Abstract
PTEN/MMAC1/TEP1 is a tumor suppressor that possesses intrinsic phosphatase activity. Deletions or mutations of its encoding gene are associated with a variety of human cancers. However, very little is known about the molecular mechanisms by which this important tumor suppressor regulates cell growth. Here, we show that PTEN expression potently suppressed the growth and tumorigenicity of human glioblastoma U87MG cells. The growth suppression activity of PTEN was mediated by its ability to block cell cycle progression in the G1 phase. Such an arrest correlated with a significant increase of the cell cycle kinase inhibitor p27KIP1 and a concomitant decrease in the activities of the G1 cyclin-dependent kinases. PTEN expression also led to the inhibition of Akt/protein kinase B, a serine-threonine kinase activated by the phosphatidylinositol 3-kinase (PI 3-kinase) signaling pathway. In addition, the effect of PTEN on p27KIP1 and the cell cycle can be mimicked by treatment of U87MG cells with LY294002, a selective inhibitor of PI 3-kinase. Taken together, our studies suggest that the PTEN tumor suppressor modulates G1 cell cycle progression through negatively regulating the PI 3-kinase/Akt signaling pathway, and one critical target of this signaling process is the cyclin-dependent kinase inhibitor p27KIP1.
Keywords: tumor suppressor, phosphatase, signal transduction
Glioblastoma is the most common and malignant brain tumor characterized by aggressive growth and extensive vascularization through angiogenesis (1). A frequent genetic event in the progression of low-grade glioma to high grade, invasive glioblastoma is the loss of heterozygosity at chromosome 10q23 (2). A candidate tumor suppressor gene, PTEN or MMAC1, has been isolated from chromosome 10q23 locus by positional cloning (3, 4). In an independent approach to identify novel phosphatases, we have cloned TEP1, which is identical to PTEN or MMAC1 (5). The PTEN gene is mutated at high frequency in many primary human cancers and several familial cancer predisposition disorders (6). PTEN contains the sequence motif that is highly conserved in the members of the protein tyrosine phosphatase family. PTEN has been shown in vitro to possess phosphatase activity on phosphotyrosyl and phosphoseryl/threonyl-containing substrates (5, 7). More recently, PTEN has been shown, in vitro, to dephosphorylate phosphatidylinositol 3,4,5-trisphosphate, a product of phosphatidylinositol 3-kinase (PI 3-kinase) (8). Consistent with the notion that the phosphatase activity of PTEN is required for the tumor suppressor activity, many cancer-derived mutations are mapped in its conserved catalytic domain. PTEN has been implicated in cellular processes such as cell migration and spreading (9). However, the exact function of PTEN in cell growth and tumorigenesis remains unclear.
In this study, we have investigated the function of the tumor suppressor protein PTEN in cell growth. We show that expression of PTEN potently suppresses the growth and tumorigenicity of human glioblastoma cells. Such effects are correlated with its ability to mediate G1 cell cycle arrest and induce the cell cycle kinase inhibitor, p27KIP1.
MATERIALS AND METHODS
Cell Culture.
U87MG (human glioblastoma) was obtained from American Type Culture Collection (Manassas, VA) and maintained in DMEM supplemented with 10% fetal bovine serum.
Antibodies.
A synthetic peptide, CGGGDSDPENEPFDEDQHTQITKV (underlined letters corresponding to the residues 384–403 at the C terminus of PTEN) was synthesized and used to raise antibody in rabbits (YU102). The anti-PTEN antibody was affinity-purified with the synthetic peptide coupled to the SulfoLink gel (Pierce). Antibodies specific for p27KIP1 (sc-528), cyclin E (sc-198), or cyclin D (R-124) were obtained from Santa Cruz Biotechnology, and anti-p21CIP1/WAF1 (Ab-1) were from Oncogene Sciences. Anti-p42 mitogen-activated protein kinase (MAPK) antibody (B9) was obtained from Upstate Biotechnology (Lake Placid, NY). Anti-Akt/protein kinase B (PKB) and anti-phospho-MAPK antibodies were from New England Biolabs. Anti-cyclin-dependent kinase 2 (anti-CDK2) and anti-cyclin A antibodies were kindly provided by Hui Zhang at Yale University (10).
