Abstract
Context: Serum pituitary antibodies (Pit Abs) and tumor-infiltrating lymphocytes (TILs) have been described in pituitary adenomas, but their clinical significance remains unknown.
Objective: The objective of the study was to assess Pit Abs and TILs prevalence in pituitary adenomas and their influence on clinical outcome.
Design: This was a prevalence case-control study.
Patients and Setting: Two hundred ninety-one pituitary adenoma cases (110 non-secreting, 30 ACTH-69 GH-71 prolactin- and 13 TSH-secreting adenoma; 177 operated and 114 untreated), 409 healthy controls, and 14 autoimmune hypophysitis were enrolled in a tertiary referral center.
Intervention: Pit Abs were measured using immunofluorescence in all cases and controls (n = 714). The presence of TILs was evaluated using CD45 staining in a subset of adenomas surgically treated (n = 72).
Main Outcome Measure: Clinical response of pituitary adenoma after surgery was evaluated.
Results: Pit Abs prevalence was higher in adenomas (5.1%) than healthy subjects (0.7%, P < 0.0001) and lower than in autoimmune hypophysitis patients (57%, P < 0.0001). Similarly, TILs prevalence was higher in adenomas than normal pituitary (P = 0.01) and lower than in autoimmune hypophysitis (P < 0.0001). No correlation between Pit Abs and TILs was found (P = 0.78). A poor clinical outcome was more common in adenoma patients with TILs (11 of 18, 61%) than in those without (17 of 54, 31%, P = 0.026). Multivariate regression analysis identified the presence of TILs as independent prognostic factor for persistence/recurrence of pituitary adenoma.
Conclusions: TILs and Pit Abs are present in a significant number of pituitary adenoma patients. Cell-mediated immunity appears to be predictive of a less favorable clinical outcome.
Pituitary antibodies and infiltrating lymphocytes are found in a significant number of pituitary adenomata; cell-mediated immunity predicts poorer clinical outcome in these patients.
Tumors are frequently infiltrated by lymphocytes. In some tumors, like germinomas and papillary thyroid cancer (1,2), tumor infiltrating lymphocytes (TILs) are often numerous; in other tumors, TILs are scanty or have not been systematically characterized.
Whereas it is established that the immune system has the capacity to recognize tumor antigens, it remains unclear whether it protects the host from tumor growth and spread. TILs have, in fact, several phenotypes. Effector CD8 lymphocytes and natural killer lymphocytes are beneficial to the host because they directly contact the tumor cell and initiate a cytotoxic cascade that eventually kills the tumor cells (3). They also contribute to the antitumor response by an antibody-dependent target cell killing. On the contrary, suppressor (or regulatory) TILs are detrimental for the patient (3). They are characterized by the expression of CD4, CD25, and Foxp3 and inhibit effector lymphocytes in a cytokine or cell-contact dependent fashion, ultimately dampening their beneficial antitumor activities (4,5).
The prognostic relevance of tumor autoimmunity, defined here as the presence of TILs or antibodies directed against tumor antigens, remains to be elucidated for most human cancer. As is often the case for several studies on human cancer, interpretation of the data are further complicated by the heterogeneity of the patient population and the tumor itself.
Very limited information exists on the presence of tumor autoimmunity in patients with pituitary adenomas. TILs have been found in 43 of 1440 (3%) surgical pathology cases reviewed retrospectively (6). TILs were mainly T cells, localized around vessels and in the region containing the adenoma, and present with similar frequency in the different types of hormone-secreting adenomas [prolactin (PRL), GH, ACTH, and multihormonal]. Serum pituitary antibodies (Pit Abs), on the other hand, have been measured so far in pituitary adenoma patients using different techniques (mainly immunofluorescence, immunoblotting, ELISA, and radioligand assay) and were found to have a prevalence ranging between 0 and 30% (7,8,9,10,11,12,13,14,15,16,17,18,19).
Whether the presence of Pit Abs is integrated with a TILs response in pituitary adenoma and which is their clinical significance in this setting is currently unknown. We designed the present study to analyze the prevalence of Pit Abs and TILs in a large cohort of patients with functioning and non-functioning pituitary adenomas in an effort to support or dismiss the prognostic relevance of tumor autoimmunity in this frequently occurring pathological condition.
Subjects and Methods
The study was organized in two parts. The first part analyzed 714 subjects to assess the presence of Pit Abs in adenoma cases (n = 291), healthy controls (n = 409), and controls with histologically proven autoimmune hypophysitis (n = 14). Then, the prevalence of Pit Abs in patients with pituitary adenoma was compared across 13 previously published studies and the present study performing a meta-analysis.
The second part analyzed a subset of operated adenoma cases (n = 72) to assess the presence of TILs and Pit Abs and correlate it with the clinical outcome of the disease during a mean follow-up period of 34 ± 2 months.
Subjects
The main clinical and demographic features of the subjects presented in this study are summarized in Table 1.
Table 1.
