Abstract
Context: Brown adipose tissue (BAT) found by positron emission/computed tomography (PET-CT) using flouro-deoxyglucose (FDG) is inducible by cold exposure in men. Factors leading to increased BAT are of great interest for its potential role in the treatment of diabetes and obesity.
Objective: We tested whether thyroid hormone (TH) levels are related to the volume and activity of BAT in a patient with a mutation in the insulin receptor gene.
Design/Setting/Intervention: Our work was based on the case report of a patient in an observational study at the National Institutes of Health.
Patient: The patient discontinued insulin and oral antidiabetics after thyroidectomy and suppressive-dose levothyroxine therapy for thyroid cancer. PET-CT uptake in BAT was confirmed by histology and molecular analysis.
Outcomes: PET-CT studies were performed, and we measured hemoglobin A1c and resting energy expenditure before and after levothyroxine discontinuation for thyroid cancer testing. Molecular studies of BAT and white adipose samples are presented.
Result: Supraclavicular and periumbilical sc adipose tissue demonstrated molecular features of BAT including uncoupling protein-1, type 2 deiodinase, and PR domain containing 16 by quantitative PCR. Activity of type 2 deiodinase activity was increased. The discontinuation of levothyroxine resulted in decreased FDG uptake and diminished volume of BAT depots accompanied by worsening of diabetic control.
Conclusions: This case demonstrates the TH effect on BAT activity and volume in this patient and an association between BAT activity and glucose levels in this patient. Because the contribution of TH on skeletal muscle energy expenditure and fuel metabolism was not assessed, an association between BAT activity and glucose homeostasis can only be suggested.
Thyroid hormone induced changes in quantity and activity of brown adipose tissue in a patient with insulin receptor mediated diabetes.
The fundamental importance of thyroid hormone (TH) on energy regulation and glucose homeostasis is most notable in conditions of extreme derangement of thyroid function. Although TH under normal conditions plays a critical role in the maintenance of core temperature through utilization of energy substrates, its contribution to glucose homeostasis appears minor relative to the effects of other hormones. Brown adipose tissue (BAT) generates heat through the “inefficient” oxidation of fatty acids and glucose that is taken up by both insulin dependent and independent transporters (1). BAT has been identified as symmetrical uptake of isotopic glucose in the supraclavicular and upper thoracic region in a minority of 18-flouro-deoxyglucose (FDG)-positron emission tomography (PET) scans performed in adult humans (2,3). The significance of these radiographic findings of BAT to metabolism in humans is not well understood.
Studies of the factors regulating BAT biogenesis have stimulated considerable interest in the field (4,5). Very recently, studies in humans based on PET technology have shown that BAT can play a role in the modulation of energy expenditure in adult humans (6,7,8). The possibility of a role of BAT in glucose homeostasis is presented in the case of a unique patient with minimal levels of insulin signaling due to an insulin receptor mutation and extreme insulin resistance who showed a dramatic change in glucose homeostasis in the period after starting levothyroxine suppressive therapy for thyroid cancer. The long duration of observation supported by clinical, histological, molecular, and functional data indicate that in this case, TH played a critical role to achieve and maintain euglycemia independent of insulin receptor action.
Patient and Methods
The patient was studied at the Clinical Center at the National Institutes of Health. All studies were performed after informed consent was obtained for protocols approved by the National Institute of Diabetes and Digestive and Kidney Diseases-National Institute of Arthritis and Musculoskeletal and Skin Diseases institutional review board.
Imaging studies
Images were obtained using a standard oncology imaging protocol on a PET/computed tomography (CT) scanner in the fasted state and with plasma glucose in the normal range. Approximately 16 mCi of F-18 FDG was administered for each study, and scanning was performed after a 60-min uptake period. The volume of active BAT was determined using MEDx image processing and visualization software (Medical Numerics, Germantown, MD). The whole body composition in grams of fat, lean mass, and bone mineral content was measured using dual energy x-ray absorptiometry (Hologic QDR 4500; Hologic, Bedford MA).
Metabolic tests
Resting energy expenditure was assessed by indirect calorimetry using a ParvoMedics apparatus (ParvoMedics, Sandy, UT). Hyperinsulinemic euglycemic clamp was performed according to established methods (9) using an insulin infusion rate of 500 mU · m2 · min−1. Core body temperature was measured using ingestible telemetric sensors (VitalSense; Mini Mitter, Bend OR).
