Abstract
Context: Treatment of osteoporosis with an anabolic agent, teriparatide [human PTH 1-34 (TPTD)], is effective in reducing incident fractures, but patient resistance to daily sc injections has limited its use. A novel transdermal patch, providing a rapid, pulse delivery of TPTD, may provide a desirable alternative.
Objective: The aim of the study was to determine the safety and efficacy of a novel transdermal TPTD patch compared to placebo patch and sc TPTD 20-μg injection in postmenopausal women with osteoporosis.
Design: Our study consisted of 6-month, randomized, placebo-controlled, positive control, multidose daily administration.
Patients: We enrolled 165 postmenopausal women (mean age, 64 yr) with osteoporosis.
Interventions: A TPTD patch with a 20-, 30-, or 40-μg dose or a placebo patch was self-administered daily for 30-min wear time, or 20 μg of TPTD was injected daily.
Outcomes: The primary efficacy measure was mean percentage change in lumbar spine bone mineral density (BMD) from baseline at 6 months.
Results: TPTD delivered by transdermal patch significantly increased lumbar spine BMD vs. placebo patch in a dose-dependent manner at 6 months (P < 0.001). TPTD 40-μg patch increased total hip BMD compared to both placebo patch and TPTD injection (P < 0.05). Bone turnover markers (procollagen type I N-terminal propeptide and C-terminal cross-linked telopeptide of type I collagen) increased from baseline in a dose-dependent manner in all treatment groups and were all significantly different from placebo patch (P < 0.001). All treatments were well tolerated, and no prolonged hypercalcemia was observed.
Conclusion: Transdermal patch delivery of TPTD in postmenopausal women with osteoporosis for 6 months is safe and effective in increasing lumbar spine and total hip BMD.
Novel transdermal delivery of teriparatide is safe and effective in increasing lumbar spine and total hip bone mineral density.
Injectable teriparatide [human PTH 1-34 (TPTD)], the only anabolic therapy currently approved for treatment of osteoporosis in the United States, is unique in its effects on the skeleton compared with antiresorptive therapies (1). TPTD treatment first stimulates new bone formation and then bone remodeling, with a positive formation balance throughout treatment (2,3,4). TPTD produces larger increments in bone mass [particularly in the lumbar spine (LS)] than those seen with potent antiresorptive therapies (2) and improvements in cancellous and cortical microarchitecture (5,6). After 18 months of treatment, TPTD injection reduced the risk of vertebral fractures by 65% and nonvertebral fragility fractures by 50% (7). In a study comparing in vivo bone strength using finite element models of quantitative computed tomography measurements, TPTD injection increased bone strength faster and to a greater degree than did alendronate in both the spine and hip (8,9). A head to head study in glucocorticoid-induced osteoporosis showed that TPTD reduced vertebral fractures substantially more than alendronate (10).
Despite the potential benefits of TPTD over antiresorptive therapy, its clinical use is limited by practical factors (11,12), including mode of administration. Because TPTD is a peptide hormone, it cannot be given orally and currently can only be administered as a daily sc injection. As a result, many patients that could benefit from this treatment decide against it (11). The need to provide an alternative for patients led to the development of a novel TPTD transdermal microneedle delivery system (13). Early studies indicated that this transdermal system delivered TPTD with a rapid time to maximal concentration, similar area under the curve (AUC), and shorter half-life than TPTD by sc injection (13). Based on these and subsequent phase 1 data, we performed a phase 2 clinical study to determine the efficacy and safety of three doses of transdermal TPTD patches (TPTD-P) administered daily for 6 months on bone mineral density (BMD), bone turnover, and safety compared with the placebo patch (Pbo-P) and TPTD injection (TPTD-I).
Subjects and Methods
Materials
Synthetic TPTD was manufactured by Bachem Americas (Torrance, CA). TPTD-P (synthetic TPTD-coated microneedle patch) was prepared by Zosano Pharma under good manufacturing practice. The transdermal system was composed of a 5-cm2 adhesive patch with TPTD coated on a 2-cm2 titanium microneedle array with 1300 microneedles of 190 μm average length. The tips of the microneedles were coated with TPTD at 20, 30, and 40 μg and dried using a process previously described (14). TPTD injection pens (recombinant human PTH 1-34, Forteo; Eli Lilly, Indianapolis, IN) were purchased from a commercial source and stored and used per the manufacturer’s instructions to supply 20-μg TPTD with each sc injection.
