Abstract
Context: GH-releasing peptide (GHRP), GHRH, and somatostatin are physiological regulators of pulsatile GH secretion.
Hypothesis: Age, independently of abdominal visceral fat (AVF) and basal (nonpulsatile) GH secretion, damps pulsatile GH secretion driven by physiological (rather than pharmacological) amounts of GHRP and GHRH in an experimentally controlled estradiol (E2) milieu.
Design and Setting: A prospectively randomized, double-blind parallel-cohort study was conducted at an academic medical center.
Participants: Community-dwelling healthy premenopausal (PRE, age 24 ± 0.8 yr, n = 20) and postmenopausal (POST, age 63 ± 1.8 yr, n = 22) women participated in the study.
Interventions: Gonadal-axis down-regulation with leuprolide was followed by randomized addback of placebo or transdermal E2 and separate-day iv bolus injections of a half-maximally stimulatory dose of GHRP-2 or GHRH (each 0.33 μg/kg).
Analysis: Three-way analysis of covariance included main factors age, E2 status, and secretagogue type and covariates AVF and basal GH secretion.
Results: Submaximally stimulated pulsatile GH secretion was positively determined by PRE vs. POST age (P < 0.001), E2 repletion vs. depletion (P = 0.001) and GHRP-2 vs. GHRH stimulation (P < 0.001), after adjustment for AVF and basal secretion. E2 vs. placebo elevated fasting mean GH concentrations in both PRE and POST women (P = 0.006) but increased basal (nonpulsatile) GH secretion in PRE only (P = 0.002). PRE vs. POST age prolonged GHRH-driven GH secretory bursts by 36% (P = 0.006).
Conclusion: PRE vs. POST age, E2 availability, and physiological peptide drive are triple determinants of pulsatile GH secretion independently of abdominal visceral fat and nonpulsatile GH secretion in healthy women.
Near-physiologic bolus injections of GHRH or GHRP-2 (a ghrelin mimetic) evoke GH secretion in proportion to premenopausal > postmenopausal age, estrogen-rich > estrogen-deficient status, and GHRP-2 > GHRH even after adjustment for abdominal visceral fat.
In healthy individuals, at least 80% of daily GH secretion proceeds in discrete bursts, which are superimposed on basal secretion (1). Ghrelin, a 28-amino-acid Ser3-octanoylated GH-releasing peptide (GHRP), and GHRH, a 44-amino-acid sequence, evoke burst-like GH secretion (2). Pituitary responses to GHRP and GHRH are noncompetitively inhibited by the hypothalamic tetradecapeptide somatostatin (3). Together these three primary effectors mediate physiologically pulsatile GH secretion (2,4). GHRP-2 (pralmorelin, KP-102) is a synthetic hexapeptide analog of ghrelin (5), for which dose-response data exist in healthy women (6). Dose-response data also exist for GHRH in women (7,8). Such information has been used in the past to select maximally stimulating (pharmacological) secretagogue doses for the evaluation of possible hypopituitarism. However, maximal drive of pulsatile GH secretion rarely, if ever, occurs under normal physiological conditions even in the fasting state (1,4,9). If mechanisms regulating normal day-to-day GH secretion reflect principally physiological rather than pharmacological peptide signaling, then mechanistic investigations should use submaximally effective, rather than maximally stimulatory, amounts of secretagogues.
GH plays a significant role in minimizing visceral adiposity and in maintaining muscle bulk, bone mass, and well-being (10). However, pulsatile GH secretion declines gradually with age in men and premenopausal (PRE) women (11). The age-related decline in women is accelerated at midlife (1,12). A plausible tenet is that estrogen availability, secretagogue actions, body composition, and other factors jointly determine the age-associated decline in pulsatile GH secretion (1,4). However, no studies have tested the prime supposition that age, independently of lower estradiol (E2) concentrations and greater abdominal visceral fat (AVF), regulates physiological actions of GHRH, ghrelin/GHRP, and/or somatostatin. This is because most clinical studies, including our own, have used pharmacological rather than physiological stimulus intensities (13,14,15,16).
To examine the impact of age on physiological peptidyl actions, the present investigation 1) injected submaximally stimulatory amounts of GHRH and GHRP iv, 2) implemented a controlled paradigm of short-term E2 depletion/repletion, 3) studied 42 healthy PRE and postmenopausal (POST) women, and 4) applied analysis of covariance to adjust for repeated measures and the major potential confounders, E2 status, AVF and concurrent basal GH secretion (1,4).