Clinical features and Pit Ab prevalence of the study population at the time of serum collection
| Disease | No. (F, M) | Age (mean ± sd) | Pituitary surgery | Adenoma size 10 mm or greater | Positive for pituitary antibodies by IF |
|---|---|---|---|---|---|
| ACTH-secreting adenoma | 29 (24, 5) | 44 ± 11 | 29 | 10 | 2 (7%) |
| GH-secreting adenoma | 68 (39, 29) | 53 ± 13 | 52 | 44 | 3 (4%) |
| PRL-secreting adenoma | 71 (54, 17) | 39 ± 14 | 14 | 26 | 6 (8%) |
| TSH-secreting adenoma | 13 (6, 7) | 51 ± 15 | 10 | 2 | 0 |
| Non-secreting adenoma | 110 (74, 36) | 51 ± 19 | 66 | 80 | 4 (4%) |
| Healthy controls | 409 (312, 97) | 45 ± 15 | 0 | NA | 3 (1%) |
| Hypophysitis controls | 14 (11, 3) | 35 ± 12 | 14 | NA | 8 (57%) |
IF, Immunofluorescence assay; NA, not applicable.
Cases included 291 patients (94 men and 197 women) with pituitary adenoma first evaluated at the Department of Endocrinology of the University of Pisa between January 2003 and March 2008. Serum was collected at the time of the initial outpatient visit in all patients. Pituitary surgery had already been performed in 177 of the 291 adenomas (at the University of Pisa in 91 patients and in other centers in 86 patients), whereas the remaining 114 were non-operated adenomas. The adenomas were non-functioning (n = 110), PRL-secreting (n = 71), GH-secreting (n = 68), ACTH-secreting (n = 29), or TSH-secreting (n = 13). Their diameter, as assessed by magnetic resonance imaging (MRI), was 10 mm or greater in 162 patients (macroadenomas, Table 1), and less than 10 mm (microadenoma) in the remaining 129. Of the 71 patients with PRL-secreting adenoma, 22 were untreated and 49 were under cabergoline treatment.
Controls included 409 healthy subjects (negative controls) and 14 patients with histologically proven lymphocytic hypophysitis (positive controls). Healthy subjects (97 men, aged 47 ± 15 yr, and 317 women, aged 44 ± 14 yr) were selected based on unremarkable physical examinations, normal endocrine function, and an absence of the following antibodies: thyroglobulin, thyroperoxidase, gastric parietal cells, glutamic decarboxylase, acetylcholine receptor, transglutaminase and 21-hydroxilase. Hypophysitis patients (eight women and six men, aged 31 ± 12 yr) were described in a recently published cohort [patients 1–14 (13)]. The study was approved by the institutional ethics committee on a previously obtained informed consent.
Pituitary antibodies measured by indirect immunofluorescence
Pit Abs were measured in all cases (n = 291), healthy controls (n = 409), and hypophysitis controls [n = 14 (13)] by indirect immunofluorescence, using a Macaca mulatta anterior pituitary and the commercially available kit (Euroimmun, Lubeck Germany) previously described (13). Sera were considered positive when a diffuse cytoplasmatic staining was observed at a 1:10 dilution (20). Positive sera were further diluted at 1:30 and 1:90 to assess pituitary antibody titer. All slides were read blindly by two of the investigators (L.M. and I.L.). Minor differences between observers were resolved by mutual agreement.
Pituitary histopathology and lymphocytic infiltration measured by CD45 staining
Surgical blocks were available in 72 of the total 91 adenoma cases operated at the University of Pisa. This subset of 72 cases included 14 ACTH-, 18 GH-, four PRL-, and four TSH-secreting and 32 nonsecreting adenoma. The specimens were stained for CD45, a cell surface marker commonly used to establish the hematopoietic origin of a cell. Twelve non-diseased pituitary glands were obtained at autopsy as normal, non-adenoma controls.
CD45 immunostaining was performed on non-consecutive pituitary sections cut from formalin-fixed, paraffin-embedded pituitary specimens. Sections were deparaffinized in xylene, rehydrated with decreasing concentrations of ethyl alcohol, and then processed for antigen retrieval [15 min at 98 C in citrate buffer (pH 6.0)]. Sections were then incubated for 30 min at room temperature in bovine serum to block aspecific antibody binding and then for 60 min at room temperature with a mouse monoclonal antibody directed against human CD45 (clone PD7/26/16 + 2B11 diluted 1:50 in antibody diluent; DiaPath, Martinengo, Italy). Antibody binding was detected using the streptavidin-biotin peroxidase detection system (Vectastain Elite kit, Vector Laboratories, Burlingame, CA), followed by addition of 3,3′-diaminobenzidine chromogen. Sections were finally counterstained with Mayer’s hematoxylin, rinsed in tap water, dehydrated through increasing concentrations of ethyl alcohol, and mounted.