Subjects
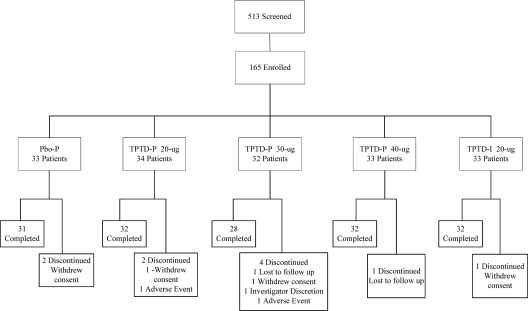
This study was performed in 13 centers in three countries (United States, n = 29; Argentina, n = 31; and Mexico, n = 105). As shown in Fig. 1, a total of 513 subjects were screened to enroll 165 postmenopausal women aged 50–81 yr whose last menstrual period was at least 1 yr earlier and who had osteoporosis by the following criteria: LS BMD T-score of −2.5 or below, or femoral neck or total hip BMD T-score of −2.5 or below, and LS T-score of −1.0 or below; or LS, femoral neck, or total hip T-score of −2.0 or below with a prevalent vertebral fracture documented by lateral spine radiographs.
Figure 1.
Consort diagram of study recruitment and retention.
Exclusion criteria included all metabolic bone diseases other than osteoporosis; any kidney stone in the past 5 yr; dermatological disorders that would interfere with the study procedures or assessments or history of contact dermatitis, known allergy, or sensitivity to tapes, adhesives, PTH, or TPTD; or active or unstable medical conditions. Study subjects were excluded if they had used any prior PTH or PTH analog for a total duration of 3 months or at all within 6 months of randomization; had ever used fluoride or strontium; or used calcitonin within 4 wk, or systemic estrogen or raloxifene within 3 months. Also excluded were subjects who had received any iv bisphosphonate within the previous 2 yr or more than two total doses. For subjects who had used oral bisphosphonates for more than 6 months, time off bisphosphonates had to be more than 6 months; if oral bisphosphonates were used for 6 to 12 months, time off had to be at least 2 yr; if previous oral bisphosphonate use exceeded 12 months, then time off had to be at least 5 yr. Excluded as well were subjects with serum calcium values above the normal range (>10.3 mg/dl), as determined by a central laboratory (Mayo Clinical Trials Services, Rochester, MN); phosphate below normal range (<0.81 mmol/liter); 25-hydroxyvitamin D level below 16 or above 80 ng/ml; bone specific alkaline phosphatase levels above the normal range (>118 U/liter); urinary calcium/creatinine ratio greater than 1.1 mmol/mmol on second morning voided specimen; substantially impaired renal function [creatinine clearance (CrCl) <30 ml/min]; hemoglobin below 10 g/dl; intact PTH above 70 pg/ml; or if on thyroid hormone replacement therapy with TSH level outside the normal range.
All subjects signed informed consent forms, and study conduct was assessed at all study centers by independent Institutional Review Boards and a clinical research organization (Kendle Corp., Cincinatti, OH). The study was sponsored by Zosano Pharma Inc. (Fremont, CA).
Protocol
All study subjects were taught self-administration techniques (beginning at the screening visit) and thereafter self-administered medication. Eligible subjects were randomized (computer allocation) to one of five groups: TPTD-P 20-, 30-, or 40-μg coated, or Pbo-P, or TPTD-I. The study was blinded to the TPTD-P or Pbo-P dose but not to TPTD- I. Patches were applied to the lateral abdomen, alternating left and right sides on a daily basis, for 30-min patch wear time, and the injection was administered in the abdomen or thigh (subjects’ choice) once each morning, except for the day of study visits. TPTD-P was supplied as individual unit doses, stored at room temperature, with a hand-held, reusable applicator supplied separately. TPTD-I pens containing a 28-d drug supply were stored in the refrigerator. Subjects were also provided 500 mg/d of calcium citrate and 1000 IU/d of vitamin D; they were instructed to take supplements with the evening meal but were asked to hold the supplements on the evening before study visits (as requested by some bone density testing centers).
Subject evaluations
Subjects were assessed monthly for 6 months after randomization. For the first month, subjects were called weekly to review use of study drug, record any concomitant medications, discuss any systemic or topical adverse events (AEs), and allow questions to be answered. Thereafter, study personnel called subjects every other week between assessments.