Tissue procurement
The patient’s suprascapular adipose tissue was collected by CT-guided needle biopsy. Subcutaneous adipose tissue from the patient and a volunteer receiving levothyroxine replacement therapy was collected by needle biopsy from the periumbilical area. Control normal thyroid tissue samples for research were obtained from patients at the National Institutes of Health Clinical Center undergoing thyroid surgery for benign and malignant nodules after informed consent on an institutional review board-approved protocol. Tissue samples were snap-frozen in liquid nitrogen and stored in a liquid nitrogen tank.
Expression studies
Total RNA was extracted from the fat tissue biopsies using TRIzol (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. The RNA was treated with TURBO DNase (Ambion, Austin, TX) to eliminate any genomic DNA contamination. For expression analysis, the mRNA was reverse transcribed using the High-Capacity cDNA Archive kit (Applied Biosystems, Inc., Foster City, CA). RT-PCR analysis was performed on a 7900HT Thermocycler (Applied Biosystems, Inc.). Uncoupling protein-1 (UCP1), PR domain containing 16 (PRDM16), and 18s mRNA levels were measured with FastStart SYBR Green Universal MasterMix (Roche, Manheim Germany). Type 2 deiodinase (D2) (no. Hs00255341ml) and glyceraldehyde-3-phosphate dehydrogenase (no. 4333764F) gene expression levels were measured using TaqMan gene expression probes from Applied Biosystems, Inc. The relative gene expression levels were determined after normalization using 18S levels or glyceraldehyde-3-phosphate dehydrogenase using the Δ-Δ CT method. The following primers were used for the analysis: human UCP1, forward, 5′-GGA ACA ATC ACC GCT GTG GT-3′, and reverse, 5′-ATC CTG AGA GAG GCG GAG CT-3′; human PRDM16, forward, 5′-GCG GTC TGT TAG CTT TGG AG-3′, and reverse, 5′-GAG GAG TGT CTT CGG AAA GGG-3′; for 18S, forward, 5′-AGT CCC TGC CCT TTG TAC ACA-3′, and reverse, 5′-CGA TCC GAG GGC CTC ACT A-3′.
Deiodinase assay
125I-T4 (Perkin-Elmer, Boston, MA) was purified using LH-20 columns (Pharmacia, Uppsala, Sweden). Before each experiment, tissue samples were briefly homogenized in 0.25 m sucrose, 0.02 m Tris/HCl (pH 7.0), and 1 mm EDTA. After centrifugation at 1000 × g, the supernatant was collected and stored at −80 C. Deiodinase activity of tissue extracts was measured according to previously described methods (10). Each reaction was performed in the presence or absence of 5 mm propylthiouracil (used as a D1 inhibitor). Results were expressed as velocity of deiodination (fmol/min/mg protein) ± sd after correction for nonspecific deiodination.
Results
Clinical history
A woman with type A insulin resistance secondary to a homozygous mutation of the α-subunit of the insulin receptor, which severely impaired its transport to the cell surface, was followed at the National Institutes of Health since age 17 (11). Her clinical course was typical for a patient with type A insulin resistance and was notable for extreme insulin resistance, poor diabetes control despite massive doses of insulin, and hyperandrogenism (12).
At age 32, the patient was diagnosed with papillary thyroid carcinoma and was treated by total thyroidectomy and resection of affected lymph nodes in the mediastinum. Additional therapy included radioiodine and levothyroxine (0.2 to 0.25 mg/d) to suppress TSH. Over the subsequent 10 yr, slightly elevated thyroglobulin levels indicated persistent disease that could not be localized by radioiodine scanning.
Initially, in an effort to improve glucose control, metformin was added to high-dose insulin immediately after surgery. In the interval of approximately 30 months after the initial treatment of thyroid cancer, remarkable improvements in glycemia were noted, and insulin therapy was tapered and eventually discontinued. Throughout the follow-up, there was no evidence of liver, renal, or adrenal insufficiency. After several years of continuous excellent glycemia control, metformin was also discontinued. The clinical course is summarized in Table 1.
Table 1.
