Abstract
Background: It is not known whether acute tissue injury is associated with endoplasmic reticulum (ER) stress.
Objective: Our objective was to determine whether open, sc fat biopsies cause ER stress.
Approach: Five healthy subjects underwent three open sc fat biopsies. The first biopsy, taken from the lateral aspect of a thigh, was followed 4 h later by a second biopsy from the same incision site and a third biopsy from the contralateral leg. Expression markers of ER stress, inflammation, hypoxia, and adipokines were measured in these fat biopsies. In addition, we tested for signs of systemic ER stress and inflammation in plasma and in circulating monocytes.
Results: mRNA/18s ratios of IL-6, monocyte chemoattractant protein-1, CD-14, hypoxia-induced factor 1-α, the spliced form of Xbox protein-1, glucose-regulated protein 78, CCAAT enhance binding protein homologous protein, and activating factor-4 were all severalfold higher, whereas mRNA/18s ratios of adiponectin and leptin were lower in fat biopsies taken from the same site 4 h after the first biopsy but were unchanged in the second biopsy that was taken from the contralateral site. The biopsies were not associated with changes in plasma and monocyte IL-6 concentrations or in monocyte ER stress markers. Also, whole-body insulin-stimulated glucose uptake was the same in 15 subjects who had biopsies compared with 15 different subjects who did not.
Conclusion: Open, sc fat biopsies produced inflammation, hypoxia, ER stress, and decreased expression of adiponectin and leptin. These changes remained confined to the biopsy site for at least 4 h.
Open, subcutaneous fat biopsies cause acute and localized inflammation, hypoxia, and endoplasmic reticulum stress in human subjects.
Open biopsies are frequently needed for diagnostic or research purposes. As any other tissue injury, open biopsies can be expected to elicit prompt inflammatory responses (1). They may also produce endoplasmic reticulum (ER) stress because tissue injuries create cellular calcium and redox state imbalances and produce an accumulation of reactive oxygen species (ROS), conditions that are known to cause ER stress (2). ER stress in turn activates intracellular pathways, collectively called the unfolded protein response (UPR), which generally assist in restoring tissue homeostasis and integrity but that can also cause inflammation (reviewed in Ref. 3). ER stress/UPR, therefore, may be one mechanism by which tissue injury is sensed and transformed into inflammation. It is presently not known whether an acute tissue injury such as an open fat biopsy is associated with ER stress. It was, therefore, the objective of this study to determine whether ER stress (indicated by a rise in UPR mRNAs) develops in adipose tissue after an open sc fat biopsy in healthy human subjects.
Subjects and Methods
Five healthy obese volunteers [two males, three females, age 34 ± 5 yr, body mass index (BMI) 34 ± 1.7 kg/m2] underwent three open sc fat biopsies at 4-h intervals. In addition, insulin-stimulated glucose uptake was measured during 4 h euglycemic-hyperinsulinemic clamping in 30 healthy subjects. Of those, 15 (13 males, two females, age 33.5 ± 2.9 yr, BMI 25.2 ± 1.1 kg/m2) had two open sc fat biopsies (before and after clamp) and the other 15 (11 males, four females, 28.7 ± 1.6 yr, BMI 24.1 ± 1.0 kg/m2) were not biopsied. None of the participants had a family history of diabetes or other endocrine disorders or were taking medications. Their body weights were stable for at least 2 months before the biopsies. Informed written consent was obtained from all subjects after explanation of the nature, purpose, and the potential risks of these studies. The study protocol was approved by the Institutional Review Board of Temple University Hospital.
Fat biopsies
The subjects were admitted to the Temple University Hospital Clinical Research Center on the day before the studies. At approximately 0800 h on the day after admission, venous blood samples were obtained and an open sc fat biopsy was performed by a surgeon from the lateral aspect of one upper thigh (∼15 cm above the patella). The skin was cleaned with betadine and anesthetized with 1% lidocaine (without epinephrine) in a field block pattern (at 2 × 3 in.). An incision (∼1 in.) was made through the skin, and 200–300 mg sc fat was excised (4). The excised fat was dropped immediately into isopentane and kept at its freezing point (−160 C) by liquid nitrogen. The wound was then temporarily closed with adhesive tape. Four hours later, another fat biopsy was obtained approximately 3 in. above the first biopsy site, and a third biopsy was obtained from the contralateral leg. The frozen fat was stored at −80 C until analyzed.