CD45 expression was scored semiquantitatively on a scale ranging from 0 to 5. A score of 0 indicates a normal pituitary, in which only a few hematopoietic cells are present; a score of 5 indicates that most of the pituitary is infiltrated by hematopoietic cells. All immunostainings were analyzed independently by I.L. and M.M. Minor differences between observers were resolved by mutual agreement.
Statistical analysis
The prevalence (presence/absence) of Pit Abs among the three diagnostic categories (cases, healthy controls, and hypophysitis controls), the five secretory types of adenoma, the two sizes of adenoma, or the coexistence of autoimmune thyroid disease were compared by χ2 test and univariate logistic regression. The intensity of CD45 pituitary staining among the three diagnostic categories was compared using the Wilcoxon’s rank sum test.
The main outcome measure of the study was the clinical response of the 72 pituitary adenoma patients in which the presence of CD45-positive cells was evaluated by immunohistochemistry after pituitary surgery during a mean follow-up time of 34 ± 2 months.
Clinical outcome was evaluated on follow-up based on the MRI size of the adenoma (disappeared, relapsed, or with a persistent and increased residual adenomatous tissue after incomplete debulking) in patients with nonsecreting adenoma and on the status of the initial hormonal hypersecretion (cured or persistent/relapsed) and the MRI size of the adenoma in patients with secreting adenoma.
Binary logistic regression was used to test the hypothesis that the clinical outcome is influenced by the presence or absence of CD45-positive cells. Other covariates evaluated in the regression model were sex, age quintiles, adenoma size, adenoma secretory types, days of follow-up, coexistence of thyroid autoimmunity, and Pit Abs positivity. A backward selection approach was used to identify the covariates that significantly contributed to the model, which turned out to be only the adenoma size. Results of the multivariate binary logistic regression were expressed as odds (or probability) of a poor clinical outcome based on the presence or absence of CD45-positive cells, both as crude or adjusted for adenoma size.
Quantitative variables were expressed as mean ± sd.
All analyses were performed using Stata Statistical Software, release 10 (StataCorp, College Station, TX).
Meta-analysis
Review of the complete literature about pituitary autoimmunity/hypophysitis, downloadable from the Johns Hopkins hypophysitis research center (http://pathology2.jhu.edu/hypophysitis), identified 13 publications that had Pit Abs in both cases with pituitary adenoma (n = 287) and healthy controls (n = 962) (7,9,10,11,12,13,14,15,16,17,18,19,22). Pit Abs were measured by immunofluorescence (n = 354), in vitro transcription translation (n = 153), ELISA (n = 140), immunoblotting (n = 83), or a combination of the above techniques (n = 519).
The Mantel-Haenszel method was used to compute weighted odds ratios for each study and the overall odds ratio. Odds ratio values significantly greater than 1 indicated that the prevalence of Pit Abs in patients with pituitary adenoma was significantly higher than that in healthy controls.
Results
Prevalence of pituitary antibodies
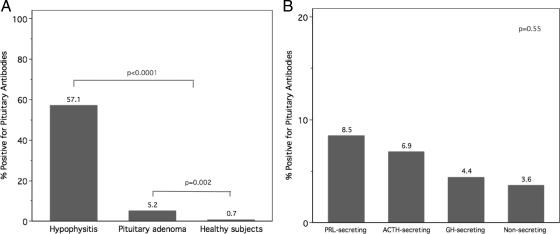
Pit Abs were found in 15 of 291 adenoma patients (5.2%), eight of 14 hypophysitis patients (57.1%), and three of 409 healthy controls (0.7%) (Table 1). The antibody titer was 1:10 in 11 adenoma cases and the three healthy subjects and 1:30 in the four remaining adenoma cases (one ACTH, one GH, and two PRL secreting) and the eight hypophysitis patients.
The prevalence of Pit Abs in the adenoma cases was significantly higher than that in the healthy controls (P = 0.002, Fig. 1A) and lower than that in the hypophysitis group (P < 0.0001, Fig. 1A).
Figure 1.
A, Prevalence of Pit Abs in patients with pituitary adenoma (all types), autoimmune hypophysitis, and healthy controls. B, Prevalence of Pit Abs in patients with ACTH- (n = 29), GH- (n = 68), PRL- (n = 71), and non-secreting adenoma (n = 110).
There was no difference in Pit Abs prevalence among the five pituitary adenoma secretory types (P = 0.55, Fig. 1B). In particular, Pit Abs were found in two of 29 patients with ACTH-secreting adenoma (6.9%), three of 68 patients with GH-secreting adenoma (4.4%), six of 71 PRL-secreting adenoma (8.4%), four of 110 non-secreting pituitary adenoma (3.6%), and none of the TSH-secreting adenoma patients.