Diabetes control, therapy, and studies performed
| Age (yr) | 18 | 23 | 29 | 32 | 34 | 35 | 37 | 40 | 44 |
|---|---|---|---|---|---|---|---|---|---|
| Insulin serum,fasting (nl 6–27) (μU/ml) | 940 | NA | NA | NA | 272 | 544 | NA | 60 | 124 |
| Glycated hemoglobin (nl 4.8–6.4) (%) | 8.7 | 8.7 | 9.4 | 9.9 | 7.1 | 5.6 | 5.2 | 5.3 | 5.5 |
| Insulin (U/d) | Up to 3000 | Up to 3000 | Up to 3000 | Up to 3000 | Up to 3000 | Discontinued | Discontinued | Discontinued | Discontinued |
| Metformin (mg/d) | 1850 | 1850 | 1850 | Discontinued | Discontinued | Discontinued | Discontinued | ||
| Levothyroxine (μg/d) | 200 | 200 | 250 | 250 | 250 | 200 | |||
| PET/PET-CT | Xa | X (2) | |||||||
| Insulin clamp study | X | X |
NA, Not available; nl, normal.
FDG-PET only because PET-CT was not available.
Identification and characterization of BAT
FDG-PET/CT studies
A PET/CT study was performed as part of the 10-yr follow-up of the patient’s persistent thyroid cancer. The study revealed symmetrical 18-FDG tracer uptake in the lower neck, suprascapular, mediastinal, and thoracic paravertebral regions (Fig. 1A). On CT scan, the areas that coregistered with FDG uptake were masses of adipose tissue based on Hounsfield units. Taken together, the uptake was thought to indicate the presence of BAT although persistent thyroid cancer could not be excluded. Slightly increased FDG uptake was also noted in the sc fat, in particular in the pelvic area and over the lower extremities.
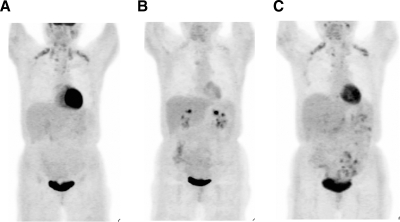
Figure 1.
FDG-PET scans normalized for intensity show the profound effect of TH status on uptake of glucose throughout the body. A, On levothyroxine suppression therapy; B, off levothyroxine; C, on levothyroxine therapy for 14 d. For the brown adipose volume calculations, a three-dimensional region of interest encompassing the areas with intense uptake on the PET image were marked, and a standardized uptake value was calculated for each pixel, corrected for body weight and injection dose. The 3-dimensional region of interest was copied from the PET image onto the CT scan (data not shown). Pixels within the region of interest were defined as BAT if the standardized uptake value was greater than 1.5 and the corresponding Hounsfield unit on CT was between −50 and −150, levels indicative of adipose tissue. The volume of designated brown fat pixels was calculated by multiplying the number of pixels by the volume of each pixel in cubic centimeters. BAT volume changed with TH levels: A, 29 cm3; B, 9 cm3; C, 27.5 cm3.
Histology and molecular analysis
A biopsy of the suprascapular focus of FDG activity was obtained under CT guidance. The specimen was identified as BAT on the basis of the characteristic high vascularity and small multivesicular lipid droplets (Fig. 2A). No thyroid cancer was identified in the specimen. A sample of periumbilical sc adipose tissue was obtained to serve as control tissue for molecular studies and showed an intermediate histological appearance between brown and white adipose tissue (Fig. 2B).
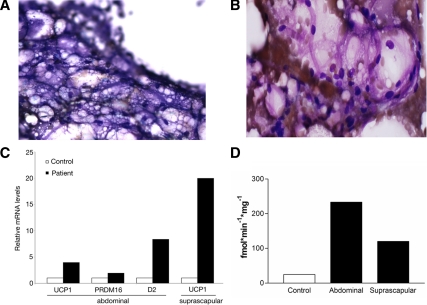
Figure 2.
Histological and molecular studies on adipose from the patient with type A insulin resistance. A, Dif-quick staining at 120× magnification of BAT from the suprascapular needle biopsy showing multivesicular cells containing lipid. B, Hematoxylin and eosin staining at 200× magnification of adipose tissue from periumbilical sc abdominal needle biopsy showing an atypical appearance consistent with brown adipose. The adipocytes lacked features of white adipose (large empty cell with classical signet-ring morphology). C, Molecular signature of brown fat in the adipose depots of the patient: real-time PCR analysis of mRNA levels of the brown fat molecular markers UCP1, PRDM16, and D2 in abdominal sc fat tissue and of UCP1 in suprascapular fat depot in a control subject and the patient. D, Type 2 5′ deiodinase activity in adipose biopsies obtained from the patient’s suprascapular and sc abdominal depots compared with control sc abdominal adipose biopsy. The patient’s type 2 5′ deiodinase activity is higher in both abdominal (white adipose) and suprascapular (brown adipose) regions compared with control.