Euglycemic-hyperinsulinemic clamping
Regular human insulin was infused iv at a rate of 7 pmol/kg · min for 4 h. Plasma glucose concentrations were clamped at approximately 5.5 mmol/liter by a feedback-controlled glucose infusion. Blood samples were obtained before (−90, −30, and 0 min) and at hourly intervals after insulin infusion for the determination of plasma glucose and insulin concentrations.
Monocytes
Human mononuclear cells were isolated from freshly heparinized whole blood as described (5).
RT-PCR
Total RNA was isolated from frozen adipose tissues, and real-time RT-PCR was performed with a SYBR Green One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA) and a Light-Cycler (Roche, Indianapolis, IN) as described (4). Primers for the spliced form of X-box binding protein-1 (XBP1s) (NM-005080) were sense TTGAGAACCAGGAGTTAAG and antisense CCTGCACCTGCTGCGGACT; for glucose-regulated protein 78 (GRP78) (NM-005347), sense 5′-GTTGGTGGCTCGACTCGAAT-3 and antisense 5′-CGCTACAGCTTCATCTGGG-3′; for CEBP homologous protein (CHOP) (NM-004083), sense 5′-GGAGAACCAGAAACGGAAAC-3′ and antisense 5′-TCTTCAGCTAGCTGTGCAC-3′; for activating transcription factor 4 (ATF-4) (NM-001675), sense 5′-CCACGTTGGATGACAC-3′ and antisense 5′-GGCTTCCTTCTCCTTCAG-3′; for IL-6 (NM-000600), sense 5′-CAGACAGCCACTCACCTCTT-3′ and antisense 5′-CCAGGCAAGTCTCCTCATTG-3′; for monocyte chemoattractant protein-1 (MCP-1) (NM-002982), sense 5′-GATCTCAGTGCAGAGGCTCG-3′ and antisense 5′-TGCTTGTCCAGGTGGTCCAT-3′; for adiponectin (NM-004797), sense 5′-CTGACTGCAGTCTGTGGTTC-3′ and antisense 5′-GAGTCGTGGTTTCCTGGTCA-3′; for leptin (NM-000230), sense 5′-CTGGCTTCCAGGTATCTCCA-3′ and antisense 5′-ACCTTGGTGGGAATCCCAGA-3′; and for CD14, SuperArray catalog item PPHO5723A.
Analytical procedures
Plasma glucose was measured with a glucose analyzer (YSI, Yellow Springs, OH). Insulin was determined in serum by RIA with a specific antibody that cross-reacts minimally (<0.2%) with proinsulin (Linco, St. Charles, MO). IL-6 in serum was measured by ELISA (R+D Systems, Minneapolis, MN).
Statistical analysis
All data are expressed as means ± se. Pre- and 4-h postbiopsy values were compared using the paired t test. Normality was tested with the Kolmogorov-Smirnov test. The Wilcoxon’s signed-rank test was used to determine significance in the data that were not normally distributed.
Results
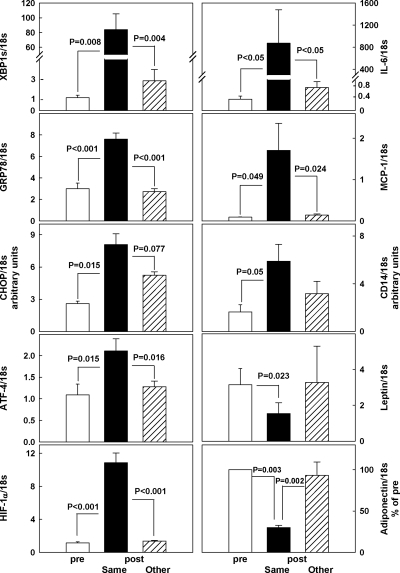
Tissue injury causes localized inflammation and hypoxia (Fig. 1, right)
Figure 1.
Human adipose tissue mRNA/18s ratios of IL-6, MCP-1, CD14, leptin, and adiponectin (right) and of XBP1s, GRP78, CHOP, ATF-4, and HIF-1α (left) in the initial adipose tissue biopsies (white bars), in biopsies obtained 4 h after the initial biopsy from the same incision site (black bars), and in biopsies obtained 4 h after the initial biopsy from the contralateral (other) site (hatched bars). n = 5 for all groups. Statistical analysis shows no significant differences in before vs. after all measurements at the other site. Actual adiponectin mRNA/18s levels were 1.02 ± 0.47 (before), 0.31 ± 0.14 (after at same site), 1.01 ± 0.51 (after at other site).
