrow adiposity and BMD (6,29,30). Yet it is provocative that in AN, in which marrow adipose depots are elevated, visceral and sc fat depots are very low (4). This suggests that the role of marrow fat in the human is distinct from that of sc and visceral depots and may include the local regulation of bone formation.
Osteoblasts and adipocytes are derived from a common progenitor, hMSCs. Many factors have been shown to affect differentiation of the hMSC into either osteoblasts or adipocytes. Peroxisome proliferator-activated receptor-γ agonists and glucocorticoids have been shown to induce adipocyte differentiation (31,32), whereas in vitro studies have demonstrated that estrogen administration leads to osteoblastogenesis with concomitant inhibition of adipogenesis (33). Yet it is still unclear whether this process of differentiation is a switch process or independently regulated because there are states, such as puberty, during which both marrow adipocyte differentiation and osteoblast differentiation are increased, arguing against a mutually exclusive switch process. New evidence is also emerging that independent preosteoblast and preadipocyte populations of mesenchymal stem cells may exist, providing a possible explanation for states of concomitant osteogenesis and adipogenesis (34).
Pref-1, a member of the EGF-like family of proteins, has also been shown to be an important regulator of adipocyte and osteoblast differentiation. Pref-1 is highly expressed in osteoblastic cell lines, preadipocytes, and hMSCs (9) and is a negative regulator of adipocyte and osteoblast differentiation. Osteoblast-specific Pref-1 overexpression in a mouse model results in significantly low-body-weight mice and significantly reduced BMD (11). Moreover, Pref-1’s role as a regulator of energy stores has been further elucidated with the finding that overexpression of Pref-1 in a mouse model leads to lower adipose tissue mass than wild-type mice but increased insulin resistance (35). Therefore, it appears that Pref-1, an in vitro inhibitor of both osteoblast and adipocyte differentiation, may also be an important in vivo regulator of several metabolic processes.
Leptin, which is a major regulator of appetite, has been shown to be decreased in AN, most likely as a result of reduced total body fat (36). With respect to the skeleton, leptin acts centrally to enhance sympathetic tone and reduce bone formation, although leptin may also have direct effects on distinct skeletal sites (21,37,38). Our findings of a positive association between leptin and BMD in the low leptin state of AN are consistent with findings that leptin, when provided sc to women with low leptin levels, stimulates markers of bone formation (22) and with observational studies that demonstrate a positive association between leptin levels and bone mass in postmenopausal women (39). Our finding that leptin is inversely associated with marrow fat is also consistent with in vitro studies in which leptin blocks hMSC differentiation into adipocytes and stimulates osteogenesis (40). Whereas we found an inverse association between leptin and marrow fat at an axial site, we did not find an association between leptin and a peripheral site of marrow fat. One explanation for this may be that the sample size of our study population was simply too small to detect this difference. Another possible explanation is that leptin acts differentially at different marrow fat sites. Hamrick et al. (38) demonstrated that when compared with wild-type mice, leptin-deficient ob/ob mice had increased marrow adipocyte number in the femur, whereas in the vertebra there was decreased marrow adipocyte number. Therefore, it is possible that leptin is differentially associated with the various marrow depots in the human as well.
IGF-I is known to be a stimulator of osteoblastogenesis (12,13,14) and is known to be low in states of nutritional deprivation, such as AN (15,16), whereas IGF-BP2, a binding protein that binds to IGF-I in the circulation, has been shown to be elevated in adults with AN (15,16,17). We have shown that IGF-I is positively associated with BMD of the spine and hip and is inversely associated with L4 marrow adiposity in AN and that IGF-BP2 is positively associated with marrow adiposity. Interestingly, IGF-I was positively associated with L4 marrow adiposity in healthy controls, suggesting that IGF-I may have differential actions, depending on an individual’s nutritional state and/or the hormonal milieu.