To characterize the transcription pattern of the samples, quantitative RT-PCR was performed from RNA samples obtained from the patient’s suprascapular and periumbilical fat, and from the periumbilical sc adipose tissue of a hypothyroid patient on levothyroxine replacement therapy. As shown in Fig. 2C, the mRNA levels of three critical brown fat molecular markers, UCP1, D2, and PRDM16, were elevated in the periumbilical depot of this patient compared with the levels measured in the fat of the control subject, indicating that the white adipose tissue depot of this patient has the molecular characteristics of BAT. As expected, high expression levels of UCP1 were found in the suprascapular area, with increased FDG uptake proving the diagnosis of BAT.
Consistent with the quantitative RT-PCR data, both the suprascapular and periumbilical adipose tissue samples of the patient showed a significant D2 activity that was not present in a control sample obtained from another subject (Fig. 2D). The 5′-deiodinase activity observed in the patient’s samples was comparable to a control sample of normal thyroid tissue (data not shown). The lower D2 activity observed in the patient’s suprascapular sample compared with the one observed in her sc sample was most likely secondary to the heavy hemoglobin contamination.
TH modulates the BAT activity
To assess the significance of TH for the activity of BAT, metabolic and imaging tests were performed before and after levothyroxine withdrawal according to conventional thyroid cancer surveillance protocol. FDG-PET/CT performed in the hypothyroid state showed decreased tracer uptake and estimated volume of fat in the areas previously identified as BAT (Fig. 1B). TH therapy was reinstituted, and a repeat FDG-PET/CT at 14 d showed an increase of BAT activity and volume (Fig. 1C).
Modulatory effects of TH therapy on glucose homeostasis and metabolism
During the hospital admission, a series of metabolic studies were performed to assess the clinical impact of TH on glycemia and BAT activity. While on levothyroxine 200 μg, the patient’s TSH was suppressed, but she did not show signs or symptoms of hyperthyroidism. The resting energy expenditure in this condition was measured at 1450 kcal/24 h, 12% greater than that predicted by the Harris-Benedict equation. A low respiratory quotient (0.7) was noted, suggesting that under TH suppressive therapy the patient’s metabolism primarily relied on fatty acid oxidation.
On levothyroxine alone, the glycated hemoglobin was 5.5%, indicating superior glycemic control. Despite a normal fasting glucose of 94 mg/dl, an oral glucose tolerance test demonstrated a diabetic pattern with the 2-h glucose reaching 320 mg/dl. The steady-state glucose disposal rate observed during a high-dose hyperinsulinemic euglycemic clamp (500 μU/m2/min) was negligible, 3 mg/kg/min, and was comparable to that observed in a previous study performed before the thyroid cancer diagnosis.
Studies were again performed after TH withdrawal. The patient was clinically hypothyroid with an elevated TSH (53 μIU/ml; reference values, 0.4–4.0) and undetectable levels of circulating THs. A clear deterioration of the fasting glucose (160 mg/dl) and glycated hemoglobin (6.7%) was noted; no difference was observed in the 2-h postglucose load (360 mg/dl). In addition, the resting energy expenditure was severely reduced to 650 kcal/24 h, and the respiratory quotient rose to 0.88, indicating a reduction in fatty acid oxidation. The clinical studies are summarized in Table 2.
Table 2.
Effect of levothyroxine (l-T4) on metabolic parameters and laboratory tests
| Reference range for adults | With l-T4 | Off l-T4 | |
|---|---|---|---|
| Weight (kg) | 58.5 | 62.3 | |
| Lean mass (%) | 62 | 62.8 | |
| Fat mass (%) | 36 | 35.8 | |
| Resting energy expenditure (kcal in 24 h) | 1450b | 650 | |
| Respiratory quotienta | 0.7 | 0.88 | |
| Core temperature [mean (sd)] (C) | 37.25 (0.21) | 37.36 (0.19) | |
| TSH (μIU/ml) | 0.4–4.0 | 0.29 | 53.8 |
| Glycated hemoglobin (%) | 4.8–6.4 | 5.5 | 6.7 |
| Glucosefasting (mg/dl) | 70–115 | 94 | 160 |
| Insulinfasting (μU/ml) | 6–27 | 144 | 297 |
| Glucose2-h (mg/dl)c | 320 | 361 | |
| Euglycemic insulin clamp (500 mU · m2 · min−1) | |||
| Glucose infusion rate (mg · kg · min−1) | 3d | ||
| Urinary catecholamines | |||
| Epinephrine (μg in 24 h) | 0–20 | 0 | 3.8 |
| Norepinephrine (μg in 24 h) | 5–80 | 35 | 42 |
To convert the values for glucose to millimoles per liter, multiply by 0.05551; for insulin to picomoles per liter, multiply by 7.217; for TSH to milliunits per liter, multiply by 1; for epinephrine to nanomoles per day, multiply by 5.46; for norepinephrine to nanomoles per day, multiply by 5.91; and for dopamine to nanomoles per day, multiply by 6.53.