Retrovirus Infection.
Bing cells or Bosc-23 cells were transfected with the retroviral plasmids, pBabePuro, pBabePuro-PTEN, or pBabePuro-PTEN(CS), and the retroviruses packaging and infection were carried out according to the methods described (11). For colony formation assay, an equal aliquot of the amphotropic viruses packaged in Bing cells were used to infect in duplicates U87MG or DLD1 cells. The infected cells were selected in medium containing 1 μg/ml puromycin. U87-EcoR cells were established from U87MG cells by using the murine ecotropic receptor-expressing retroviruses, which were produced in Bing cells after transfection with the pWZL-Neo-EcoR plasmid (12). The infected cells were selected in medium containing 400 μg/ml neomycin, and the drug-resistant colonies were pooled and expanded (U87-EcoR cells). To infect U87-EcoR cells, ecotropic retroviruses were packaged in Bosc-23 cells.
Soft Agar and Nude Mice Studies.
Logarithmically growing cells (1 × 104 cells) were plated as a single-cell suspension in 0.3% agarose in DMEM supplemented with 10% fetal bovine serum. Colonies were inspected under the microscope and photographed 4 weeks later. For tumor formation in nude mice assay, cells were trypsinized and resuspended in PBS, and 5 × 105 cells (in 0.1 ml) were injected into each flank of the nude mice (The Jackson Laboratory, nu/nu). Tumors were monitored weekly after inoculation.
Flow Cytometry, Immunoprecipitation, and Western Blot Analyses.
Fluorescence-activated cell sorting analysis was performed by using fluorescein-conjugated anti-bromodeoxyuridine (BrdUrd) antibody (Becton Dickinson) following the supplier’s protocol. Immunoprecipitation, Western blot analysis, and histone H1 kinase assay were carried out as described (10).
RESULTS
PTEN Selectively Suppresses the Growth of Certain Tumor Cell Lines.
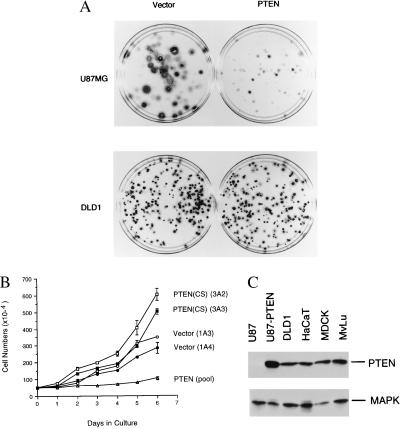
To investigate whether the loss of PTEN may provide a growth advantage to tumor cells that contain inactivating PTEN mutations, we examined the function of the PTEN gene in human glioblastoma cell line U87MG. U87MG has been shown to carry a deletion in the PTEN gene (3). Using an anti-PTEN antibody YU102, we found that U87MG cells did not contain any detectable PTEN protein, confirming that these cells are indeed deficient in PTEN (Fig. 1C). To investigate whether deletion of the PTEN gene confers any growth advantage, we reintroduced the wild-type PTEN gene into the U87MG cells and analyzed its effect on cell growth. To increase the gene delivery efficiency, the PTEN cDNA was cloned into a retroviral vector (pBabePuro), which carries the puromycin selection marker. The recombinant PTEN retroviruses, as well as the control (the empty retroviral vector) viruses, were produced from Bing cells as amphotropic viruses and used to infect the U87MG cells. After infection, stable colonies were selected in puromycin-containing medium and counted after 2–4 weeks. U87MG cells infected with the vector control retroviruses produced many large and densely growing colonies in 2 weeks in the selection medium. However, in PTEN retrovirus infected cells, few colonies were observed during the same time period. Very small colonies did appear in PTEN infected cells after a longer incubation (4 weeks) (Fig. 1A Upper). These observations suggest that PTEN exerted potent growth suppression in U87MG cells. In contrast, in human colon carcinoma cells, DLD1, there were much less differences in the number of drug resistant colonies between the vector control and PTEN virus infected cells (Fig. 1A Lower). The results from DLD1 cells also indicate that the titers for the vector control and PTEN-expressing viruses were similar. In addition to DLD1, a variety of other cell lines such as human colon carcinoma cells HCT116 and mink lung epithelial cells (MvLu), appeared to be relatively resistant to PTEN-mediated growth suppression (data not shown). These studies suggest that PTEN can suppress cell growth in a cell-type dependent manner. Our observations are consistent with the previous report showing that expression of the PTEN gene led to 60–70% reduction of cell numbers in PTEN-deficient human glioma cells in a transient transfection assay (13).