Subjects and Methods
Subjects
Subjects provided Institutional Review Board-approved written informed consent and were compensated according to Institutional Review Board guidelines for time spent in the study. Exclusion criteria included any history, symptoms, or signs of breast neoplasia; arterial or venous thrombotic events; cerebrovascular, cardiac, hepatic, renal, or pulmonary disease; chronic or acute infections; untreated triglyceride-predominant hyperlipidemia; cholelithiasis; anemia (hemoglobin <11.8 g/dl); psychiatric disease; substance abuse, including illicit drugs and alcohol; exposure to neuroactive drugs within five biological half-lives; more than 3 kg weight change in 6 wk; nightshift work; and lack of written informed consent. Participants were community-dwelling, healthy women recruited within the age windows of 18–30 and 50–80 yr and body mass index (BMI) of 18–32 kg/m2. PRE and POST status were confirmed, respectively, by cyclic menses (six 28 ± 5-d cycles over the last 6 months) and by amenorrhea for at least 2 yr, FSH higher than 45 IU/liter, LH higher than 20 IU/liter, and E2 lower than 35 pg/ml (multiply the last value by 3.68 for picomoles per liter).
Clinical protocol
The design was a prospectively randomized, double-blind, parallel-cohort, placebo-controlled comparison of the effects of PRE and POST age on GH responses to submaximal doses of GHRH and GHRP-2 administered by iv bolus injection during controlled E2 depletion and repletion. Subjects, nurses in the Clinical Research Unit, and investigators were blinded to peptide infusions. To achieve age-independent estrogen depletion, 3.75 mg leuprolide acetate, a GnRH agonist, was administered im twice 3 wk apart to both PRE and POST women. The first injection was administered to PRE volunteers within 8 d of menstrual bleeding and within 24 h of a negative serum pregnancy test and to POST women 3 or more weeks after discontinuation of any estrogen supplementation. Ten PRE and 10 POST subjects were randomized to estrogen repletion and 10 PRE and 12 POST women to estrogen depletion, beginning on the day of the second leuprolide injection.
Estrogen repletion comprised graded transdermal E2 supplementation designed to mimic rising E2 concentrations across the follicular phase of the menstrual cycle. E2 or placebo patches (Novartis, Basel, Switzerland) were applied beginning on the evening of the second leuprolide injection (6 wk after any previous estrogen exposure) at an initial dose of 0.05 mg daily for 4 d with 0.05-mg increments made every 4 d. The highest dose (0.2 mg daily) was maintained for a total of 10 d. Morning bolus peptide injection sessions were scheduled fasting within the 6-d inclusive interval, defined as d 18–23 after the second leuprolide injection. Intravenous bolus injections of GHRH and GHRP-2 were administered at least 2 d apart. A similar design was used earlier to study continuous iv peptide drive in the same subjects at a different time (14).
The night before peptide injections, volunteers received a standardized outpatient meal (8 kcal/kg: 20% protein, 50% carbohydrate, and 30% fat) at 1800 h and then remained fasting overnight until 1400 h the next day. Participants arrived in the Clinical Research Unit by 0700 h on the morning of study. Catheters were placed in contralateral forearm veins to allow simultaneous injection of a secretagogue and sampling of blood (1 ml) every 10 min for 6 h from 0800–1400 h. Lunch was provided at 1400 h at the end of the sampling procedure. In accordance with good medical practice, 12 d of micronized progesterone (100 mg orally nightly) were initiated upon completion of study in women with an intact uterus.
Infusion sessions
Randomly ordered secretagogue injection sessions comprised continuous iv infusion of saline from 0800–1000 h followed by either iv bolus GHRP-2 (0.33 μg/kg) or iv bolus GHRH (0.33 μg/kg) injections at 1000 h. The peptide doses are submaximally stimulatory in POST women (6,7).
Single-slice computed tomography of the abdomen at L3–L4 was performed to estimate relative visceral adiposity (AVF) expressed in units of cross-sectional area (square centimeters) (17).
Hormone assays
Plasma GH concentrations were measured in duplicate by automated double-monoclonal immunoenzymatic chemiluminescence assay using 22-kDa recombinant human GH as assay standard (Sanofi Diagnostics Pasteur Access, Chaska, MN) (15). All samples (n = 73) from one subject were analyzed together. Sensitivity (3 sd above the zero-dose tube) was 0.010 μg/liter. Interassay coefficients of variation (CV) for GH concentrations of 3.4 μg/liter and 12 μg/liter were 7.9 and 6.3%, respectively. Intraassay CV were 4.9 and 4.5% at 1.1 μg/liter and 20 μg/liter, respectively. No values fell less than 0.020 μg/liter. Cross-reactivity with 20-kDa GH is less than 5%.
Screening serum E2, prolactin, LH, and FSH concentrations were quantified by automated chemiluminescence assay (ACS 180; Bayer, Norwood, MA), using as standards E2 and the First and Second International Gonadotropin Reference Preparations, respectively. Procedural sensitivities for E2, prolactin, LH, and FSH are 35 pg/ml (189 pmol/liter), 2.0 μg/liter, and 0.2 and 0.4 IU/liter, respectively (15).
Liquid-chromatography tandem mass spectrometry was used to quantify serum E2 and testosterone (T) on both days of the infusions (18). SHBG and albumin were measured as previously reported (18). Total IGF-I, IGF-binding protein (IGFBP)-1, and IGFBP-3 concentrations were assayed by immunoradiometric assay (Diagnostic Systems Laboratories, Webster, TX), as described (19). Mean values from the two sessions were analyzed.