The respiratory quotient (RQ) is the ratio of the volume of carbon dioxide produced to the volume of oxygen consumed using indirect calorimetry and reflects the fuel source being metabolized. Under normal physiological conditions, the range of RQ is 0.7 (fat oxidation) to 1.0 (glucose oxidation).
12% greater than the value calculated with the Harris-Benedict equation, which predicts basal energy expenditure using body height, weight, age, and sex.
The glucose measurement was obtained 2 h after the administration of 75 g of glucose orally. Values over 200 mg/dl at the 2-h time point are diagnostic for diabetes mellitus.
Study performed in patient at age 44 yr, after the diagnosis and treatment of thyroid cancer.
Discussion
This report shows, for the first time, the impact of TH on BAT activity and volume and leads to speculation of its effects on glucose metabolism in an adult human. Although both white and brown adipose store triglycerides, the unique function of BAT is the generation of heat through uncoupled oxidation of substrates via the integrated effects of the autonomic nervous system and TH. The improvement in glucose control observed in this patient cannot be explained by reversal of the severe hereditary defect of insulin signaling, as demonstrated by the hyperinsulinemic-euglycemic clamp and oral glucose tolerance test results, which indicate a state of extreme insulin resistance despite the normal fasting glucose levels. The functional brown adipose in this patient raises the intriguing possibility that the improvement is secondary to non-insulin-mediated glucose disposal and metabolism due to increased brown adipose volume and activity. We hypothesized that chronic exposure to levels of TH required to suppress TSH for treatment of thyroid cancer in combination with the limited insulin signaling associated with the mutation in this patient led to increased brown adipose activity and volume via the expression of TH responsive genes such as UCP1. This leads to increased substrate oxidation and ultimately improved glycemia control. Although this is a promising hypothesis, alternative explanations such as TH effect on skeletal muscle energy expenditure and fuel metabolism must be considered for its possible contribution to the observed improvement in glycemia.
There is a strong temporal relationship between the dramatic clinical improvement in diabetes control and the initiation of suppressive doses of TH. It is impossible to know whether and by what extent brown adipose activity was present before the first PET study performed for clinical purposes of surveillance for thyroid cancer. Although we cannot definitively demonstrate the causality, our investigation including serial FDG PET-CT analysis shows that both the activity on PET scan and the volume of BAT appears to be responsive to changes in TH levels. A third study performed 14 d after restarting TH demonstrated a return of brown adipose to near baseline levels (27 cm3). The metabolic consequences of levothyroxine withdrawal included a marked decrease in resting energy expenditure, greater than predicted based on hypothyroidism alone (13,14), and an increase in the respiratory quotient reflecting the marked decrease in brown adipose activity and diminished fatty acid oxidation.
The withdrawal of levothyroxine decreases metabolism in all metabolically active tissues, including the heart and skeletal muscle. The FDG-PET/CT scan demonstrated the marked effect of TH on both cardiac and brown adipose metabolism. Under resting conditions, the heart uses approximately 5% of total caloric needs (15), amounting to less than 75 kcal in this patient on levothyroxine. Assuming that cardiac metabolism is decreased by half, the deficit of metabolized carbohydrate is small and unlikely to contribute to the overall glycemic control. The contribution of TH-induced skeletal muscle thermogenesis (see review by Silva in Ref. 16) through induction of genes such as sarcoplasmic/endoplasmic reticulum calcium ATP-ase 1, β-adrenergic receptors, and uncoupling protein 3 was not assessed and presents a major limitation to our report. Recent work by Visser et al. (17) on global gene expression patterns in muscle before and after withdrawal of TH demonstrated the great number of known and previously unknown genes associated with energy utilization. It is also possible that the molecular changes we observed in the sc adipose tissue are mirrored in the skeletal muscle through the stimulation of brown adipose progenitors (4,18,19).
