Abstract
Objective: The objective of the study was to examine the effects of an exercise/diet lifestyle intervention on free fatty acid (FFA)-induced hepatic insulin resistance in obese humans.
Research Design and Methods: Obese men and women (n = 23) with impaired glucose tolerance were randomly assigned to either exercise training with a eucaloric (EU; ∼1800 kcal; n = 11) or hypocaloric (HYPO; ∼1300 kcal; n = 12) diet for 12 wk. Hepatic glucose production (HGP; milligrams per kilogram fat-free mass−1 per minute−1) and hepatic insulin resistance were determined using a two-stage sequential hyperinsulinemic (40 mU/m2 · min−1) euglycemic (5.0 mm) clamp with [3-3H]glucose. Measures were obtained at basal, during insulin infusion (INS; 120 min), and insulin plus intralipid/heparin infusion (INS/FFA; 300 min).
Results: At baseline, basal HGP was similar between groups; hyperinsulinemia alone did not completely suppress HGP, whereas INS/FFA exhibited less suppression than INS (EU, 4.6 ± 0.8, 2.0 ± 0.5, and 2.6 ± 0.4; HYPO, 3.8 ± 0.5, 1.2 ± 0.3, and 2.3 ± 0.4, respectively). After the intervention the HYPO group lost more body weight (P < 0.05) and fat mass (P < 0.05). However, both lifestyle interventions reduced hepatic insulin resistance during basal (P = 0.005) and INS (P = 0.001) conditions, and insulin-mediated suppression of HGP during INS was equally improved in both groups (EU: −42 ± 22%; HYPO: −50 ± 20%, before vs. after, P = 0.02). In contrast, the ability of insulin to overcome FFA-induced hepatic insulin resistance and HGP was improved only in the HYPO group (EU: −15 ± 24% vs. HYPO: −58 ± 19%, P = 0.02).
Conclusions: Both lifestyle interventions are effective in reducing hepatic insulin resistance under basal and hyperinsulinemic conditions. However, the reversal of FFA-induced hepatic insulin resistance is best achieved with a combined exercise/caloric-restriction intervention.
Lifestyle interventions combining exercise and diet are effective in reducing basal, insulin, and free fatty-acid induced hepatic insulin resistance in older obese men and women.
Lipid accumulation in the liver is considered to be one of the primary mechanisms that drives obesity-related insulin resistance and type 2 diabetes (1). Understanding how lipids can induce insulin resistance in the liver, and how preventive strategies such as exercise and diet can reverse this effect, is essential in advancing treatment and prevention of lipid-related disease. Exercise training can reduce basal hepatic glucose production (HGP) and augment insulin suppression of HGP (2,3). Recently a single bout of endurance exercise was shown to completely reverse free fatty acid (FFA)-induced peripheral insulin resistance in young healthy individuals (4). Dietary interventions also reduce hepatic insulin resistance in obese humans (5). However, the effects of a combined exercise and caloric restriction intervention on HGP is not known. In addition, there are no data on the effects of such an intervention on FFA-induced hepatic insulin resistance in obese humans. In this study we hypothesized that exercise training without caloric restriction would reduce basal HGP, improve insulin suppression of HGP, and enhance the ability of insulin to overcome FFA-induced hepatic insulin resistance and that a greater negative energy balance induced by the addition of caloric restriction would result in even greater reversal of hepatic insulin resistance.
Subjects and Methods
Study participants
A total of 23 older (66 ± 1 yr), obese (34 ± 1 kg/m2) men and women with impaired glucose tolerance were entered into a 12-wk lifestyle intervention. Subjects in the present report represent part of a larger cohort that were involved in our studies on FFA-induced insulin resistance, and some data from a subgroup (n = 16) were presented in a previous publication (6). The protocol was approved by the Institutional Review Board of MetroHealth Medical Center, and all subjects provided written informed consent.
Lifestyle intervention
The lifestyle intervention involved 12 wk of aerobic exercise training combined with dietary counseling to facilitate adherence to either normal dietary intake [eucaloric diet (EU)] or a reduction in caloric intake by about 500 kcal/d [hypocaloric diet (HYPO)]. Exercise (5 d/wk) and caloric intake were carefully supervised (6). Changes in body composition and abdominal adiposity were assessed by hydrostatic weighing and computed tomography, as previously described (7). All metabolic testing was performed after a 12-h overnight fast during a controlled 3-d inpatient stay in the General Clinical Research Center.