Model-free analysis
Unstimulated baseline (presecretagogue infusion) GH concentrations were averaged over the two 2.5-h saline-infusion intervals (0800–1030 h). Means for each separate infusion session were obtained for peptide-specific stimulated 3.5-h GH concentrations (1040–1400) in each subject.
Deconvolution analysis
Deconvolution analysis used an automated Matlab-based method, which has been mathematically verified and empirically validated using electrophysiological recordings, hypothalamo-pituitary sampling, and simulated pulsatile time series (19,20). Discriminative accuracy is approximately 93%. Analysis was applied to each 6-h GH concentration time series to permit estimation of both basal and pulsatile GH secretion. In this methodology, multiple sets of potential GH pulse times are first created by a nonlinear-diffusion method. Deconvolution analysis is then applied to each of the provisional pulse-time sets, assuming potentially asymmetric burst-like secretion (generalized γ-distribution), basal GH release, and biexponential GH kinetics comprising a 3.5-min rapid-phase and a 20.8-min slow-phase half-life (rapid/total amplitude-decay fraction = 0.37 and slow/total amplitude-decay fraction = 0.63), based upon directly measured GH-disappearance curves (21). After all candidate pulse-time sets are deconvolved, the optimal pulse-time set is decided using the Akaike information criterion (22). Pulsatile GH secretion was defined as the summed mass of GH (micrograms) secreted in bursts per unit distribution volume (liters) during the 3.5-h interval after each peptide stimulus. Secondary endpoints were asymmetric shape (waveform) of underlying secretory bursts and basal (nonpulsatile) GH secretion. Shape was quantified by the mathematical mode of GH-secretory bursts, viz., time delay in minutes from the objectively defined pulse onset to maximal secretion.
Statistical analysis
The goal was to evaluate the effect of age category (PRE vs. POST) on pulsatile GH secretion in controlled E2 (deplete vs. replete) milieus after submaximal secretagogue (GHRH and GHRP-2) stimulation. According to the experimental design, age category and E2 milieu were modeled as between-subject effects, and secretagogue type was modeled as a within-subject repeated-measures effect. To limit the dispersion of residual variance, log-transformed values of pulsatile GH secretion were used. The primary model was three-way analysis of covariance (ANCOVA) using AVF and basal (nonpulsatile) GH secretion as joint covariates with repeated measures corresponding to the two secretagogues received by each subject. AVF is a strongly negative determinant of pulsatile GH secretion in men and women (1,4). Moreover, basal GH secretion codetermines total GH secretion and is positively influenced by E2 concentrations in prepubertal children, adolescents, and healthy adults (23,24). Main-effect terms were age category, E2 status, and secretagogue type. The model also included terms for possible two-way interactions, viz., age by E2, secretagogue by age, and secretagogue by E2. To aid in the interpretation of interactive effects, supplemental two-way ANCOVA models were fit separately to assess individual secretagogue-specific effects in relation to age and E2 status. Post hoc linear contrasts were performed between PRE vs. POST age groups using Tukey’s honestly significantly different test (25). Experiment-wise two-tailed P ≤ 0.05 was considered statistically significant. For generality, we assessed other ANCOVA models, which included covariate combinations of BMI, AVF, basal GH secretion, and baseline GH concentrations. In all cases, the same main effects of age, E2 status, and secretagogue type were observed. According to the Akaike information criterion (22), the statistically optimal model for pulsatile GH secretion contained both AVF and basal GH secretion as covariates. Hence, this model was used here. Analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
Subject characteristics before intervention are given in supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Expected differences existed in age and AVF as well as in FSH, LH, E2, T, prolactin, IGF-I, IGFBP-1, IGFBP-3, and albumin concentrations between POST and PRE women. Hormone measurements after leuprolide/placebo and leuprolide/E2 interventions are summarized in supplemental Table 2. FSH was suppressed less by E2 in POST than PRE women; E2 and T were elevated slightly more in PRE than POST women; and LH, SHBG, and IGFBP-1 concentrations were similar by age category. Exposure to E2 vs. placebo increased E2, SHBG, IGFBP-1, and albumin and decreased FSH and T in both age groups. There was a nonsignificant trend (P = 0.064) for E2 to suppress IGFBP-3.
Initial statistical analyses were performed using two-way ANCOVA to assess whether AVF or BMI differed by age category (PRE vs. POST) or E2 status (deplete vs. replete). AVF differed strongly according to age (P < 0.001, higher in POST vs. PRE) and weakly by E2 status (P = 0.026, higher in deplete vs. replete). BMI did not differ with respect to age (P = 0.22) or E2 status (P = 0.35). AVF (P = 0.035) but not BMI (P = 0.68) explained the negative effect of age on mean baseline (prestimulus) GH concentrations (P = 0.033). Model exploration demonstrated that even when AVF was included as a covariate, basal GH secretion also significantly determined estimates of pulsatile GH secretion and the mass of GH secreted per burst (each P < 0.001) but not GH secretory-burst mode (P = 0.06). Thus, the final ANCOVA model included the stimulus type, age, and E2 as primary factors and used both AVF and basal GH secretion as covariates in assessing pulsatile and burst-mass responses.