The histological and transcriptional analysis of the periumbilical sc adipose tissue in this patient is more consistent with brown adipose than white adipose tissue. This surprising finding suggests an additional dimension to the concept of plasticity of the adipose organ; potential transdifferentiation of white adipose tissue to BAT, or alternatively a recruitment of stromovascular precursors to BAT, could have occurred in this patient’s white adipose depot. Although brown adipose was not previously thought to play a significant role in human metabolism, the recent work of van Marken Lichtenbelt et al. (8) demonstrated that volume and activity of cold-induced BAT is associated with an increase in resting energy expenditure by up to 30% without evidence of shivering in healthy men. Using similar methods, we estimated the volume (30 g) of brown adipose in the suprascapular/neck region of this patient showing fluctuation with TH levels; however, methods are not yet available to quantify all of the metabolically active brown adipose-like sc tissue. Taken together, we reason that the volume of brown adipose may have a significant impact on energy expenditure and substrate utilization in this patient. Mechanistic studies to assess the impact of cold exposure and resultant increased BAT activity on glucose homeostasis have not been performed.
It is recognized that both brown and white adipose tissues are able to dispose of glucose through the insulin-dependent glucose transporter 4 (GLUT4) (1) as well as insulin-independent mechanisms stimulated by cold exposure, adrenergic stimulation, and TH (20,21,22). Most of the glucose in brown adipocytes is converted to pyruvate, then to lactate, and is subsequently released (23), but some enters the tricarboxylic acid cycle (24) or remains in the cytosol and is converted to fatty acids, the main substrate for thermogenesis. The facilitator of such transport is unclear, although there is evidence that GLUT1 may be involved (25,26). To date, there is no information in humans on the role of BAT in glucose clearance. In rodents, it is estimated to account for 5–15% of the total glucose turnover rate. Although insulin is not required in the cold exposure and sympathetic nervous system stimulation of glucose uptake (27), insulin increases expression of both GLUT4 and UCP1. Notably, GLUT4 transcription is also driven by TH (28). The presence of brown adipose in our subject suggests that a fully functional insulin receptor is not necessary for brown adipose function in humans.
The patient described in this report displays a greatly reduced insulin receptor signal transduction that has led to a life of uncontrollable hyperglycemia and hyperinsulinemia with devastating consequences of blindness, disfiguring hirsutism, and acanthosis nigricans (12). However, after receiving high doses of TH as treatment of thyroid cancer, the severe hyperglycemia resolved and the patient now requires no therapy to maintain her glucose levels on target. The hypothesis that prolonged supraphysiological TH therapy has led to the induction of BAT in this particular patient with a unique genetic and metabolic milieu is highly plausible but cannot be conclusively proven. Nonetheless, the sequence of events in this patient and the results of metabolic tests while on suppressive TH therapy and during withdrawal provide strong indirect evidence that the metabolic and trophic effects of TH on BAT and possibly skeletal muscle play a critical role in non-insulin-mediated glucose utilization in this patient, ultimately leading to near-normal glucose levels. The clinical and biochemical data derived from this case support further clinical and basic research on the roles of TH and BAT in the maintenance of glucose and energy homeostasis.
Acknowledgments
Special thanks go to Dr. Richard Chang, Clinical Center Department of Radiology, and the Clinical Associates and Nursing staff who have cared for this special patient, and especially to the patient herself who continues to selflessly participate in research in the hope of helping others.
Footnotes
This work was supported by intramural National Institute of Diabetes and Digestive and Kidney Diseases Grants Z01 DK075005-04 and Z01-DK047057-01.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 6, 2009
Abbreviations: CT, Computed tomography; D2, type 2 deiodinase; FDG, 18-flouro-deoxyglucose; GLUT, glucose transporter; PET, positron emission tomography; PRDM16, PR domain containing 16; TH, thyroid hormone; UCP1, uncoupling protein-1.