Euglycemic-hyperinsulinemic clamp with intralipid/heparin infusion
Hepatic insulin resistance was assessed via a two-stage euglycemic hyperinsulinemic clamp. A primed-continuous infusion (40 mU/m−2 · min−1) of insulin was continued for 7 h, whereas saline was infused (INS) at a rate of 90 ml/h to control for fluid volume changes during the subsequent lipid infusion (6). Plasma glucose levels were clamped at 5 mmol/liter. After 2 h of INS, plasma FFA concentrations were raised by infusion of 20% Intralipid (90 ml/h) and heparin (200 IU bolus and continuous infusion at 15 IU/min) for an additional 5 h (INS/FFA). HGP was determined using a primed (25 μCi), continuous infusion (0.25 μCi · min−1) of [3-3H]glucose, which was started 2 h before beginning the insulin infusion. Isotopic-specific activity was maintained by infusion of 20% dextrose enriched with [3-3H]glucose.
Calculations and analytical determinations
Plasma glucose kinetics were determined using the steady-state equations of Steele (8,9). Basal HGP was determined from the rate of glucose appearance during the final 30 min of the 2-h basal period. Insulin suppression of HGP during the INS and INS/FFA periods was determined from the difference between rate of glucose appearance and the exogenous glucose infusion rate. Hepatic insulin resistance was calculated during each clamp stage as the product of HGP and the steady-state insulin concentration (10). Analytical determinations for plasma glucose, insulin, and FFAs were performed as described previously (6). Plasma tritiated glucose-specific activity was determined by liquid scintillation counting after plasma deproteinization with 3.0 m perchloric acid (9).
Statistical analyses
Data are presented as mean ± sem. No differences were found between groups for any of the measured variables at baseline. Hence, all the data were analyzed using three-way [group × trial (basal, INS, and INS/FFA) × time] repeated-measures ANOVA to examine the effects of the interventions on HGP and hepatic insulin resistance. A two-way (group × time) repeated-measures ANOVA was used to analyze changes in subject characteristics. Spearman’s rank correlation was used to assess associations between variables. Statistical significance was determined a priori to be P < 0.05. When appropriate, Bonferroni post hoc tests were applied to identify specific differences between means. Analyses were performed using StatView version 5.0.1 (SAS Institute Inc., Cary, NC).
Results
Body composition and weight loss
Compared with the EU group, the HYPO group lost 4.7 kg more body weight and 4.3 kg more body fat. The HYPO group also demonstrated additional losses in total abdominal fat (EU: −9 ± 4%, HYPO: −20 ± 4%, P < 0.05). Total sc (EU: 418 ± 34 to 363 ± 32 cm2, HYPO: 418 ± 38 to 340 ± 42 cm2), superficial (EU: 244 ± 22 to 240 ± 24 cm2, HYPO: 246 ± 29 to 218 ± 28 cm2), and deep sc (EU: 188 ± 20 to 164 ± 18 cm2, HYPO: 180 ± 14 to 140 ± 17 cm2), and visceral adipose tissue (EU: 103 ± 23 to 83 ± 18 cm2, HYPO: 134 ± 18 to 105 ± 15 cm2) were also significantly reduced (P < 0.05 vs. before intervention) after the 12-wk lifestyle intervention; however, no additional benefit (P > 0.05) was gained from caloric restriction. Before the intervention, total abdominal fat correlated with basal HGP (Rho: 0.60, P = 0.006), in line with evidence that upper body adiposity may contribute to increased HGP (11). This relationship persisted after the lifestyle intervention and was independent of caloric restriction (Rho: 0.56, P = 0.01).