At each study visit, drug accountability was assessed by counting returned used patches or pens, and subjects were questioned about AEs. Compliance with the patches was self-reported in a daily written medication log. Subjects were considered compliant if they wore the patch for between 25 and 35 min and, for TPTD-I, if they wrote “yes” to taking their injection that day. Vital signs were assessed before and 15, 30, and 60 min after medication administration at each study visit, and medication administration sites were examined for topical effects. An assessment of patch adhesion was also performed at each study visit.
Safety
Total serum calcium was assessed at each visit, approximately 24 h after the last study dose. Clinical laboratory tests were assessed at baseline and at 1, 3, and 6 months. A 24-h urine collection for calcium was obtained at baseline and at 1 month. An algorithm for management of hypercalcemia and hypercalciuria was specified, which included assurance of adequate hydration, repeat serum and urine calcium measurement, and temporary or permanent discontinuation of calcium and vitamin D supplements or study medication. Electrocardiograms were obtained at screening and at 6 months. Hypercalciuria was defined as greater than 300 mg in a 24-h urine collection. Serum collection for human antibodies to TPTD was obtained at baseline and at 1 and 6 months. Samples were tested using an antibody bridging ELISA assay format developed by ALTA Analytical (San Diego, CA), using antibody capture to bound TPTD and detection with biotin-TPTD and horseradish peroxidase-streptavidin conjugate.
Efficacy variables
BMD measured by dual-energy x-ray absorptiometry (DXA; Hologic, Inc., Bedford, MA; or GE Lunar, Madison, WI) of the LS (L1–L4), left total hip, and femoral neck and left forearm (or right side if history of left-sided fracture) was obtained at screening and at 6 months. An additional DXA measurement of the LS was performed at the 3-month visit. All DXA measurements were analyzed at a central site by a radiologist blinded to the treatment group.
Quality control of the DXA instruments including cross-calibration using the Bona Fide Phantom (15) across study centers was performed by Bio-Imaging Technologies (now named BioClinica, Newtown, PA). The coefficients of variation (CVs) were 1% for the LS and 2–2.5% for the hip and forearm. Thoracolumbar lateral and anteroposterior radiographs were performed at baseline, and lateral radiographs were repeated at 6 months. Any abnormal vertebrae identified by a blinded, independent reader were excluded from the DXA analysis at all time points.
Bone formation marker, serum procollagen type I N-terminal propeptide (P1NP) samples were obtained at baseline, and at 1, 3, and 6 months and assessed by UniQ RIA (Orion Diagnostica, Espoo, Finland) with an intra- and interassay CV of 2.3 and 6.8%, respectively. Bone resorption marker serum C-terminal cross-linked telopeptide of type I collagen (CTX) samples were obtained at baseline and at 3 and 6 months and assessed by Serum CrossLap ELISA (Nordic Bioscience Diagnostics, Herlev, Denmark) with an intra- and interassay CV of 2.7 and 3.0%, respectively. Serum calcium was determined by a photometric assay on a Hitachi/Roche Modular system with intra- and interassay CVs of 0.85 and 1.15%, respectively. Normal range for serum calcium was defined as 8.9–10.1 mg/dl.
Pharmacokinetic variables
At wk 4 and 24, a plasma sample for the measurement of TPTD was collected before drug administration as well as 10 and 30 min, between 1 and 2 h, and between 3 and 4 h after drug administration in every subject. Plasma TPTD was assayed using a previously described specific ELISA assay (16). The inter- and intraassay variability was less than 4.8% and less than 7.7%, respectively. The least detectable concentration was 11.3 pg/ml.
Analysis of pharmacokinetic data
The data collected in this study were jointly analyzed with pharmacokinetic data from four phase 1 studies in postmenopausal women to better characterize the time course of the TPTD concentrations. In the phase 1 studies, a total of 106 women received 103 TPTD-I and 237 TPTD-P. A one-compartment model with first-order absorption was fitted to the TPTD-I plasma concentration data, and a one-compartment model with a biphasic slow and fast first-order absorption was fitted to the TPTD-P plasma concentration data using a nonlinear mixed effects regression analysis (NLME, S-PLUS 7.0; Insightful Corp., Palo Alto, CA). The model included random effects on the pharmacokinetic parameters by subject and by visit within subject. Subject-specific estimates of AUC and Cmax were obtained and compared across the treatment arms. For TPTD-P, the predicted concentrations at 10 min were used to represent maximum concentration (Cmax) based on phase 1 study data.