Similarly, no difference related to the adenoma size was observed: Pit Abs were indeed found in eight of 162 patients with macroadenoma (4.9%) and seven of 129 microadenoma (5.4%, P = 0.85). A history of pituitary surgery had no influence on the prevalence of Pit Abs: antibodies were, in fact, found in eight of 114 not operated patients (7%) and seven of 177 (4%) operated patients (P = 0.284). Similarly, when we considered the subgroup of PRL-secreting adenoma, we could not find a significant association between cabergoline treatment and Pit Abs prevalence; Pit Ab were found in three of 49 (6%) patients who were under cabergoline treatment and three of 22 (13.6%) who were untreated; this difference, however, was not statistically significant (P = 0.293).
Meta-analysis of Pit Abs
A meta-analysis combining 13 studies and our current study was performed to compare the prevalence of Pit Abs in adenoma cases and healthy controls. Two studies (9,16) were dropped by the meta-analysis because no Pit Abs were found in either adenomas or healthy controls. The Mantel-Haenszel pooled estimate indicated that the odds of having Pit Abs were 7-fold greater in patients with pituitary adenoma than in healthy controls (95% confidence interval from 4.9 to 11.6, Table 2). The χ2 test of homogeneity was not significant (P = 0.243), indicating minimal variability among the odds ratios from the individual studies.
Table 2.
Meta-analysis of 14 studies testing pituitary antibodies prevalence in pituitary adenoma patients and in controls
| Year | First author | Method | OR | 95% Conf. interval | % weight |
|---|---|---|---|---|---|
| 1987 | Scherbaum | IF | 36.12 | 2.08–626.5 | 2.55 |
| 1988 | Komatsu | IF | 7.93 | 0.38–162.1 | 2.99 |
| 1993 | Mau | IB | 18.2 | 0.67–494.8 | 1.32 |
| 1998 | Crock | IB | 2.35 | 0.56–9.84 | 15.35 |
| 1998 | Yabe | IB & ELISA | 0.26 | 0.01–4.61 | 21.41 |
| 2000 | Kikuchi | IB & ELISA | 9.77 | 4.20–22.7 | 15.67 |
| 2002 | Keda | ELISA | 6.88 | 1.73–27.42 | 10.6 |
| 2003 | Tanaka | ITT | 18.86 | 3.15–112.72 | 3.27 |
| 2003 | De Bellis | IF | 10.28 | 1.86–56.72 | 5.53 |
| 2003 | Tatsumi | ITT | 77.34 | 4.39–1360 | 1.29 |
| 2008 | Lupi | IB | 2.69 | 0.15–46.26 | 3.59 |
| 2009 | Lupi (current study) | IF | 7.35 | 2.11–25.64 | 16.34 |
| 2002 | Tanaka | ITT | Excluded | ||
| 2007 | De Bellis | IF | Excluded | ||
Mantel-Haenszel pooled odds ratio = 7.02 (95% confidence interval from 4.9 to 11.6). IF, immunofluorescence; IB, immunoblotting; ELISA, Enzyme-linked immunosorbent assay; ITT, in vitro transcription translation.
Prevalence and severity of mononuclear cell infiltration in the pituitary gland
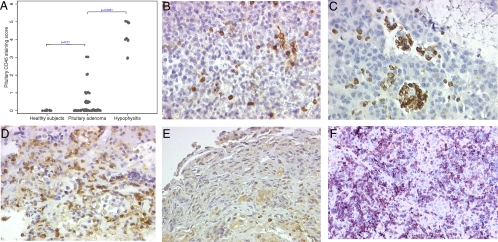
Mononuclear cell infiltration, assessed by CD45 immunostaining, was found in 18 of 72 pituitary adenoma specimens (25%). There was no statistically significant difference (P = 0.275) among the various secretory types: one of 14 ACTH-secreting (7%), five of 18 GH-secreting (28%), two of four PRL-secreting (50%), two of four TSH-secreting (50%), and eight of 32 nonsecreting adenoma (25%), although the infiltration tended to be lower in ACTH adenomas. There was also no difference (P = 0.423) based on the size of the adenoma: CD45-positive cells were, in fact, observed in three of 17 microadenomas (18%) and 15 of 55 macroadenomas (27%). The mononuclear infiltration was mild (a score of 1 or 0.5) in the majority of the cases (13 of 18, 72%, Fig. 2B) and moderate (a score of 2 or 3) in the remaining five cases: a score of 2 was found in two PRL- and one nonsecreting adenoma and a score of 3 in two nonsecreting adenoma (Fig. 2, C and D). Normal (non-diseased) pituitary collected at autopsy showed very few CD45-positive cells (4 ± 1, Fig. 2E), whereas pituitary gland of patients with autoimmune hypophysitis (the positive control) was overrun by lymphocytes (Fig. 2F).
Figure 2.
A, CD45 immunostaining infiltration score in pituitary adenomas (n = 72), normal pituitary at autopsy (n = 12), and autoimmune hypophysitis (n = 14). Pituitary adenoma CD45 immunostaining infiltration scores 1 (B), score 2 (C), score 3 (D), normal pituitary at autopsy score 0 (E), and autoimmune hypophysitis score 5 (F) are shown.