HGP
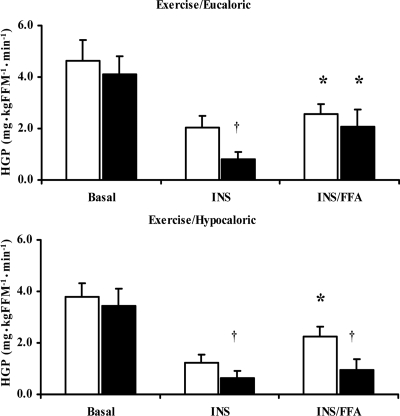
The results of the three-way (group × trial × time) ANOVA for HGP showed a main effect for trial (P < 0.0001) and time (P = 0.01) as well as a significant group × trial × time interaction (P = 0.04) (Fig. 1). Before the intervention, basal HGP was similar for both groups (Fig. 1), whereas insulin suppression of HGP was incomplete during the first stage of the clamp: 63 ± 5% (EU) and 71 ± 7% (HYPO). When FFAs were infused, insulin suppression of HGP was further impaired and HGP was increased, although the increase was not statistically significant. After the intervention, basal HGP was not changed (P > 0.05). However, both interventions improved the ability of insulin to suppress HGP under normal hyperinsulinemic conditions (EU: −42 ± 22%; HYPO: −50 ± 20%, P < 0.05). When FFAs were elevated, the lifestyle intervention did not alter insulin suppression of HGP in the EU group; however, in the HYPO group, insulin suppression of HGP was enhanced (−58 ± 19%, P < 0.05). In addition, the absolute change in the FFA-induced increase in HGP from insulin-stimulated conditions was significantly less in the HYPO group after the lifestyle intervention [EU: 1.29 ± 0.62, HYPO: 0.31 ± 0.29 mg/kg fat-free mass per minute, P < 0.05].
Figure 1.
HGP during basal, insulin, and intralipid/heparin stimulated conditions before and after two 12-wk exercise/diet interventions. White bars, Preintervention means; black bars, postintervention means. The x-axis indicates the basal, insulin-stimulated (INS), and intralipid/heparin (INS/FFA) stages of the clamp. Data are presented as mean ± se (EU group: n = 11; HYPO group: n = 12). *, P < 0.01 vs. INS; †, P < 0.05 vs. before intervention.
Hepatic insulin resistance
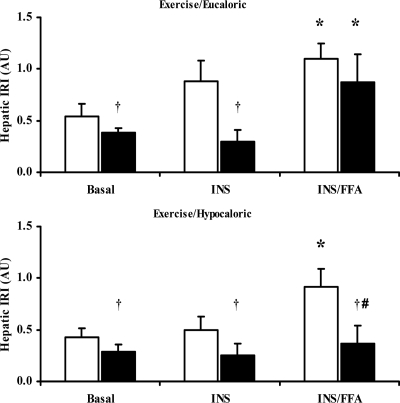
A significant group × trial × time interaction was also found for hepatic insulin resistance (P = 0.006) with main effects for group (P = 0.018), trial (P = 0.004), and time (P = 0.002) (Fig. 2). Reductions in hepatic insulin resistance were consistent with the changes seen with HGP after both lifestyle interventions (Fig. 2). Before the study, hepatic insulin resistance was significantly elevated during INS/FFA when compared with the INS clamp stage in both groups. After intervention, hepatic insulin resistance was reduced in the basal and INS conditions in the EU group, but it was not reduced during the INS/FFA stage. In contrast, hepatic insulin resistance was significantly attenuated during the basal, INS, and INS/FFA clamp stages in the HYPO group. The postintervention decreases in basal and INS hepatic insulin resistance were largely due to reductions in hyperinsulinemia (data not shown) in both intervention groups.
Figure 2.
Hepatic insulin resistance index (IRI) derived during basal, insulin and intralipid/heparin stimulated conditions before and after two 12-wk exercise/diet interventions. White bars, Preintervention means; black bars, postintervention means. The x-axis indicates the basal, insulin-stimulated (INS), and Intralipid/heparin (INS/FFA) stages of the clamp. Data are presented as mean ± se (EU group: n = 11; HYPO group: n = 12). *, P < 0.01 vs. INS; †, P < 0.05 vs. before intervention; #, P < 0.05 vs. EU.