Statistical analysis
Baseline subject characteristics were assessed using an ANOVA. For categorical data, the Cochran-Mantel-Haenszel test stratified by pooled clinic site was used. The primary efficacy variable was mean percentage change in LS BMD from baseline at 6 months for TPTD-P groups compared with Pbo-P group using the Last Observation Carried Forward approach and was analyzed using both intent to treat (primary analysis) and per-protocol (secondary analysis) populations. Sample size was determined by an anticipated 4.5% LS BMD increase in the TPTD-P groups with a 4.5% sd and an expected 15% withdrawal rate (7). The 95% confidence interval of the mean difference (TPTD-P − Pbo-P and TPTD-P − TPTD-I) was calculated using a contrast statement within an analysis of covariance model with treatment and center as fixed factors and baseline LS BMD as a covariate. The secondary efficacy variables were analyzed with the same methodology as the primary endpoints.
All safety measurements including abnormal laboratory values were descriptively summarized by treatment group. MedDRA dictionary (version 9.1) was used for coding AEs. The Cochran-Mantel-Haenszel test, adjusted by pooled clinical site, was used to examine differences among treatment groups.
Results
Of the 165 women enrolled, 155 (94%) completed the 6-month protocol (Fig. 1). Baseline data for the five groups of subjects are shown in Table 1. Mean baseline biochemistry levels were all within the normal or expected range, and there were no differences among groups.
Table 1.
Study subject characteristics
| Characteristic | Pbo-P | TPTD-P 20-μg | TPTD-P 30-μg | TPTD-P 40-μg | TPTD-I 20-μg |
|---|---|---|---|---|---|
| n | 33 | 34 | 32 | 33 | 33 |
| Age (yr) | 64.8 (7.1) | 64.1 (7.5) | 63.6 (5.8) | 64.6 (7.3) | 63.2 (6.8) |
| Weight (kg) | 63.2 (9.4) | 59.1 (9.2) | 58.6 (7.8) | 62.0 (9.9) | 63.0 (9.5) |
| Height (cm) | 154 (6.2) | 152 (7.3) | 154 (6.8) | 156 (7.5) | 152 (7.7) |
| Race (%)a | |||||
| Caucasian | 12.1 | 26.5 | 31.3 | 42.4 | 24.2 |
| Hispanic | 87.9 | 73.5 | 68.8 | 57.6 | 72.7 |
| Asian | 3 | ||||
| Vertebral fractures | 8 | 6 | 4 | 7 | 8 |
| T-score | |||||
| LS | −3.2 (0.7) | −3.0 (0.5) | −3.2 (0.5) | −3.2 (0.7) | −3.0 (0.6) |
| Total hip | −1.6 (0.7) | −1.5 (0.7) | −1.7 (0.5) | −1.8 (0.7) | −1.7 (0.6) |
| BMD (gm/cm2) | |||||
| LS | 0.71 (0.1) | 0.74 (0.1) | 0.72 (0.1) | 0.74 (0.1) | 0.74 (0.1) |
| Total hip | 0.70 (0.1) | 0.70 (0.09) | 0.74 (0.1) | 0.73 (0.1) | 0.75 (0.1) |
| Femoral neck | 0.60 (0.1) | 0.60 (0.1) | 0.63 (0.1) | 0.64 (0.1) | 0.65 (0.1) |
| Biomarkers | |||||
| P1NP (ng/ml) | 49.5 (19.4) | 45.7 (22.0) | 43.9 (17.6) | 42.2 (19.6) | 45.8 (19.1) |
| CTX (ng/ml) | 0.60 (0.3) | 0.62 (0.3) | 0.63 (0.2) | 0.65 (0.4) | 0.65 (0.3) |
| Total serum calcium (mg/dl) | 9.4 (0.5) | 9.6 (0.5) | 9.3 (0.5) | 9.5 (0.5) | 9.6 (0.4) |
All values are expressed as mean (sd) unless otherwise stated.
There was no statistical difference among any of the groups. For race, P < 0.1.
Delivery profile
TPTD-P delivery was rapid and produced a dose-proportional pulsatile plasma profile that was well described by the pharmacokinetic model (Fig. 2). The pharmacokinetic profile for all patch doses (measured at both 4 and 24 wk) showed a faster time to peak concentration and a shorter apparent half-life than TPTD-I. The geometric mean AUC-ratio for TPTD-P 40 μg compared with TPTD-I was 0.81 (95% confidence interval, 0.68 to 0.98). The AUC-ratios were 0.42 (0.35 to 0.50) and 0.63 (0.52 to 0.76) for the 20- and 30-μg patch doses. The between-subject variability in AUC was similar between the TPTD-P and TPTD-I. The coefficient of variation was 39% for injection and 34, 33, and 36% for the 20-, 30-, and 40-μg TPTD patches. The relative AUCs of the TPTD patch and injections in the current study were similar to those observed in phase 1 studies. The geometric mean Cmax-ratio for TPTD-P 40-μg (at 10 min) compared with TPTD-I was 1.56 (1.31 to 1.85). The Cmax-ratios were 0.82 (0.69 to 0.98) and 1.21 (1.01 to 1.45) for the 20- and 30-μg TPTD-P doses.