Overall, the CD45 staining in pituitary adenomas was significantly greater than that in normal pituitary (Fig. 2A, P = 0.01) and lower than that in autoimmune hypophysitis (Fig. 2A, P < 0.0001).
Only one of 18 adenoma cases with CD45-positive cells had also positive Pit Abs: thus, no correlation between the presence of Pit Abs and CD45-positive cells in the pituitary was observed in adenoma patients (P = 0.78).
Clinical outcome in pituitary adenomas with mononuclear cell infiltration
The clinical outcome was evaluated in a follow-up visit in all 72 patients who underwent surgery and in which CD45 staining was performed. The mean follow-up period was 34 ± 2 months.
Poor clinical outcome was observed in 28 of 72 (38%), whereas a favorable outcome was observed in 44 of 72 operated patients (62%). In particular, we classified as adenoma with a poor outcome 13 secreting adenoma (12 macroadenoma and one microadenoma) in which was observed biochemical persistence (one ACTH, six GH, two PRL secreting) or recurrence of the disease after surgery (four ACTH secreting). Among the non-secreting adenoma, we classified as adenoma with poor outcome 15 cases, all macroadenoma, in which was observed persistence and growth of residual adenomatous tissue after incomplete surgical debulking (n = 13), or recurrence of the adenoma after surgery (n = 2). On the contrary, we classified as secreting adenoma with favorable outcome 27 cases, 15 microadenoma and 12 macroadenoma, in which was observed cure of the initial hypersecretion after surgery (nine ACTH, 12 GH, two PRL, four TSH secreting adenoma). Finally, we classified as non-secreting adenoma with favorable outcome 17 macroadenoma in which complete mass debulking, and no relapse after surgery was observed.
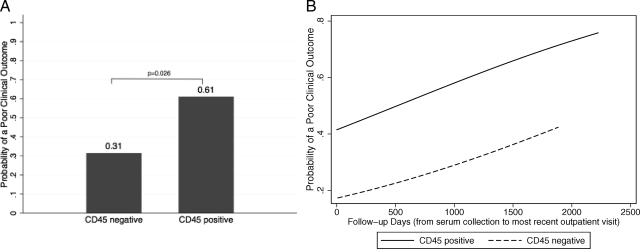
A poor clinical outcome was significantly more common in adenoma patients with CD45-positive cells (11 of 18, 61%) than in those without (17 of 54, 31%, P = 0.026 by χ2 test, Fig. 3A). A poor clinical outcome was observed in three of five (60%) patients with Pit Abs and 25 of 67 patients without Pit Abs (37%): however, we could not observe a significant correlation between the presence of Pit Abs and poor clinical outcome (P = 0.315). Pit Abs, measured during follow-up in the five positive Pit Abs patients, remained positive in two of three with poor clinical outcome and in one of two with good clinical outcome.
Figure 3.
A, Probability of a poor clinical outcome in patients with CD45-positive cells (11 of 18, 61%) and CD45-negative cells (17 of 54, 31%). B, Probability of a poor clinical outcome, adjusted for adenoma size, increases over time in patients with CD45-positive cells (n = 18, solid line) and CD45-negative cells (n = 54, dashed line).
Binary logistic regression analysis showed that the odds of a poor clinical outcome were 3.4-fold greater in patients with CD45-positive cells than in those without (95% confidence interval from 1.12 to 10.35, P = 0.03).
Results remained significant when the effect of CD45 positivity on the clinical outcome was adjusted for the adenoma size: the odds of a poor clinical outcome were, in fact, 3.3-fold greater than that observed in adenoma without mononuclear cell infiltration (95% confidence interval from 1.03 to 10.7, P = 0.044) as shown in Table 3.
Table 3.
Multivariate logistic regression analysis showing the predictors of a poor clinical outcome in patients with pituitary adenoma
| Predictor | Adjusted odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| CD45 positive cells | 3.3 | 1.03–10.7 | 0.044 |
| Adenoma size ≥10 mm | 6.5 | 1.32–32.6 | 0.021 |
The probability of a poor clinical outcome increased over time (Fig. 3B). Addition of follow-up days (the time from serum collection to the most recent outpatient visit) to the multivariate logistic regression model showed that, after adjusting for the adenoma size, the clinical outcome tended to worsen with time, although not reaching the 0.05 significance level (regression analysis showed that for every 100 d increase in follow-up, the odds of a poor clinical outcome increased 1.0007-fold, 95% confidence interval from 0.99 to 1.001, P = 0.167, Figure 3B).
Discussion
This study reports that cellular pituitary autoimmunity is a predictor of poor clinical outcome in patients with pituitary adenomas. TILs were, in fact, more prevalent in adenomas than in healthy controls and predicted a less satisfactory clinical outcome upon follow-up.