Discussion
We evaluated the effects of two highly generalizable lifestyle interventions (exercise alone or exercise combined with a hypocaloric diet) on two central elements of hepatic function, hepatic insulin resistance and HGP, in older obese men and women with impaired glucose tolerance. These studies were performed under important in vivo experimental physiological conditions: basal fasting conditions, hyperinsulinemia with suppressed FFA (reasonably representative of a carbohydrate rich meal), and hyperinsulinemia with supraphysiological elevated FFA (proof of concept for FFA induced insulin resistance). Our data are the first to demonstrate that both lifestyle interventions can initiate the reversal of hepatic insulin resistance under basal and hyperinsulinemic conditions, but a combined exercise/diet intervention is more effective in reversing FFA-induced hepatic insulin resistance than exercise alone. Our data are also the first to show that the reversal of FFA-induced hepatic insulin resistance is related to significant decreases in central adiposity. Although neither intervention was sufficient to reverse fasting hyperglycemia or significantly reduce basal HGP in these subjects, both interventions were successful in improving insulin’s ability to suppress HGP during hyperinsulinemia with suppressed FFA. Finally, the exercise/caloric-restriction intervention proved more effective in regulating HGP during hyperinsulinemia with elevated FFA. These data suggest that older obese patients with elevated FFA levels may require greater weight loss and greater reductions in abdominal adiposity to facilitate greater clearance of FFA, thus reducing FFA-induced gluconeogenesis and HGP.
It is known that when FFAs are elevated, insulin’s ability to suppress HGP is markedly attenuated, and this effect is most evident in subjects with impaired glucose tolerance and type 2 diabetes (12,13,14,15). Human data demonstrating the effects of lifestyle interventions on the effects of FFA on hepatic insulin resistance and HGP are lacking. Our data provide strong experimental evidence that FFA-induced hepatic insulin resistance and insulin’s ability to suppress HGP can be reversed by a combination of exercise and caloric restriction. The mechanism responsible for this effect may be related to increased clearance of FFA. We observed that during the controlled intralipid/heparin infusion, the prevailing FFA levels during the postintervention INS/FFA clamp were reduced in both intervention groups. This may reflect increased FFA uptake by liver, adipose tissue, or skeletal muscle. One of the classic metabolic adaptations to exercise is the increased capacity of skeletal muscle to take up and oxidize fat (16,17). Furthermore, exercise training has been shown to decrease liver FFA uptake and increase muscle FFA uptake during euglycemic hyperinsulinemic clamps, thus causing a preferential storage of FFA in skeletal muscle (18). It was recently shown that an acute bout of exercise prevents FFA-induced insulin resistance in skeletal muscle by increasing muscle triglyceride synthesis (4). Therefore, it is most likely that lower FFAs during the postintervention clamp were the result of increased muscle FFA uptake and storage as triglycerides, with concurrent sparing of lipid accumulation in the liver, thus facilitating the enhanced ability of insulin to suppress HGP.
There is an increased clinical awareness that abdominal adiposity is one of the primary mechanisms underlying insulin resistance (9). Abdominal adiposity correlates with HGP, and it is also known that FFA flux is increased in obese subjects with upper-body adiposity (19). Our data are consistent with these observations because we demonstrated that basal HGP was significantly correlated with total abdominal adiposity. Interestingly, changes in visceral adipose tissue (VAT) and sc adipose tissue alone were not correlated with changes in hepatic insulin resistance or HGP under any of the three experimental conditions studied. This is in contrast to a previous report in which exercise-induced changes in VAT were strongly associated with improved insulin sensitivity assessed from an oral glucose tolerance test, and animal data showing that surgical removal of VAT resulted in enhanced hepatic insulin sensitivity (7,20). Our results are more in line with the idea that in humans both VAT and sc adipose tissue are important determinants of hepatic function, and therefore, intervention strategies need to target both sites.
In conclusion, we provide novel insight into the role of fatty acids in the development of hepatic insulin resistance and evidence for recommending an appropriate lifestyle intervention to reverse the problem. The data show that whereas exercise alone can improve hepatic insulin sensitivity under fasting and typical postprandial insulin levels, one may need a combination of exercise and caloric restriction to prevent hepatic insulin resistance in patients with elevated FFA levels. Greater weight loss and greater reductions in abdominal adiposity, together with greater clearance of FFAs, may facilitate less FFA-induced gluconeogenesis and HGP, and exercise/diet has the added benefit of an improved adipose tissue distribution. Whereas more detailed mechanistic studies are needed to probe the root cause of the observed changes in hepatic insulin resistance, these data provide efficacy for the use of a combined exercise/diet treatment strategy for older obese patients with insulin resistance and elevated FFAs.