Figure 2.
Comparison of TPTD pharmacokinetic profile administered by transdermal microprojection patch and sc injection. The symbols (○, TPTD-I 20-μg; ▪, TPTD-P 20-μg; •, TPTD-P 30-μg; and ▴, TPTD 40-μg) correspond to the mean observed TPTD plasma concentration at different time points after administration. The corresponding continuous lines are averaged data from four previous phase 1 pharmacokinetic studies including 106 women and is based on a one-compartment model. The mean observed TPTD-I PTH values are slightly lower than the predicted model value but are not significant.
Efficacy variables
LS BMD (Fig. 3A) increased in all active treatment arms within 3 months compared with baseline. LS BMD continued to increase in a dose-dependent and linear fashion between 3 and 6 months in the TPTD-P and TPTD-I groups. At 6 months, mean percentage (sd) change from baseline BMD was 2.96% (3.5), 3.47% (3.6), and 4.97% (4.1) in the TPTD-P 20-, 30-, and 40-μg groups, respectively, and 3.55% (3.7) in the TPTD-I group. All were significantly different compared with the Pbo-P group (−0.33%) (P < 0.001).
Figure 3.
A, LS BMD mean percentage change from baseline (±se). After 24 wk of daily administration, all active treatment groups produced increases in LS BMD (P < 0.001) with no significant change in the Pbo-P group. B, Total hip BMD mean percentage change from baseline (±se). *, After 24 wk of daily treatment, the TPTD-P 40-μg group increased hip BMD (P < 0.05) compared with Pbo-P. C, Femoral neck and distal forearm BMD mean percentage changes from baseline (±se). There were no statistically significant BMD changes in any of the treatment groups from baseline value.
There was an increase in total hip BMD in the patch groups, with a final increase of 1.33% (2.1) at 6 months in the TPTD-P 40-μg dose. This increment was significantly greater than the Pbo-P group at −0.63% (2.0) (P < 0.05). The change in the TPTD-I group was 0.09% (2.6) (Fig. 3B). There were no statistically significant BMD changes at the femoral neck or forearm in any of the treatment groups.
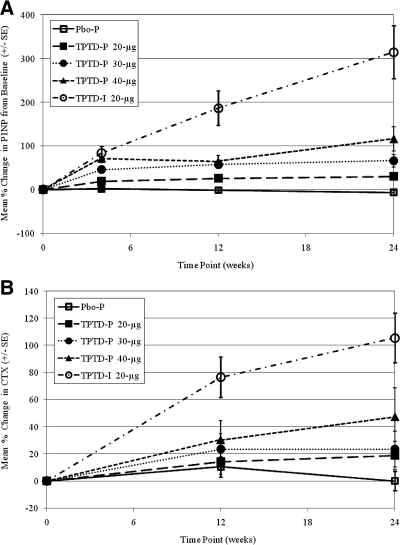
Serum P1NP levels increased in all treatment groups. At 1 month, the percentage change in P1NP from baseline for the TPTD-I group was 84% compared with 72% for the 40-μg patch dose (Fig. 4A), whereas at 6 months, the P1NP level for the TPTD-I group was 314% vs. 116% for the 40-μg patch. The percentage increase in serum CTX levels at 3 and 6 months was highest for the TPTD-I group at 76 and 105%, respectively, compared with the TPTD-P 40-μg group at 30% for 3 months and 47% for 6 months (Fig. 4B).
Figure 4.
A, P1NP mean percentage change from baseline (±se). After 4 wk of daily treatment, all active treatments showed an increase in P1NP. At 6 months, the P1NP level for the TPTD-I group was 314% vs. 116% for the 40-μg patch. B, CTX mean percentage change from baseline (±se). At 3 and 6 months, CTX levels were highest for the TPTD-I group at 76% and 105%, respectively, compared with the TPTD-P 40-μg group at 30% for 3 months and 47% for 6 months.