TILs are found, with various extent, in numerous solid tumors like germinoma, melanoma, ovarian, breast, and colon cancer (23,24,25,26). TILs and other mononuclear cells, like tumor-associated macrophages and dendritic cells, are recruited to the tumor site through several mechanisms including specific recognition of tumor antigens, aspecific inflammatory response to necrotic tumor cells, and chemotaxis. The phenotype and functional consequences of TILs are complex. Some TILs behave as effector cells and are thus beneficial for the host because they induce direct cytotoxicity of the tumor cells. Other TILs, however, have a T regulatory phenotype and are harmful to the host because they inhibit the antitumor action of effector T cells. Not surprisingly, the prognostic significance of TILs is contradictory: TILs present in ovarian cancer are associated with a more favorable outcome and longer survival (26), and in seminoma a low TILs count has been associated with a higher risk of relapse (25); on the contrary, TILs are more abundant in colon adenomas found in hereditary nonpolyposis colorectal cancer syndrome than in those found in the general population (27).
Very limited information is available for TILs present in pituitary adenomas. A slight grade of lymphocytic infiltration, also referred to as secondary hypophysitis, can be found adjacent to pituitary adenomas (28,29,30,31) as well as in other tumors such as Rathke’s cleft cyst (32,33,34) and craniopharyngioma (35) and is considered to be a peritumoral inflammatory reaction. A different condition seems to be the case of lymphocytic infiltration within the adenomatous lesion. TILs have been described to be present within the adenoma lesion in 3% of a cohort of 1400 secreting and nonsecreting adenomas. However, no difference was found in relation to the type of tropin secretion (6). The study was retrospective; thus, a correlation with humoral pituitary autoimmunity or disease outcome could not be investigated. In our study we similarly could not demonstrate an association between the presence of pituitary autoimmunity, either humoral or cell mediated, and the adenoma type. In particular, we could not find any association between the hypersecretion of GH, which is a proposed autoantigen in autoimmune hypophysitis (13,36), and the presence of pituitary autoimmunity. These findings suggest that the type of hormonal secretion per se might not be determinant in pituitary autoimmunity development. Interestingly, results from the multivariate analysis showed that the presence of TILs influences the disease progression independently from the adenoma size. From a clinical perspective, it implies that when TILs are found in macroadenomas the risk of a poor outcome becomes higher.
Not only the cell-mediated autoimmunity but also the humoral immunity represents a response to the tumor presence/growth. Autoantibodies, either those against tumor antigens or those against ubiquitous antigens, are often found in tumors (23,37,38). Interestingly, detecting cancer antigen–related autoantibodies has proven useful in early diagnosis of certain malignant tumors as lung cancer (39). The prevalence of humoral autoimmunity holds in the range of 10–30% for all types of cancer and may precede the clinical manifestation of the tumor (38). However, the overall low sensitivity of individual autoantibodies make them, at present, a poor diagnostic marker for clinical purposes.
In this study Pit Abs were measured, using the same assay, in a large cohort of pituitary adenoma patients; interestingly, we found that their prevalence is in accordance with the results obtained from the meta-analysis. Although Pit Abs measurement suffers from limitations due to the non-antigen specificity of the currently available methods, the results from the meta-analysis are highly consistent among the different studies. Hence, with a risk of having Pit Abs 7-fold higher in adenoma patients than control subjects, the finding of Pit Abs in adenoma patients appears not to be an infrequent event.
Autoantibodies are a classic marker of autoimmune diseases and are of great help for the diagnosis, prediction and, to some extent, monitoring of the remission of the disease. It comes into view that tumor immunology is on the way to developing similar tools that will be valuable in the serological identification of tumors. Limitation/efficacy of immunological markers primarily relies on the antigen specificity of the methods used, and therefore, Pit Abs cannot, at present, be used in clinical practice (21). Taking these restrictions into account, this study establishes a positive correlation between pituitary cell-mediated autoimmunity and adenoma disease progression. Also, our data well support the hypothesis that pituitary adenomas act as other tumors in predisposing to autoimmunity development. Based on our findings, pituitary adenomas seem to be an intermediate condition that holds between normal pituitary and hypophysitis. Our findings need further investigations such as the analysis of the immune cell subtypes to determine the mononuclear cell types that influence tumor progression.
Acknowledgments
We thank Professor Aldo Pinchera (University of Pisa) for his continuous encouragement and advice and Dr. Sonia Albertini for her precious contribution in performing Pit Abs assay.
Footnotes
This work was supported in part by grants from the Ministero dell’Istruzione, Università e Ricerca (Rome, Italy), University of Pisa (Fondi d’Ateneo per la Ricerca) (to E.M.), and National Institutes of Health Grant DK080351 (to P.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 29, 2009
Abbreviations: MRI, Magnetic resonance imaging; Pit Abs, pituitary antibodies; PRL, prolactin; TILs, tumor-infiltrating lymphocytes.