Figure 1.
Growth inhibition by PTEN. (A) PTEN selectively suppresses colony formation in U87MG cells. Glioblastoma U87MG (Upper) or colon carcinoma DLD1 (Lower) cells were infected with either empty-vector control (Left) or PTEN-expressing retroviruses (Right). The infected cells were then selected in puromycin containing medium for either 4 weeks (U87MG) or 2 weeks (DLD1). Cells were fixed, stained with Giemsa dye, and photographed. (B) PTEN expression inhibits U87MG cell doublings. Two independent stable U87MG cell clones that contain the empty vector (Vector, clones 1A3 and 1A4) and the catalytically inactive PTEN(CS) mutant [PTEN(CS), clones 3A2 and 3A3], or the pooled clones from U87MG derivatives expressing PTEN (PTEN, pool), were analyzed. 5 × 105 cells from each cell line (in duplicates) were seeded onto culture dishes and the average cell numbers (with the standard deviation) were presented. (C) Western blot analyses. Cell lysates (50 μg each) from the indicated cell lines were examined by Western blot analysis with anti-PTEN antibody or anti-p42 MAPK antibody, respectively.
To further examine the growth properties of the PTEN-expressing cells, we recovered the drug resistant cell clones after retrovirus infection. Because the PTEN-retrovirus infected U87MG cells grow extremely slow, the drug resistant clones were pooled and cultured further. As the controls, we isolated cell colonies that were derived from cells infected either with retroviruses carrying the empty vector or with retroviruses expressing PTEN(CS), a mutant derivative of PTEN that contains Cys-124 to serine substitution at the phosphatase catalytic center. We have previously shown that the C124S mutation in PTEN completely abolished the phosphatase activity of the enzyme (5). To determine whether PTEN expression causes an increase of cell doubling time, we compared the growth rate of the control and PTEN-expressing cells. Equal number of cells from each cell line were plated in culture dishes and the number of cells were monitored every 24 hr after seeding. As shown in Fig. 1B, whereas the vector control or PTEN(CS)-expressing cells have a doubling time of 24–36 hr, the PTEN-expressing derivatives have a doubling time of ≈6 days. In addition, the PTEN(CS)-expressing cells appeared to grow to higher saturation density than the vector control cells, suggesting that the catalytically inactive PTEN(CS) mutant may provide a growth advantage to these cells.
To examine the level of PTEN expression in U87MG cells, we performed Western blot analysis with an anti-PTEN antibody. As shown in Fig. 1C, U87-PTEN stable cell lines express PTEN at a level that was comparable to those detected in a variety of human and rodent cell lines, including DLD1, HaCaT (human keratinocytes), Madin–Darby canine kidney (MDCK) epithelial cells and MvLu cells. As control, the level of p42 MAPK is also examined in parallel (Fig. 1C). DLD1, HaCaT, MDCK, and MvLu cells all contain active PTEN protein, as determined by the phosphatase activity assay of the anti-PTEN immunoprecipitates (data not shown). In addition, PTEN and PTEN(CS) were expressed at similar levels in U87MG derivatives (see Fig. 4B). These results further indicate that the expression of the wild-type PTEN gene confers a potent growth suppression effect in U87MG cells.
Figure 4.