Safety assessments/withdrawals
There were no deaths. As shown in Fig. 1, there were two serious AEs unrelated to study drugs; one patient in the Pbo-P group suffered a subdural hematoma from a motor vehicle accident, and one patient in the TPTD-P 20-μg patch group had gastroenteritis. There were 10 early dropouts distributed across the groups; only two were due to AEs (one patient developed tachycardia in the TPTD-P 20-μg group, and one patient developed moderate skin hyperpigmentation in the TPTD-P 30-μg group).
TPTD-P doses were well tolerated. The mean compliance was above 85% in all TPTD-P groups and above 95% for the TPTD-I group at all time points. There were no episodes of delayed hypersensitivity or skin infection reported, nor were there any incidents of human antibodies to TPTD in any of the treatment arms. There were few incidents of edema, punctate bruising, or pinpoint bleeding at the site of administration. All of the subjects had a transient mild to moderate erythema at the patch site that was well tolerated, with no difference between the Pbo-P and TPTD-P groups. One subject in the TPTD-I group reported mild injection site erythema. Overall treatment related that AEs of special interest were not different between TPTD-P groups and TPTD-I groups (Table 2).
Table 2.
Treatment emergent AEs of special interest
| AEs | Pbo-P | TPTD-P 20-μg | TPTD-P 30-μg | TPTD-P 40-μg | TPTD-I 20-μg |
|---|---|---|---|---|---|
| n | 33 | 34 | 32 | 33 | 33 |
| Calcium related | |||||
| Blood calcium decreased | 0 | 0 | 0 | 0 | 1 (3) |
| Hypercalcemia | 2 (6)a | 4 (12) | 0 | 4 (12) | 6 (18) |
| Hypercalciuriab | 2 (6) | 4 (12) | 2 (6) | 1 (3) | 1 (3) |
| Urine calcium/creatinine ratio increased | 0 | 1 (3) | 0 | 1 (3) | 0 |
| Other AEs of interest | |||||
| Arthralgia/pain in extremities | 3 (1) | 5 (15) | 5 (16) | 3 (9) | 6 (18) |
| Crystal urine | 0 | 0 | 0 | 0 | 1 (3) |
| Dizziness | 0 | 3 (9) | 1 (3) | 1 (3) | 0 |
| Headache | 2 (6) | 5 (15) | 4 (12) | 3 (9) | 4 (12) |
| Hypotension | 0 | 0 | 0 | 0 | 1 (3) |
| Nausea | 0 | 3 (9) | 2 (6) | 0 | 2 (6) |
| Nephrolithiasis | 0 | 0 | 0 | 1 (3) | 0 |
| Syncope | 0 | 0 | 0 | 0 | 1 (3) |
Number of subjects (% of subjects).
Hypercalciuria was defined as >300 mg in a 24-h urine collection.
Mean serum calcium increased slightly in all active treatment groups but remained within the normal range. Postbaseline serum calcium elevations (above the upper limit of normal) occurred transiently in 20 subjects: eight (24.2% %) in the TPTD-I group, eight (24.2%) in the TPTD-P 40-μg group, none in the TPTD-P 30-μg group, three (8.8%) in the TPTD-P 20-μg group, and one (3.0%) in the Pbo-P group. In 17 of these 20 subjects, serum calcium returned to normal spontaneously by the next serum measurement or after discontinuation of the calcium and/or vitamin D supplements (prespecified protocol). In three patients (one in the Pbo-P group, one in the TPTD-P 20-μg group, and one in the TPTD-I group), study medication was temporarily withheld (for 7–25 d), in addition to stopping the supplements due to elevated serum calcium levels. The calcium levels subsequently returned to within normal limits, and there was no recurrence of hypercalcemia after restarting treatment. No patients were discontinued from the study due to hypercalcemia or hypercalciuria. Ten subjects developed hypercalciuria (two in the Pbo-P group, four in the TPTD-P 20-μg group, two in the TPTD-P 30-μg group, and one each in the TPTD-P 40-μg and TPTD-I 20-μg groups) based on either an above the upper limit of normal value of 24-h urine calcium (7.5 mmol/d) or urine-calcium/creatinine ratio (1.1 mmol/mmol). Calcium supplements were temporarily or permanently stopped in these subjects, and their urine calcium values returned to normal by the next visit. One subject in the TPTD-P 40-μg group with a history of hypercalciuria developed a kidney stone, which led to temporary discontinuation of study medication for 11 d.
During the 6 months of therapy, there was no clinically significant hypercalcemia observed. There were no changes in liver function, renal function, blood counts, or electrocardiograms.