References
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA 2003 Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA 100:8372–8377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, Matsuzuka F, Kakudoh K, Kuma K, Tamai H 1995 The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab 80:3421–3424 [DOI] [PubMed] [Google Scholar]
- Le Bitoux MA, Stamenkovic I 2008 Tumor-host interactions: the role of inflammation. Histochem Cell Biol 130:1079–1090 [DOI] [PubMed] [Google Scholar]
- Kosmaczewska A, Ciszak L, Potoczek S, Frydecka I 2008 The significance of Treg cells in defective tumor immunity. Arch Immunol Ther Exp (Warsz) 56:181–191 [DOI] [PubMed] [Google Scholar]
- Uppaluri R, Dunn GP, Lewis Jr JS 2008 Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in head and neck cancers. Cancer Immun 8:16 [PMC free article] [PubMed] [Google Scholar]
- Heshmati HM, Kujas M, Casanova S, Wollan PC, Racadot J, Van Effenterre R, Derome PJ, Turpin G 1998 Prevalence of lymphocytic infiltrate in 1400 pituitary adenomas. Endocr J 45:357–361 [DOI] [PubMed] [Google Scholar]
- De Bellis A, Bizzarro A, Conte M, Perrino S, Coronella C, Solimeno S, Sinisi AM, Stile LA, Pisano G, Bellastella A 2003 Antipituitary antibodies in adults with apparently idiopathic growth hormone deficiency and in adults with autoimmune endocrine diseases. J Clin Endocrinol Metab 88:650–654 [DOI] [PubMed] [Google Scholar]
- De Bellis A, Colao A, Pivonello R, Savoia A, Battaglia M, Ruocco G, Tirelli G, Lombardi G, Bellastella A, Bizzarro A 2007 Antipituitary antibodies in idiopathic hyperprolactinemic patients. Ann NY Acad Sci 1107:129–135 [DOI] [PubMed] [Google Scholar]
- De Bellis A, Sinisi AA, Conte M, Coronella C, Bellastella G, Esposito D, Pasquali D, Ruocco G, Bizzarro A, Bellastella A 2007 Antipituitary antibodies against gonadotropin-secreting cells in adult male patients with apparently idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 92:604–607 [DOI] [PubMed] [Google Scholar]
- Keda YM, Krjukova IV, Ilovaiskaia IA, Morozova MS, Fofanova OV, Babarina MB, Marova EI, Pankov YA, Kandror VI 2002 Antibodies to pituitary surface antigens during various pituitary disease states. J Endocrinol 175:417–423 [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Yabe S, Kanda T, Kobayashi I 2000 Antipituitary antibodies as pathogenetic factors in patients with pituitary disorders. Endocr J 47:407–416 [DOI] [PubMed] [Google Scholar]
- Komatsu M, Kondo T, Yamauchi K, Yokokawa N, Ichikawa K, Ishihara M, Aizawa T, Yamada T, Imai Y, Tanaka K, et al 1988 Antipituitary antibodies in patients with the primary empty sella syndrome. J Clin Endocrinol Metab 67:633–638 [DOI] [PubMed] [Google Scholar]
- Lupi I, Broman KW, Tzou SC, Gutenberg A, Martino E, Caturegli P 2008 Novel autoantigens in autoimmune hypophysitis. Clin Endocrinol (Oxf) 69:269–278 [DOI] [PubMed] [Google Scholar]
- Mau M, Phillips TM, Ratner RE 1993 Presence of anti-pituitary hormone antibodies in patients with empty sella syndrome and pituitary tumours. Clin Endocrinol (Oxf) 38:495–500 [DOI] [PubMed] [Google Scholar]
- Scherbaum WA, Schrell U, Gluck M, Fahlbusch R, Pfeiffer EF 1987 Autoantibodies to pituitary corticotropin-producing cells: possible marker for unfavourable outcome after pituitary microsurgery for Cushing’s disease. Lancet 1:1394–1398 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Tatsumi KI, Kimura M, Takano T, Murakami Y, Takao T, Hashimoto K, Kato Y, Amino N 2002 Detection of autoantibodies against the pituitary-specific proteins in patients with lymphocytic hypophysitis. Eur J Endocrinol 147:767–775 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Tatsumi KI, Takano T, Murakami Y, Takao T, Yamakita N, Tahara S, Teramoto A, Hashimoto K, Kato Y, Amino N 2003 Anti-α-enolase antibodies in pituitary disease. Endocr J 50:697–702 [DOI] [PubMed] [Google Scholar]
- Yabe S, Kanda T, Hirokawa M, Hasumi S, Osada M, Fukumura Y, Kobayashi I 1998 Determination of antipituitary antibody in patients with endocrine disorders by enzyme-linked immunosorbent assay and Western blot analysis. J Lab Clin Med 132:25–31 [DOI] [PubMed] [Google Scholar]
- Crock PA 1998 Cytosolic autoantigens in lymphocytic hypophysitis. J Clin Endocrinol Metab 83:609–618 [DOI] [PubMed] [Google Scholar]