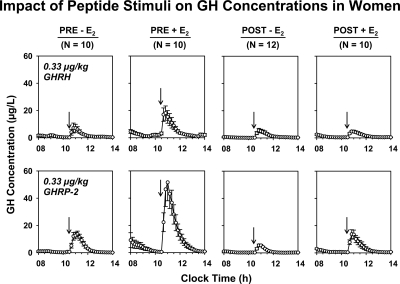
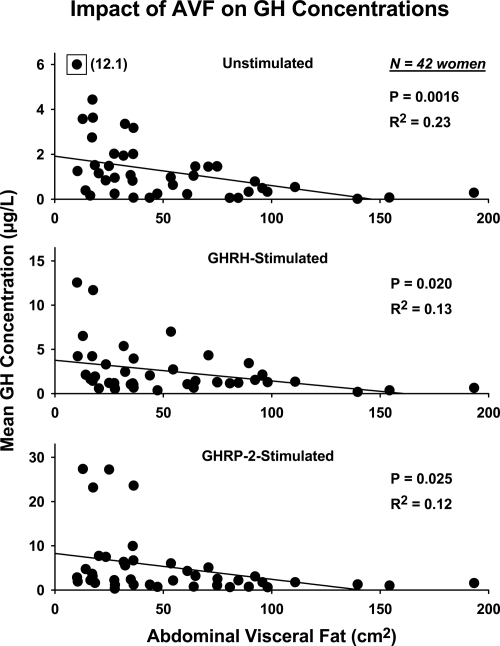
Mean (± sem) 10-min 6-h GH concentration profiles are shown in Figure 1. Prestimulus (baseline 2.5-h saline infusion) and individual poststimulus mean 3.5-h GH concentrations were negatively determined by AVF (unstimulated P < 0.001, R2 = 0.23; GHRH-stimulated P = 0.020, R2 = 0.13; and GHRP-2-stimulated P = 0.025, R2 = 0.12; Fig. 2). By two-way ANCOVA with age and E2 status as primary variables and AVF and basal GH secretion as covariates, mean prestimulus (average of the two saline infusions) GH concentrations (μg/liter) were significantly defined by E2 status: 0.65 ± 0.11 (placebo) and 2.3 ± 0.22 (E2) (P = 0.006).
Figure 1.
Six-hour GH concentration profiles for each of the four cohorts of women studied under a leuprolide clamp, stratified by age (PRE vs. POST), E2 status (deplete vs. replete), and secretagogue type (GHRH vs. GHRP-2). Data are 10-min serum GH measurements given as the mean ± sem at each time point. Saline was infused from 0800–1030 h. The indicated secretagogue was injected at 1030 h. Each group comprised the indicated number (N) of subjects.
Figure 2.
Regression of (2.5- and 3.5-h) mean GH concentrations on AVF. Data were obtained in 42 healthy women sampled for 2.5 h during saline infusion (top) followed by sampling for 3.5 h after bolus GHRH (middle) or bolus GHRP-2 (bottom) injection. The single boxed datum (top) with a y-axis value of 12.1 was excluded on statistical grounds at P < 10−4 by Studentized residuals.
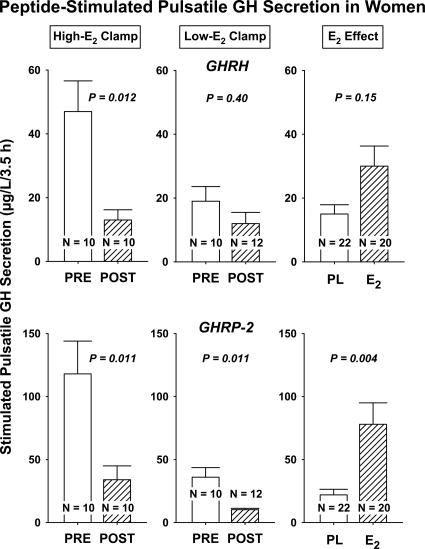
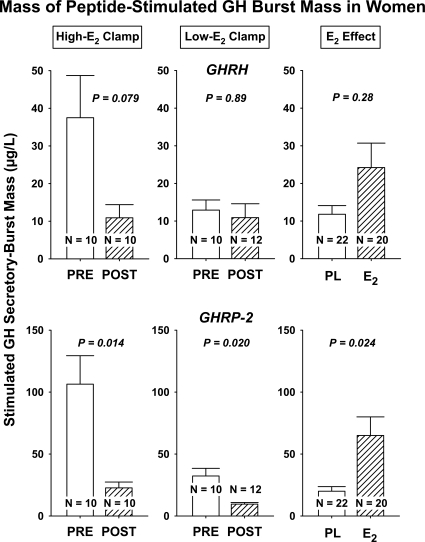
Three-way ANCOVA with peptide type, age, and E2 as primary factors and joint covariates AVF and basal secretion revealed that submaximal peptide-stimulated pulsatile GH secretion, as estimated by deconvolution analysis, was determined strongly by each of age (P < 0.001, higher in PRE vs. POST), E2 status (P = 0.001, higher for replete vs. deplete), and secretagogue type (P < 0.001, higher for GHRP-2 vs. GHRH). There were no two-factor interactions: supplemental Table 3 and Fig. 3. Post hoc contrasts demonstrated that pulsatile GH secretion was significantly greater in PRE than POST women after 1) GHRH infusion in the +E2 milieu (P = 0.012), 2) GHRP-2 without E2 (P = 0.011), and 3) GHRP-2 with E2 (P = 0.011), but not 4) GHRH without E2 (P = 0.40) (supplemental Table 4). Further assessment showed that the amount (mass) of GH secreted per pulse (micrograms) per unit distribution volume (liters) largely explained observed GHRP-2-stimulated differences in pulsatile GH secretion (Fig. 4). In addition, there was a nearly significant (P = 0.051) interaction between age and E2, wherein POST age and low-E2 milieu together reduced submaximal drive by GHRP-2 (supplemental Tables 3 and 4). The mass of GH secreted per burst was greater in PRE than POST women whether or not E2 was provided, in the case of the GHRP-2, but not GHRH, stimulus [P = 0.020 (placebo); P = 0.014 (E2); supplemental Table 4).