Discussion
We have shown that the transdermal microneedle patch is an effective delivery system for TPTD. With 6 months of treatment, the 40-μg coated dose produced a BMD change in the LS similar to that of the approved TPTD injectable dose. The BMD change in the total hip with 40-μg patch administration was greater than that seen with injectable TPTD. Although the relationship between BMD change and bone strength with osteoporosis medication is not linear, the similar or greater BMD change with TPTD-P 40-μg suggests that it may have similar efficacy. The explanation for the greater total hip BMD increase with the TPTD-P 40-μg vs. TPTD-I is not clear. In other clinical trials, TPTD-I has produced a more delayed increase in hip compared with LS BMD (2,7,17). It is unclear at this time whether our results are due to increased cortical remodeling at the hip site, expansion of bone diameter, or bone of low mineral content that becomes more fully mineralized over time. Further study of transdermal vs. injectable TPTD effect on hip BMD and other parameters of bone quality in a larger study over a longer treatment period is warranted to understand this phenomenon.
Pharmacokinetic differences between the two delivery systems could provide an explanation for distinctive biological effects. The higher peak concentration and shorter half-life of TPTD-P vs. TPTD-I administration might be important in mediating an even greater anabolic effect. Multiple in vivo preclinical studies have confirmed that a rapid rise in serum TPTD and a very short serum half-life and rapid fall-off is required for an anabolic rather than catabolic effect of this peptide. These findings are consistent with the catabolic effect (at least on cortical bone) of endogenous hyperparathyroidism where PTH levels are elevated continuously, compared with the anabolic effect of exogenous sc administered PTH where the PTH elevation is resolved within several hours (18,19,20).
The changes in the biochemical markers of both bone formation and bone resorption markers were lower with TPTD-P vs. TPTD-I. At the tissue level, the magnitude of bone accrual reflects a balance between bone formed and bone resorbed. Both patch and injection increased serum measurements of bone turnover, P1NP, and CTX, but it is not possible to determine from these serum measurements what the net effect will be when both are elevated. The observation that the gain in BMD at the hip was greater for the TPTD-P 40-μg group suggests that the overall BMD effect is more positive than the effect with sc administration for this 6-month treatment period. The pharmacokinetic differences between the delivery systems could perhaps explain this difference.
The strengths of this study included evaluation of a novel transdermal microneedle patch that delivered TPTD rapidly, with a short plasma half-life, and produced a significant increase in LS BMD and total hip BMD. Due to the small sample size and short duration of this study, there is a chance that the BMD difference between the TPTD-P and TPTD-I results will change in future studies with larger sample sizes. Another study limitation was the lack of blinding with regard to TPTD-I, although both efficacy and safety outcomes were objective.
We conclude that the transdermal TPTD microneedle patch is an effective delivery system. Over 6 months, the 40-μg dose provided a significant LS BMD and total hip BMD change that was similar to the TPTD-I treatment currently approved for the treatment of osteoporosis. Additional studies are now planned to explore the efficacy and safety of TPTD-P with a longer treatment period.
Acknowledgments
The following additional investigators participated in the study: N. Binkley, Madison, Wisconsin; C. Fogarty, Spartanburg, South Carolina; J. C. Gallagher, Omaha, Nebraska; E. M. Araujo-Arias, G. M. Guzman, and N. A. Martinez-Trejo, Mexico City, Mexico; M. de la Pena-Rodriguez and S. R. Gutierrez-Urena, Guadalajara, Jalisco; and Z. Man and C. A. Mautalen, Buenos Aires, Argentina.
The authors thank Mary Southam, Asha Ramdas, Stephen Hwang, Jaap Mandema, Beth Anne Piper, and Colin G. Miller for their valuable insights and contributions to this manuscript.
Footnotes
This study was funded by Zosano Pharma Inc. The authors were fully responsible for all manuscript content and had full access to the primary data and analyses and editorial decisions. The authors received no compensation for the development of this paper.
Clinical Trial: NCT 00489918.
Disclosure Summary: F.C., N.E.L., and K.S. were consultants to Zosano Pharma in the development, performance, and interpretation of the data of this trial. F.C. had partial grant support from National Institutes of Health Grant AR-051454. N.E.L. had partial support for this work from National Institute of Arthritis and Musculoskeleton and Skin Diseases/National Institutes of Health Grant K24 AR-048841 and a grant from the endowment for Aging at the University of California at Davis Medical Center. M.A.B., J.R.Z., and P.A.G.-H. have nothing to declare. J.A.M., K.G., and P.E.D. are employed by and have equity interest in Zosano Pharma.