IL-6, MCP-1, and CD14 mRNA concentrations were severalfold higher, whereas adiponectin and leptin mRNA concentrations were lower in the second fat biopsies obtained from the same site 4 h after the first but were not different when the first and second biopsies were obtained from contralateral sites. The increase in the proinflammatory cytokines/chemokines and the monocyte/macrophage marker CD14 indicated development of an acute inflammatory response and the rise of hypoxia-induced factor-1α (HIF-1α) in the development of tissue anoxia.
Tissue injury causes localized ER stress (Fig. 1, left)
When both biopsies were obtained from the same incision site at 4-h intervals, mRNA/18s ratios of XBP1s, GRP78, CHOP, ATF-4, and HIF-1α were all severalfold higher in the second compared with the first biopsy. In contrast, there were no significant changes in mRNA/18s ratios levels between the first and the second biopsies when the second biopsy was obtained from the contralateral site.
These results suggested that open sc fat biopsies caused ER stress, inflammation and hypoxemia that were confined to the site of tissue injury.
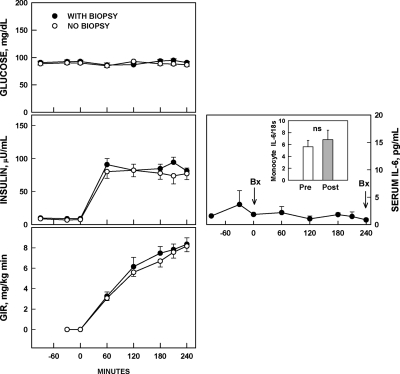
Tissue injury is not associated with systemic markers of inflammation or insulin resistance (Fig. 2)
Figure 2.
Left, Serial plasma glucose and insulin concentrations and glucose infusion rates (GIR) needed to maintain euglycemia during euglycemic-hyperinsulinemic clamping in healthy volunteers who underwent open sc adipose tissue biopsies at 0 and at 240 min (n = 15, •) and in healthy volunteers who were not biopsied (n = 15, ○). Right, Serial serum IL-6 concentrations during the 4 h between two sc adipose tissue biopsies and monocyte IL-6 mRNA content before the first and after the second open sc adipose tissue biopsy (n = 4). Bx, Biopsy.
To test for signs of systemic inflammation, we measured IL-6 concentrations in serum and IL-6 mRNA/18s ratios in circulating monocytes. Neither IL-6 serum concentrations nor IL-6 mRNA/18s ratios in monocytes rose during the 4 h after tissue injury. Also, there were no significant changes in monocyte mRNA/18s ratios of XBP1s [2.4 vs. 1.6, nonsignificant (NS)] CHOP (1.4 vs. 1.8, NS), ATF-4 (1.0 vs. 1.2, NS), and GRP78 (1.7 vs. 2.0, NS).
To further examine whether open sc fat biopsies had systemic effects, we compared rates of whole-body insulin-stimulated glucose uptake during euglycemic-hyperinsulinemic clamping in 15 healthy volunteers who either had two fat biopsies separated by 4 h or had no biopsies. As can be seen in Fig. 2 (left panels), rates of glucose infusion rates needed to maintain euglycemia during hyperinsulinemia were the same whether or not the volunteers had two biopsies. These results suggested that ER stress and inflammation were confined to the biopsy site.
Discussion
Inflammation is known to be an early feature of acute tissue injury (1), and disruption of normal blood supply is likely to produce tissue anoxia. As expected, therefore, fat biopsy samples obtained from the same incision site, 4 h after the first biopsy, showed signs of inflammation (increased transcriptional expression of IL-6, CD14, and MCP-1) and hypoxia, (increased expression of HIF-1α) (6). The inflammation seen in the second biopsy samples taken 3 in. upstream from the first biopsy site was presumably caused by extravasated blood and injured cells releasing proinflammatory signals (1).
A new finding was that transcriptional expression of the ER chaperones and transcription factors GRP78, XBP1s, ATF-4, and CHOP had also increased severalfold in the second fat biopsy within 4 h of tissue injury. This represented strong evidence for the presence of ER stress and, to our knowledge, was the first demonstration of ER stress developing acutely in vivo in a human tissue.