Figure 3.
Pulsatile GH secretion evaluated according to age (PRE vs. POST), E2 status (high vs. low), and peptidyl secretagogue (GHRH vs. GHRP-2). Responses were evaluated by three-way ANCOVA with AVF and basal GH secretion as covariates. P values were estimated using Tukey’s post hoc honestly significantly different multiple-comparison test for age effects (PRE vs. POST) in relation to secretagogue type and E2 status. Values are the mean ± sem.
Figure 4.
Mass of GH secreted per burst assessed as described in Fig. 2.
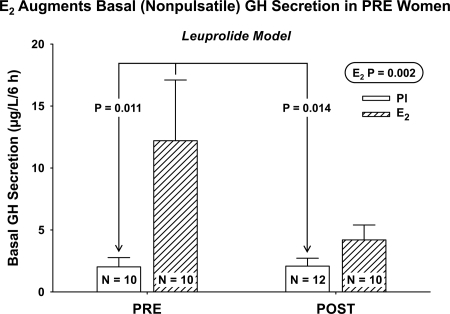
ANCOVA revealed that AVF and basal GH secretion did not covary (P = 0.12). Therefore, ANOVA was used to evaluate the effects of age and E2 on basal secretion. There was a positive effect of E2 (P = 0.002) but not of age (P = 0.32) or their interaction (P = 0.18) on basal GH secretion. Post hoc analysis indicated that basal GH secretion was higher in PRE with E2 than in both PRE without E2 (P = 0.011) and POST without E2 (P = 0.014) but not higher than in POST with E2 (P = 0.23) (Fig. 5).
Figure 5.
E2 elevates basal (nonpulsatile) GH secretion only in PRE women. P values reflect ANOVA and post hoc Tukey contrasts.
The time course of peptide-stimulated burst-like GH release was quantified by the secretory-burst mode (time delay between objective pulse onset and maximal secretion rate). AVF (P = 0.033) but not basal GH secretion (P = 0.058) was a significantly negative covariate of secretory-burst mode. ANCOVA, using AVF as a covariate, disclosed significant age-by-secretagogue (P = 0.034) and age-by-E2 (P = 0.036) interactions, indicating that the effect of age on burst shape is dependent on both secretagogue type and E2 status. In particular, the significant effect of age applied to GHRH stimulation (P = 0.006; higher in PRE vs. POST) but not GHRP-2 stimulation (P = 0.57) in the low-E2 milieu (supplemental Fig. A). The absolute difference by age was 5 ± 1.4 min or a 36% prolongation of secretory bursts in PRE compared with POST women.
Discussion
This study establishes that POST compared with PRE age, independently of AVF, E2 milieu, or basal GH secretion, restricts pulsatile GH secretion induced by near-physiological GHRP drive in healthy women. In particular, deconvolution analyses revealed that all three of PRE age, E2 repletion, and GHRP (more than GHRH) stimulation amplify pulsatile GH secretion after accounting for intersubject differences in AVF and basal GH secretion. Basal (nonpulsatile) GH secretion was positively influenced by E2 status solely in PRE women. In addition, the waveform mode, or time delay from burst onset to maximal GH release within secretory bursts, was prolonged in PRE compared with POST women given GHRH in an E2-deficient setting. Accordingly, PRE and POST age, short-term E2 availability, AVF, and submaximal peptide-secretagogue actions appear to supervise distinct physiological facets of GH secretion in healthy women.