- Manetti L, Lupi I, Morselli LL, Albertini S, Cosottini M, Grasso L, Genovesi M, Pinna G, Mariotti S, Bogazzi F, Bartalena L, Martino E 2007 Prevalence and functional significance of antipituitary antibodies in patients with autoimmune and non-autoimmune thyroid diseases. J Clin Endocrinol Metab 92:2176–2181 [DOI] [PubMed] [Google Scholar]
- Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR 2005 Autoimmune hypophysitis. Endocr Rev 26:599–614 [DOI] [PubMed] [Google Scholar]
- Tatsumi KI, Tanaka S, Takano T, Tahara S, Murakami Y, Takao T, Hashimoto K, Kato Y, Teramoto A, Amino N 2003 Frequent appearance of autoantibodies against prohormone convertase 1/3 and neuroendocrine protein 7B2 in patients with nonfunctioning pituitary macroadenoma. Endocrine 22:335–340 [DOI] [PubMed] [Google Scholar]
- Gutenberg A, Bell JJ, Lupi I, Tzou SC, Landek-Salgado MA, Kimura H, Su J, Karaviti LP, Salvatori R, Caturegli P 2009 Pituitary and systemic autoimmunity in a case of intrasellar germinoma. Pituitary doi:19466616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BH 2008 The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev 222:101–116 [DOI] [PubMed] [Google Scholar]
- Parker C, Milosevic M, Panzarella T, Banerjee D, Jewett M, Catton C, Tew-George B, Gospodarowicz M, Warde P 2002 The prognostic significance of the tumour infiltrating lymphocyte count in stage I testicular seminoma managed by surveillance. Eur J Cancer 38:2014–2019 [DOI] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G 2003 Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213 [DOI] [PubMed] [Google Scholar]
- Polydorides AD, Mukherjee B, Gruber SB, McKenna BJ, Appelman HD, Greenson JK 2008 Adenoma-infiltrating lymphocytes (AILs) are a potential marker of hereditary nonpolyposis colorectal cancer. Am J Surg Pathol 32:1661–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson DJ, Ritchie D, Crooks D, Main G, Smith C, Vora J, Eljamel MS, Leese GP 2008 Lymphocytic hypophysitis occurring simultaneously with a functioning pituitary adenoma. Endocr J 55:729–735 [DOI] [PubMed] [Google Scholar]
- Moskowitz SI, Hamrahian A, Prayson RA, Pineyro M, Lorenz RR, Weil RJ 2006 Concurrent lymphocytic hypophysitis and pituitary adenoma. Case report and review of the literature. J Neurosurg 105:309–314 [DOI] [PubMed] [Google Scholar]
- McConnon JK, Smyth HS, Horvath E 1991 A case of sparsely granulated growth hormone cell adenoma associated with lymphocytic hypophysitis. J Endocrinol Invest 14:691–696 [DOI] [PubMed] [Google Scholar]
- Ballian N, Chrisoulidou A, Nomikos P, Samara C, Kontogeorgos G, Kaltsas GA 2007 Hypophysitis superimposed on a non-functioning pituitary adenoma: diagnostic clinical, endocrine, and radiologic features. J Endocrinol Invest 30:677–683 [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Takahashi JA, Shimatsu A, Hashimoto N 2007 Hypophysitis caused by Rathke’s cleft cyst. Case report. Neurol Med Chir (Tokyo) 47:136–139 [DOI] [PubMed] [Google Scholar]
- Yuyama R, Kojima H, Mizutani T, Suzuki Y, Miki Y 2002 [Secondary pan-hypophysitis caused by rupture of Rathke’s cleft cyst: case report]. No Shinkei Geka 30:95–99 [PubMed] [Google Scholar]
- Schittenhelm J, Beschorner R, Psaras T, Capper D, Nägele T, Meyermann R, Saeger W, Honegger J, Mittelbronn M 2008 Rathke’s cleft cyst rupture as potential initial event of a secondary perifocal lymphocytic hypophysitis: proposal of an unusual pathogenetic event and review of the literature. Neurosurg Rev 31:157–163 [DOI] [PubMed] [Google Scholar]
- Puchner MJ, Ludecke DK, Saeger W 1994 The anterior pituitary lobe in patients with cystic craniopharyngiomas: three cases of associated lymphocytic hypophysitis. Acta Neurochir (Wien) 126:38–43 [DOI] [PubMed] [Google Scholar]
- Takao T, Nanamiya W, Matsumoto R, Asaba K, Okabayashi T, Hashimoto K 2001 Antipituitary antibodies in patients with lymphocytic hypophysitis. Horm Res 55:288–292 [DOI] [PubMed] [Google Scholar]
- Mou Z, He Y, Wu Y 2009 Immunoproteomics to identify tumor-associated antigens eliciting humoral response. Cancer Lett 278:123–129 [DOI] [PubMed] [Google Scholar]
- Tan EM, Zhang J 2008 Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev 222:328–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Coe SP, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA 2006 Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol 1:513–519 [PubMed] [Google Scholar]