There are several limitations to our study. First, this was a cross-sectional study, and therefore, we cannot determine causation based on these data; therefore, all of the relationships demonstrated in this study are purely associational and cannot imply causation. Second, the source of Pref-1 is not clear from our studies. It is possible that Pref-1, which is found in high levels in preadipocytes, is cleaved during the maturation process and released into the circulation, explaining the elevated levels of circulating Pref-1 observed in anorexia nervosa. Yet Pref-1 is synthesized in several tissues, including the liver, and therefore, we cannot exclude the possibility that this EGF-like protein from other tissue sources may contribute to the suppressed bone formation reported in AN. Third, any conclusions about leptin’s effects on bone have to be tempered by the complex nature of its relationship to hypothalamic processing and sympathetic signaling. Fourth, because only a single estradiol level was measured, definitive conclusions regarding the relationship between estradiol and marrow adiposity cannot be made. Given the exploratory nature of our study, further studies, involving larger study populations, will be needed in further understanding the association between Pref-1, leptin, and marrow fat.
In conclusion, our data demonstrate that women with AN have significantly higher levels of Pref-1, an important negative regulator of adipocyte and osteoblast differentiation. We have shown that Pref-1 is associated with marrow adiposity and low bone mass. Further understanding of the role of Pref-1 as a possible mediator of adipocyte and osteoblast differentiation may be of significant clinical importance.
Acknowledgments
We thank the nurses and bionutritionists of the Massachusetts General Hospital Clinical Research Center for their expert care. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
This work was supported by Grant UL1 RR025758 from the National Center for Research Resources (to Harvard Clinical and Translational Science Center), and Grants R01 DK052625, R01 HL077674, M01 RR01066, ULI RR0257801, and T32 DK007028 from the National Institutes of Health.
Disclosure Summary: The authors have no conflicts to declare.
First Published Online October 22, 2009
Abbreviations: AN, Anorexia nervosa; AP, anteroposterior; BMD, bone mineral density; BMI, body mass index; CV, coefficient of variation; DXA, dual-energy x-ray absorptiometry; EGF, epidermal growth factor; hMSC, human mesenchymal stem cell; IGF-BP, IGF binding protein; Pref-1, preadipocyte factor-1; SAT, sc adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
References
- Lucas AR, Beard CM, O'Fallon WM, Kurland LT 1991 50-year trends in the incidence of anorexia nervosa in Rochester, Minn.: a population-based study. Am J Psychiatry 148:917–922 [DOI] [PubMed] [Google Scholar]
- Pope HG JH, Yurgelun-Todd D, Hudson MS 1984 Prevalence of anorexia nervosa and bulimia in three student populations. Int J Eating Disord 3:45–51 [Google Scholar]
- Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A 2005 Medical findings in outpatients with anorexia nervosa. Arch Intern Med 165:561–566 [DOI] [PubMed] [Google Scholar]
- Mayer L, Walsh BT, Pierson Jr RN, Heymsfield SB, Gallagher D, Wang J, Parides MK, Leibel RL, Warren MP, Killory E, Glasofer D 2005 Body fat redistribution after weight gain in women with anorexia nervosa. Am J Clin Nutr 81:1286–1291 [DOI] [PubMed] [Google Scholar]
- Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME 1992 Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 102(Pt 2):341–351 [DOI] [PubMed] [Google Scholar]
- Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ 2002 Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 55:693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Hosseini Ghomi R, Rosen CJ, Klibanski A 2009 Increased bone marrow fat in anorexia nervosa. J Clin Endocrinal Metab 94:2129–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellinger D, Lin CS, Hatipoglu HG, Fertikh D 2001 Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol 22:1620–1627 [PMC free article] [PubMed] [Google Scholar]
- Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M 2004 Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res 19:841–852 [DOI] [PubMed] [Google Scholar]
- Wang Y, Kim KA, Kim JH, Sul HS 2006 Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J Nutr 136:2953–2956 [DOI] [PubMed] [Google Scholar]
- Abdallah BM, Ditzel N, Traustadottir GA, Schilling AF, Amling M, Kassem M Dlk1/FA1 is a novel factor enhancing osteoclastogenesis and inhibiting bone formation in vitro and in vivo. Proc 30th Annual Meeting of the American Society for Bone and Mineral Research, 2008, Abstract 1007:S3 [Google Scholar]
- Bennett A, Chen T, Feldman D, Hintz RL, Rosenfeld RG 1984 Characterization of insulin-like growth factor I receptors on cultured rat bone cells: regulation of receptor concentration by glucocorticoids. Endocrinology 115:1577–1583 [DOI] [PubMed] [Google Scholar]