The current data extend earlier findings that maximal GH secretory capacity assessed by triple-secretagogue infusion is not diminished with age (9). The present design differs by evaluating near-physiological rather than maximally stimulated GH secretion, whereas adjusting simultaneously for covariate effects of AVF, E2 status, and basal GH secretion. These covariates are important, because AVF is a prominent negative determinant, and both E2 concentrations and basal GH secretion are major positive determinants of GH secretion and 24-h integrated or nocturnal GH concentrations in adults (1,4,24,26,27). Indeed, there was a consistently negative effect of AVF and age and a positive effect of E2 on submaximal GHRP action in healthy women (Figs. 3 and 4). Conversely, when AVF was accounted for and E2 was depleted experimentally, no age effect was detectable for submaximal GHRH stimulation. Therefore, nearly physiological GHRP drive of burst-like GH secretion is conjointly determined by age, AVF, E2, and basal GH secretion, whereas submaximal GHRH action is determined mainly by AVF in the E2-deplete and by age in the E2-replete settings. Moreover, because E2 facilitated GHRH drive in PRE but not POST women, it is possible that E2 action on this endpoint may be impaired in POST individuals.
Estrogen repletion for 3 wk and submaximal GHRP stimulation were significantly more effective than E2 withdrawal and submaximal GHRH stimulation whether subjects were of PRE or POST age. This distinction might be explained, in part, by the unique actions of GHRP to oppose hypothalamic somatostatin and synergize with available GHRH (3,4). In addition, estrogen can up-regulate expression of the human GHRP-receptor gene promoter at least in vitro (28), pituitary-specific transcription factors, pituitary GHRH receptors, and the GH gene and inhibit expression of the pituitary somatostatin-receptor subtype-5 gene (29). Beyond these effects, E2 is able to stimulate somatotrope GH secretion directly in vitro and by ectopic pituitary tissue in vivo (30,31,32), regulate neuronal GHRH gene expression via ER-α (33), and reduce free IGF-I concentrations by blocking hepatic GH receptors and elevating systemic IGFBP-1 concentrations (1,4,34). The collective effects could serve to restrain negative feedback and amplify pulsatile GH secretion. However, in other laboratory models, estrogen represses hypophyseal GHRH-receptor and induces pituitary somatostatin-receptor subtype-2 genes (35,36), changes that would predict diminished pulsatile GH secretion. Thus, the present data in healthy women are important in defining clinical mechanisms of age-related and estrogen-dependent modulation of GH secretion as well as their interrelationships with AVF and secretagogue type. Specifically, age contrasts are striking in a high-E2 milieu for both peptide agonists and also in a low-E2 milieu for GHRP-2 drive. In addition, the facilitative action of E2 is 2-fold greater on submaximal GHRP than on submaximal GHRH stimulation in both POST and PRE individuals. A plausible physiological implication of this dichotomy is that attenuation of endogenous GH pulses in older E2-deficient compared with young E2-sufficient women may principally reflect reduced potency of endogenous GHRP/ghrelin signaling.
Basal (nonpulsatile) GH secretion was up-regulated by E2 supplementation in women of PRE but not POST age, indicating a second inferable age distinction in responsiveness to estrogen. The positive effect of estrogen may explain why basal GH secretion in pubertal children and young adults correlates with endogenous E2 concentrations (4,24) and why basal GH secretion can be higher in PRE than POST women in an E2-replete state (15).
The deconvolution procedure used here allows one to estimate the time course (waveform or shape) of a secretory burst, such as that due to secretagogue-induced GH release (19,37). Waveform analysis revealed that peptide-induced GH secretory bursts are abbreviated by increased AVF as well as by POST compared with PRE status in women given GHRH in a low-E2 context. Based upon information obtained by atomic-force microscopy (38), abbreviated GH-release events in older women would likely reflect a shorter duration of discharge of somatotrope membrane-apposed secretory vesicles. Imposition of a high-E2 milieu in POST women reversed the constriction of GHRH-induced secretory waveform. Somatostatin may contribute to abbreviating GH secretory bursts in aging, inasmuch as GHRP-2 stimulation, which antagonizes central somatostatin inhibition (3,39), normalized the waveform in POST women whether or not E2 was administered.
Caveats include the need to replicate outcomes in larger cohorts (here, n = 42) and extend the duration and dose range of E2 supplementation. In addition, prospective analyses would be required to establish that age per se is responsible for the differences inferred in POST and PRE women under low and high E2 clamps.
In conclusion, in healthy women, PRE and POST age, E2 availability, type of peptide secretagogue, AVF, and basal (nonpulsatile) GH secretion are prime determinants of pulsatile GH secretion when assessed in response to near-physiological peptide stimulation. E2 dose-response and time-course analyses will be ultimately needed to appraise the longitudinal effects of aging.
Supplementary Material
Acknowledgments
We thank Donna Scott for capable support of manuscript preparation, Ashley Bryant for excellent data analysis and graphics, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementing the protocol.