Acknowledgments
The authors thank the nursing and dietary staff in the General Clinical Research Centers and the graduate students who helped with data collection. Also, special thanks go to our research volunteers for their hard work and determination on completing the study.
Footnotes
This work was supported by National Institutes of Health Grants RO1-AG12834 (to J.P.K.), General Clinical Research Center Grants MO1-RR10732, RR00080, RR018390, HL007887, and CTSA 1UL1-RR024989.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 11, 2009
Abbreviations: FFA, Free fatty acid; HGP, hepatic glucose production; VAT, visceral adipose tissue.
References
- DeFronzo RA 2004 Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract 143:9–21 [DOI] [PubMed] [Google Scholar]
- Devlin JT, Hirshman M, Horton ED, Horton ES 1987 Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes 36:434–439 [DOI] [PubMed] [Google Scholar]
- Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO 2009 Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 297:E151–E156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Horowitz JF 2007 Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117:1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljanen AP, Iozzo P, Borra R, Kankaanpää M, Karmi A, Lautamäki R, Järvisalo M, Parkkola R, Rönnemaa T, Guiducci L, Lehtimäki T, Raitakari OT, Mari A, Nuutila P 2009 Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab 94:50–55 [DOI] [PubMed] [Google Scholar]
- Solomon TP, Haus JM, Marchetti CM, Stanley WC, Kirwan JP 2009 Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocrinol Metab 297:E552–E559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP 2006 Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 100:1584–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R 1959 Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82:420–430 [DOI] [PubMed] [Google Scholar]
- Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO 1993 Insulin resistance in aging is related to abdominal obesity. Diabetes 42:273–281 [PubMed] [Google Scholar]
- Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA 2007 Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 30:89–94 [DOI] [PubMed] [Google Scholar]
- Roust LR, Jensen MD 1993 Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes 42:1567–1573 [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA 1983 Effect of fatty acids on glucose production and utilization in man. J Clin Invest 72:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G 2001 Free fatty acids—the link between obesity and insulin resistance. Endocr Pract 7:44–51 [DOI] [PubMed] [Google Scholar]
- Bajaj M, Berria R, Pratipanawatr T, Kashyap S, Pratipanawatr W, Belfort R, Cusi K, Mandarino L, DeFronzo RA 2002 Free fatty acid-induced peripheral insulin resistance augments splanchnic glucose uptake in healthy humans. Am J Physiol Endocrinol Metab 283:E346–E352 [DOI] [PubMed] [Google Scholar]
- Staehr P, Hother-Nielsen O, Landau BR, Chandramouli V, Holst JJ, Beck-Nielsen H 2003 Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes 52:260–267 [DOI] [PubMed] [Google Scholar]
- Costill DL, Fink WJ, Getchell LH, Ivy JL, Witzmann FA 1979 Lipid metabolism in skeletal muscle of endurance-trained males and females. J Appl Physiol 47:787–791 [DOI] [PubMed] [Google Scholar]
- Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O'Carroll SM, O'Leary VB, Kirwan JP 2008 Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol 104:1313–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo P, Takala T, Oikonen V, Bergman J, Grönroos T, Ferrannini E, Nuutila P, Knuuti J 2004 Effect of training status on regional disposal of circulating free fatty acids in the liver and skeletal muscle during physiological hyperinsulinemia. Diabetes Care 27:2172– 2177 [DOI] [PubMed] [Google Scholar]
- Shadid S, Kanaley JA, Sheehan MT, Jensen MD 2007 Basal and insulin-regulated free fatty acid and glucose metabolism in humans. Am J Physiol Endocrinol Metab 292:E1770–E1774 [DOI] [PubMed] [Google Scholar]
- Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, Rossetti L 1999 Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes 48:94–98 [DOI] [PubMed] [Google Scholar]