- Canalis E 1980 Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest 66:709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock JM, Centrella M, Canalis E 1988 Insulin-like growth factor I has independent effects on bone matrix formation and cell replication. Endocrinology 122:254–260 [DOI] [PubMed] [Google Scholar]
- Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler Jr GB 1992 The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab 75:762–767 [DOI] [PubMed] [Google Scholar]
- Hotta M, Fukuda I, Sato K, Hizuka N, Shibasaki T, Takano K 2000 The relationship between bone turnover and body weight, serum insulin-like growth factor (IGF) I, and serum IGF-binding protein levels in patients with anorexia nervosa. J Clin Endocrinol Metab 85:200–206 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Miller K, Herzog D, Clemmons D, Klibanski A 2003 Effects of recombinant human insulin-like growth factor (IGF)-I and estrogen administration on IGF-I, IGF binding protein (IGFBP)-2, and IGFBP-3 in anorexia nervosa: a randomized-controlled study. J Clin Endocrinol Metab 88:1142–1149 [DOI] [PubMed] [Google Scholar]
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC 1995 The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 80:898–904 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A 2002 Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab 87:2883–2891 [DOI] [PubMed] [Google Scholar]
- Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S 2008 Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int 19:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguera B, Hofbauer LC, Thomas T, Gori F, Evans GL, Khosla S, Riggs BL, Turner RT 2001 Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology 142:3546–3553 [DOI] [PubMed] [Google Scholar]
- Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS 2004 Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351:987–997 [DOI] [PubMed] [Google Scholar]
- Smith WJ, Underwood LE, Clemmons DR 1995 Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J Clin Endocrinol Metab 80:443–449 [DOI] [PubMed] [Google Scholar]
- Barthe N, Braillon P, Ducassou D, Basse-Cathalinat B 1997 Comparison of two Hologic DXA systems (QDR 1000 and QDR 4500/A). Br J Radiol 70:728–739 [DOI] [PubMed] [Google Scholar]
- Johnson J, Dawson-Hughes B 1991 Precision and stability of dual-energy X-ray absorptiometry measurements. Calcif Tissue Int 49:174–178 [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR 1991 The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA 265:1133–1138 [PubMed] [Google Scholar]
- Lucas AR, Melton 3rd LJ, Crowson CS, O'Fallon WM 1999 Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc 74:972–977 [DOI] [PubMed] [Google Scholar]
- Di Iorgi N, Mittelman SD, Gilsanz V 2008 Differential effect of marrow adiposity and visceral and subcutaneous fat on cardiovascular risk in young, healthy adults. Int J Obes (Lond) 32:1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB 2007 MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int 18:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V 2008 Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab 93:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B 2004 Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A 2006 Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab 17:144–149 [DOI] [PubMed] [Google Scholar]
- Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Löwik CW 2002 Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res 17:394–405 [DOI] [PubMed] [Google Scholar]
- Post S, Abdallah BM, Bentzon JF, Kassem M 2008 Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone 43:32–39 [DOI] [PubMed] [Google Scholar]
- Villena JA, Choi CS, Wang Y, Kim S, Hwang YJ, Kim YB, Cline G, Shulman GI, Sul HS 2008 Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): a new model of partial lipodystrophy. Diabetes 57:3258–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, Ma Z, Vignati L, Bowsher R, Herzog D, Klibanski A 1996 Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab 81:3861–3863 [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G 2000 Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207 [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Pennington C, Newton D, Xie D, Isales C 2004 Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34:376–383 [DOI] [PubMed] [Google Scholar]
- Blain H, Vuillemin A, Guillemin F, Durant R, Hanesse B, de Talance N, Doucet B, Jeandel C 2002 Serum leptin level is a predictor of bone mineral density in postmenopausal women. J Clin Endocrinol Metab 87:1030–1035 [DOI] [PubMed] [Google Scholar]
- Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL 1999 Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140:1630–1638 [DOI] [PubMed] [Google Scholar]