Footnotes
This work was supported in part by the Clinical Translational Research Center Grant MO1 RR00585 to the Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD) and R01 NIA AG29362 from the National Institutes of Health (Bethesda, MD).
Disclosure Summary: Authors have nothing to declare.
First Published Online October 26, 2009
Abbreviations: ANCOVA, Analysis of covariance; AVF, abdominal visceral fat; BMI, body mass index; CV, coefficient of variation; E2, estradiol; GHRP, GH-releasing peptide; IGFBP, IGF-binding protein; POST, postmenopausal; PRE, premenopausal; T, testosterone.
References
- Giustina A, Veldhuis JD 1998 Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K 2005 Ghrelin: structure and function. Physiol Rev 85:495–522 [DOI] [PubMed] [Google Scholar]
- Bowers CY, Chang JK, Wu S, Linse KD, Hurley DL, Veldhuis JD 2006 Biochemistry of the growth hormone-releasing peptides, secretagogues and ghrelin. In: Mantovani G, Anker SD, Inui A, Morley JE, Fanelli F, Scevola D, Schuster M, Yeh SS, eds. Cachexia and wasting: a modern approach. New York: Springer-Verlag; 219–234 [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY 2006 Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27:101–140 [DOI] [PubMed] [Google Scholar]
- 2004 Pralmorelin: GHRP 2, GPA 748, growth hormone-releasing peptide 2, KP-102 D, KP-102 LN, KP-102D, KP-102LN. Drugs R D 5:236–239 [DOI] [PubMed] [Google Scholar]
- Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD 2001 Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. J Clin Endocrinol Metab 86:551–560 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Evans WS, Bowers CY 2003 Estradiol supplementation enhances submaximal feedforward drive of growth hormone (GH) secretion by recombinant human GH-releasing hormone-1,44-amide in a putatively somatostatin-withdrawn milieu. J Clin Endocrinol Metab 88:5484–5489 [DOI] [PubMed] [Google Scholar]
- Gelato MC, Pescovitz OH, Cassorla F, Loriaux DL, Merriam GR 1984 Dose-response relationships for the effects of growth hormone-releasing factor-(1–44)-NH2 in young adult men and women. J Clin Endocrinol Metab 59:197–201 [DOI] [PubMed] [Google Scholar]
- Arvat E, Ceda GP, Di Vito L, Ramunni J, Gianotti L, Broglio F, Deghenghi R, Ghigo E 1998 Age-related variations in the neuroendocrine control, more than impaired receptor sensitivity, cause the reduction in the GH-releasing activity of GHRP’s in human aging. Pituitary 1:51–58 [DOI] [PubMed] [Google Scholar]
- Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamura H, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I 2002 Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest 109:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltman A, Weltman JY, Hartman ML, Abbott RD, Rogol AD, Evans WS, Veldhuis JD 1994 Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: effects of gender. J Clin Endocrinol Metab 78:543–548 [DOI] [PubMed] [Google Scholar]
- Ho KY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO 1987 Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 64:51–58 [DOI] [PubMed] [Google Scholar]
- Anderson SM, Wideman L, Patrie JT, Weltman A, Bowers CY, Veldhuis JD 2001 Estradiol supplementation selectively relieves GH’s autonegative feedback on GH-releasing peptide-2-stimulated GH secretion. J Clin Endocrinol Metab 86:5904–5911 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Hudson SB, Erickson D, Bailey JN, Reynolds GA, Bowers CY 2009 Relative effects of estrogen, age and visceral fat on pulsatile growth-hormone secretion in healthy women. Am J Physiol Endocrinol Metab 297:E367–E364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson D, Keenan DM, Mielke K, Bradford K, Bowers CY, Miles JM, Veldhuis JD 2004 Dual secretagogue drive of burst-like growth hormone secretion in postmenopausal compared with premenopausal women studied under an experimental estradiol clamp. J Clin Endocrinol Metab 89:4746–4754 [DOI] [PubMed] [Google Scholar]
- Erickson D, Keenan DM, Farhy L, Mielke K, Bowers CY, Veldhuis JD 2005 Determinants of dual secretagogue drive of burst-like GH secretion in premenopausal women studied under a selective estradiol clamp. J Clin Endocrinol Metab 90:1741–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Erickson D, Mielke K, Farhy LS, Keenan DM, Bowers CY 2005 Distinctive inhibitory mechanisms of age and relative visceral adiposity on GH secretion in pre- and postmenopausal women studied under a hypogonadal clamp. J Clin Endocrinol Metab 90:6006–6013 [DOI] [PubMed] [Google Scholar]
- Paulo RC, Brundage R, Cosma M, Mielke KL, Bowers CY, Veldhuis JD 2008 Estrogen elevates the peak overnight production rate of acylated ghrelin. J Clin Endocrinol Metab 93:4440–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD 2003 Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. Am J Physiol 285:R664–R673 [DOI] [PubMed] [Google Scholar]
- Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD 2009 Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab 297:E538–E544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AC, Veldhuis JD, Thorner MO, Vance ML 1989 Half-time of endogenous growth hormone (GH) disappearance in normal man after stimulation of GH secretion by GH-releasing hormone and suppression with somatostatin. J Clin Endocrinol Metab 68:535–541 [DOI] [PubMed] [Google Scholar]
- Akaike H 1974 A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723 [Google Scholar]
- Veldhuis JD, Liem AY, South S, Weltman A, Weltman J, Clemmons DA, Abbott R, Mulligan T, Johnson ML, Pincus SM, Straume M, Iranmanesh A 1995 Differential impact of age, sex-steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab 80:3209–3222 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Rogol AD 2000 Gender and sexual maturation-dependent contrasts in the neuroregulation of growth hormone secretion in prepubertal and late adolescent males and females: a general clinical research center-based study. J Clin Endocrinol Metab 85:2385–2394 [DOI] [PubMed] [Google Scholar]
- Fisher LD, van Belle G 1996 Descriptive statistics. Biostatistics: a methodology for the health sciences. New York: John Wiley, Sons; 58–74 [Google Scholar]
- Vahl N, Jørgensen JO, Skjaerback C, Veldhuis JD, Orskov H, Christiansen J 1997 Abdominal adiposity rather than age and sex predicts the mass and patterned regularity of growth hormone secretion in mid-life healthy adults. Am J Physiol 272:E1108–E1116 [DOI] [PubMed] [Google Scholar]
- O'Connor KG, Harman SM, Stevens TE, Jayme JJ, Bellantoni MF, Busby-Whitehead MJ, Christmas C, Munzer T, Tobin JD, Roy TA, Cottrell E, St Clair C, Pabst KM, Blackman MR 1999 Interrelationships of spontaneous growth hormone axis activity, body fat, and serum lipids in healthy elderly women and men. Metabolism 48:1424–1431 [DOI] [PubMed] [Google Scholar]
- Petersenn S, Rasch AC, Penshorn M, Beil FU, Schulte HM 2001 Genomic structure and transcriptional regulation of the human growth hormone secretagogue receptor. Endocrinology 142:2649–2659 [DOI] [PubMed] [Google Scholar]
- Yan M, Jones ME, Hernandez M, Liu D, Simpson ER, Chen C 2004 Functional modification of pituitary somatotropes in the aromatase knockout mouse and the effect of estrogen replacement. Endocrinology 145:604–612 [DOI] [PubMed] [Google Scholar]
- Haug E, Gautvik KM 1978 Effects of sex steroids on growth hormone production in cultured rat pituitary cells. Acta Endocrinol (Copenh) 87:40–54 [DOI] [PubMed] [Google Scholar]
- Komolov IS, Perez-Arce JA, Fedotov VP 1980 The effects of estradiol on prolactin and growth hormone secretion in cultured pituitary cells from intact and ovariectomized rats. Endokrinologie 75:278–284 [PubMed] [Google Scholar]
- Jansson JO, Carlsson L, Seeman H 1983 Estradiol - but not testosterone - stimulates the secretion of growth hormone in rats with the pituitary gland autotransplanted to the kidney capsule. Acta Endocrinol (Copenh) 103:212–218 [Google Scholar]
- Shimizu T, Kamegai J, Tamura H, Ishii S, Sugihara H, Oikawa S 2005 The estrogen receptor (ER)α, but not ERβ, gene is expressed in hypothalamic growth hormone-releasing hormone neurons of the adult female rat. Neurosci Res 52:121–125 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Frystyk J, Iranmanesh A, Ørskov H 2005 Testosterone and estradiol regulate free IGF-I, IGFBP-I and dimeric IGF-I/IGFBP-I concentrations. J Clin Endocrinol Metab 90:2941–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman DI, Sweetland M, Duckett G, Root AW 1987 Effect of estrogen on the growth hormone (GH) secretory response to GH-releasing factor in the castrate adult female rat in vivo. Endocrinology 120:1047–1051 [DOI] [PubMed] [Google Scholar]
- Kimura N, Tomizawa S, Arai KN, Kimura N 1998 Chronic treatment with estrogen up-regulates expression of sst2 messenger ribonucleic acid (mRNA) but down-regulates expression of sst5 mRNA in rat pituitaries. Endocrinology 139:1573–1580 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Bowers CY 2007 Estimation of the size and shape of GH secretory bursts in healthy women using a physiological estradiol clamp and variable-waveform deconvolution model. Am J Physiol Regul Integr Comp 293:R1013–R1021 [DOI] [PubMed] [Google Scholar]
- Cho SJ, Jeftinija K, Glavaski A, Jeftinija S, Jena BP, Anderson LL 2002 Structure and dynamics of the fusion pores in live GH-secreting cells revealed using atomic force microscopy. Endocrinology 143:1144–1148 [DOI] [PubMed] [Google Scholar]
- Dickson SL, Viltart O, Bailey AR, Leng G 1997 Attenuation of the growth hormone secretagogue induction of Fos protein in the rat arcuate nucleus by central somatostatin action. Neuroendocrinology 66:188–194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.