References
- Cannon B, Nedergaard J 2004 Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359 [DOI] [PubMed] [Google Scholar]
- Cohade C, Osman M, Pannu HK, Wahl RL 2003 Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med 44:170–176 [PubMed] [Google Scholar]
- Clarke JR, Brglevska S, Lau EW, Ramdave S, Hicks RJ 2007 Atypical brown fat distribution in young males demonstrated on PET/CT. Clin Nucl Med 32:679–682 [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM 2008 PRDM16 controls a brown fat/skeletal muscle switch. Nature 454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR 2008 New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454:1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR 2009 Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P 2009 Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525 [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJJ 2009 Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- St Germain DL 1988 Dual mechanisms of regulation of type I iodothyronine 5′-deiodinase in the rat kidney, liver, and thyroid gland. Implications for the treatment of hyperthyroidism with radiographic contrast agents. J Clin Invest 81:1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accili D, Frapier C, Mosthaf L, McKeon C, Elbein SC, Permutt MA, Ramos E, Lander E, Ullrich A, Taylor SI 1989 A mutation in the insulin receptor gene that impairs transport of the receptor to the plasma membrane and causes insulin-resistant diabetes. EMBO J 8:2509–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso C, Cochran E, Moran SA, Skarulis MC, Oral EA, Taylor S, Gorden P 2004 Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore) 83:209–222 [DOI] [PubMed] [Google Scholar]
- al-Adsani H, Hoffer LJ, Silva JE 1997 Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab 82:1118–1125 [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C, Hodges RE, Evans TC 1965 Myxedema; a study of 400 cases. Arch Intern Med 116:183–190 [DOI] [PubMed] [Google Scholar]
- Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB 1998 Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol 275:E249–E258 [DOI] [PubMed] [Google Scholar]
- Silva JE 2006 Thermogenic mechanisms and their hormonal regulation. Physiol Rev 86:435–464 [DOI] [PubMed] [Google Scholar]
- Visser WE, Heemstra KA, Swagemakers SM, Ozgür Z, Corssmit EP, Burggraaf J, van Ijcken WF, van der Spek PJ, Smit JW, Visser TJ 2009 Physiological thyroid hormone levels regulate numerous skeletal muscle transcripts. J Clin Endocrinol Metab 94:3487–3496 [DOI] [PubMed] [Google Scholar]
- Crisan M, Casteilla L, Lehr L, Carmona M, Paoloni-Giacobino A, Yap S, Sun B, Léger B, Logar A, Pénicaud L, Schrauwen P, Cameron-Smith D, Russell AP, Péault B, Giacobino JP 2008 A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells 26:2425–2433 [DOI] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM 2009 Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-β transcriptional complex. Nature 460:1154–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco-Perotto R, Zaninetti D, Assimacopoulos-Jeannet F, Bobbioni E, Jeanrenaud B 1987 Stimulatory effect of cold adaptation on glucose utilization by brown adipose tissue. Relationship with changes in the glucose transporter system. J Biol Chem 262:7732–7736 [PubMed] [Google Scholar]
- Cooney GJ, Caterson ID, Newsholme EA 1985 The effect of insulin and noradrenaline on the uptake of 2-[1–14C]deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse. FEBS Lett 188:257–261 [DOI] [PubMed] [Google Scholar]
- Liu X, Pérusse F, Bukowiecki LJ 1998 Mechanisms of the antidiabetic effects of the β 3-adrenergic agonist CL-316243 in obese Zucker-ZDF rats. Am J Physiol 274:R1212–R1219 [DOI] [PubMed] [Google Scholar]
- Ma SW, Foster DO 1986 Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can J Physiol Pharmacol 64:609–614 [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J 1979 The physiological role of pyruvate carboxylation in hamster brown adipose tissue. Eur J Biochem 94:419–426 [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Kielar D, Minokoshi Y, Shimazu T 1996 Noradrenaline increases glucose transport into brown adipocytes in culture by a mechanism different from that of insulin. Biochem J 314:485–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Satoh S, Yano H, Minokoshi Y, Cushman SW, Shimazu T 1998 Effects of noradrenaline on the cell-surface glucose transporters in cultured brown adipocytes: novel mechanism for selective activation of GLUT1 glucose transporters. Biochem J 330:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Pérusse F, Vallerand A, Bukowiecki LJ 1989 Cold exposure reverses inhibitory effects of fasting on peripheral glucose uptake in rats. Am J Physiol 257:R96–R101 [DOI] [PubMed] [Google Scholar]
- Richardson JM, Pessin JE 1993 Identification of a skeletal muscle-specific regulatory domain in the rat GLUT4/muscle-fat gene. J Biol Chem 268:21021–21027 [PubMed] [Google Scholar]