In in vitro experiments, ER stress has previously been shown to cause inflammation via different mechanisms including activation of c-Jun N-terminal kinase (JNK), and nuclear factor-κB (NF-κB) or by producing ROS (3,7). Conversely, however, inflammation may also be able to produce ER stress, because TNF-α has been shown to activate the UPR in mouse fibrosarcoma cells in a ROS-dependent manner (8).
Another interesting finding was that the biopsies decreased adiponectin and leptin transcription. Because both peptides are synthesized only in fat, the reason for the decrease in adiponectin blood levels in obesity seems paradoxical (9). Our findings suggested that it may be related to inflammation (10), ER stress, and/or hypoxia (6,11), which are all typically present in fat of obese people (1,4,11). Also, proinflammatory cytokines have been reported to decrease leptin mRNA in human fat in vitro (12).
Whether or not biopsy-induced ER stress remains localized within the adipose tissue may be important because systemic adipose tissue dysfunction caused by ER stress has been postulated to be a key pathological factor in obesity-related problems (7). The evidence that the biopsy-induced ER stress, hypoxia, and inflammation were confined to the biopsy site, at least for the initial 4 h, was based on the observation that there were no signs of ER stress, hypoxia, or inflammation in fat biopsies taken from the contralateral leg, that there were no increases in markers of inflammation or ER stress in serum or in circulating monocytes, and that the inflammation and ER stress at the biopsy site had no effect on whole-body insulin-stimulated glucose uptake, which during euglycemic-hyperinsulinemic clamping occurs predominantly into skeletal muscle (13). Although we cannot rule out that ER stress and/or inflammation may have affected other untested variables, such as protein and lipid synthesis in the biopsies taken from the contralateral leg, our results nevertheless suggested that biopsies performed in conjunction with clamps should be taken from different sites.
In summary, these studies showed that sterile tissue injury produced by surgical incisions and sc fat biopsies produced ER stress, inflammation, and hypoxemia, events that can assist in restoring tissue integrity but that can also cause insulin resistance. They also showed that these events remained localized to the biopsy site for at least 4 h.
Acknowledgments
We thank Maria Mozzoli, B.S., for excellent technical assistance and Constance Crews for typing the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants R01-HL-073367 and R01-DK-066003 and a grant from the Groff Foundation (all to G.B.).
Disclosure Summary: G.B., M.S., B.S., P.C., and C.H. have nothing to declare.
First Published Online November 6, 2009
Abbreviations: ATF-4, Activating transcription factor-4; CHOP, CCAAT enhance binding protein homologous protein; ER, endoplasmic reticulum; GRP78, glucose-regulated protein 78; HIF-1α, hypoxia-induced factor-1α; MCP-1, monocyte chemoattractant protein-1; NS, nonsignificant; ROS, reactive oxygen species; UPR, unfolded protein response; XBP1s, spliced from of X-box binding protein-1.
References
- Rock KL, Kono H 2008 The inflammatory response to cell death. Annu Rev Pathol 3:99–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ 2005 The mammalian unfolded protein response. Annu Rev Biochem 74:739–789 [DOI] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ 2008 From endoplasmic-reticulum stress to the inflammatory response. Nature 454:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S 2008 Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese insulin-resistant individuals. Diabetes 57:2438–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G 2006 Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes 55:202–208 [PubMed] [Google Scholar]
- Ye J, Gao Z, Yin J, He Q 2007 Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293:E1118–E1128 [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS 2007 Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 48:1905–1914 [DOI] [PubMed] [Google Scholar]
- Xue X, Pial JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano J 2005 Tumor necrosis factor α (TNFα) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFα. J Biol Chem 280:33917–33925 [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF 1996 Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295 [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R 2003 Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun 301:1045–1050 [DOI] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I 2007 Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911 [DOI] [PubMed] [Google Scholar]
- Bruun JM, Pedersen SP, Kristensen K, Richelsen B 2002 Effects of pro-inflammatory cytokines and chemokines on leptin production in human adipose tissue in vitro. Mol Cell Endocrinol 190:91–99 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP 1981 The effect of insulin on the disposal of intravenous glucose; results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30:1000–1007 [DOI] [PubMed] [Google Scholar]