First Published Online October 26, 2009
Abbreviations: AEs, Adverse events; AUC, area under the curve; BMD, bone mineral density; Cmax, maximum concentration; CTX, C-terminal cross-linked telopeptide of type I collagen; CV, coefficient of variation; DXA, dual-energy x-ray absorptiometry; LS, lumbar spine; Pbo-P, placebo patch; P1NP, procollagen type I N-terminal propeptide; TPTD, teriparatide; TPTD-I, TPTD injection; TPTD-P, TPTD patch.
References
- Cosman F 2008 Parathyroid hormone treatment of osteoporosis. Curr Opin Endocrinol Diabetes Obes 15:495–501 [DOI] [PubMed] [Google Scholar]
- McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF 2005 Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768 [DOI] [PubMed] [Google Scholar]
- Arlot M, Meunier PJ, Boivin G, Haddock L, Tamayo J, Correa-Rotter R, Jasqui S, Donley DW, Dalsky GP, Martin JS, Eriksen EF 2005 Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res 20:1244–1253 [DOI] [PubMed] [Google Scholar]
- Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, Nieves JW, Dempster DW 2006 A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res 21:366–373 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF 2003 Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 18:1932–1941 [DOI] [PubMed] [Google Scholar]
- Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetiæ K, Müller R, Bilezikian J, Lindsay R 2001 Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in subjects with osteoporosis: a paired biopsy study. J Bone Miner Res 16:1846–1853 [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH 2001 Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- Keaveny TM, Hoffman PE, Kepperdahl DL, Donley DW, Krohn K, Glass EV, Mitlak BH 2007 Comparison of the effects of teriparatide and alendronate on parameters of total hip strength assessed by finite element analysis (Abstract). J Bone Miner Res 22(Suppl 1):S75 [DOI] [PubMed] [Google Scholar]
- Keaveny TM, Hoffmann PF, Singh M, Palermo L, Bilezikian JP, Greenspan SL, Black DM 2008 Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res 23:1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, Dalsky GP, Marcus R 2007 Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357:2028–2039 [DOI] [PubMed] [Google Scholar]
- Fraenkel L, Gulanski B, Wittink D 2006 Patient treatment preferences for osteoporosis. Arthritis Rheum 55:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel L, Gulanski B, Wittink D 2007 Patient willingness to take teriparatide. Patient Educ Couns 65:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V, Hwang S, Loughrey H 2004 Administration of ThPTH to humans using Macroflux transdermal technology results in the rapid delivery of biologically active PTH. J Bone Miner Res 19(Suppl 1):S460 [Google Scholar]
- Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, Daddona P 2004 Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release 97:503–511 [DOI] [PubMed] [Google Scholar]
- Miller CG 2007 Organization of the clinical trial by the sponsor. In: Pearson D, Miller CG, eds. Clinical trials in osteoporosis. 2nd ed. New York: Springer; 75–89 [Google Scholar]
- Chen K, Nguyen H, Zhu J, Shi Y, Prince P, Scott G, Beaver C 2006 Development and validation of a specific/sensitive/robust ELISA for quantification of human PTH (1-34) in human plasma: issues, possible solutions. AAPS J 8(Suppl 2):1995 [Google Scholar]
- Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, Audran M, Barker C, Anastasilakis AD, Fraser WD, Nickelsen T; EUROFORS Investigators 2008 Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 23:1591–1600 [DOI] [PubMed] [Google Scholar]
- Kitazawa R, Imai Y, Fukase M, Fujita T 1991 Effects of continuous infusion of parathyroid hormone-related peptide on rat bone in vivo: comparative study by histomorphometry. Bone Miner 12:157–166 [DOI] [PubMed] [Google Scholar]
- Hock JM, Gera I 1992 Effects of continuous and intermittent administration and inhibition of resorption of the anabolic response of bone to parathyroid hormone. J Bone Miner Res 7:65–72 [DOI] [PubMed] [Google Scholar]
- Fraher LJ, Avram R, Watson PH, Hendy GN, Henderson JE, Chong KL, Goltzman D, Morley P, Willick GE, Whitfield JF, Hodsman AB 1999 Comparison of the biochemical responses to human parathyroid hormone-(1-31)NH2 and hPTH-(1-34) in health humans. J Clin Endocrinol Metab 84:2739–2743 [DOI] [PubMed] [Google Scholar]