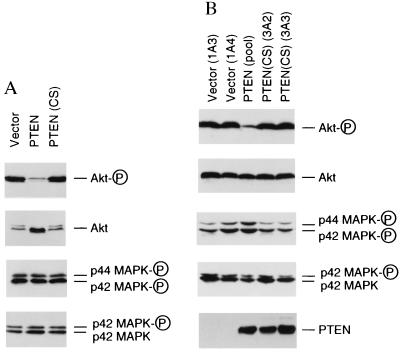
Phosphorylation status of Akt and MAPKs. (A) Phosphorylation status of Akt and MAPKs in PTEN retroviruses-infected cells. U87-EcoR cells were infected with retroviruses carrying empty vector, PTEN, or PTEN(CS) and harvested 48 hr later. Cell lysates (50 μg each) were analyzed by Western blot analysis with specific antibodies against phospho-Akt or phospho-MAPKs, respectively. As controls, duplicate filters were examined in parallel with anti-Akt or anti-p42 MAPK antibodies that recognize both phospho- and nonphosphoproteins. (B) Phosphorylation status of Akt and MAPKs in U87MG derivatives that stably express PTEN or PTEN(CS). U87MG cell clones that contain the empty vector (clones 1A3 and 1A4) and the catalytically-inactive PTEN mutant, PTEN(CS) (clones 3A2 and 3A3); or the pooled clones from PTEN-expressing U87MG derivatives, PTEN (pool), were analyzed by Western blot analysis by using antibodies specific for the phosphorylated forms for Akt or MAPKs, respectively. As control, duplicate filters were examined in parallel with regular anti-Akt or anti-p42 MAPK antibodies. The expression of PTEN or PTEN(CS) were detected by Western blot analysis using affinity-purified anti-PTEN antibody.
PTEN Suppresses the Tumorigenicity of Human Glioblastoma U87MG Cells.
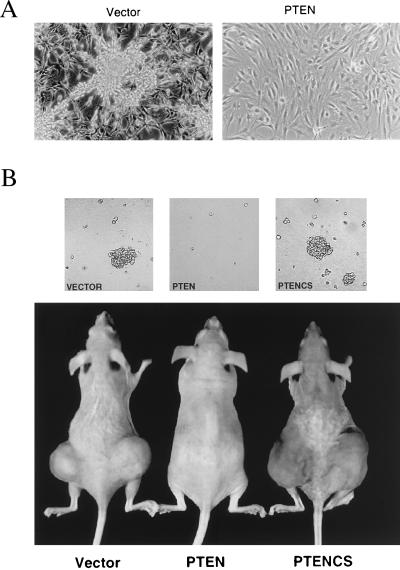
In addition to reduction in growth rate, expression of the PTEN gene in U87MG cells caused morphological changes. The vector control cells usually grow into aggregates to form “cell clumps” in culture (Fig. 2A). Similar morphology has also been observed in cells-expressing the catalytically inactive mutant, PTEN(CS) and the parental cells (data not shown). However, the PTEN-expressing cells showed a much flattened cell morphology and nonrefractile appearance (Fig. 2A). These observations suggest that the expression of PTEN profoundly alters the cell shape and/or cell–matrix interactions.
Figure 2.
Alteration of growth properties in PTEN-expressing U87MG cells. (A) Morphological changes. The vector control U87MG cells (Left) were highly refractile and many grew in “cell clumps.” The PTEN-expressing cells (Right) were much flatter and non-refractile. Photographs were taken at the same magnification (×200). (B) PTEN suppressed cell growth in soft agar and in nude mice. (Upper) 104 cells (a representation from duplicated samples) from U87MG cell clones that contain either the empty vector, or cells expressing PTEN or the PTEN(CS) mutant were assayed for their ability to grow in soft agar. Photographs were taken 4 weeks after seeding. (Lower) Tumor formation in nude mice. Cells (5 × 105) were injected into each site in the nude mice (two sites per mouse) and mice were monitored weekly after inoculation. A photograph from representative mice 8 weeks after injection with the indicated cells is shown.
Changes in cell morphology have been shown to be associated with malignant transformation in many tumor cells. A hallmark of transformation is that transformed cells can grow in the absence of cell anchorage, as reflected by their ability to grow in soft agar and in nude mice. To investigate whether the morphological changes induced by PTEN expression correlates with a reduction in the transformation properties of these cells, we compared the ability of U87MG cells carrying either the empty vector, or expressing PTEN or the catalytically-inactive mutant PTEN(CS) to grow in soft agar and in nude mice. Whereas the vector control and PTEN(CS)-expressing cells readily grew in soft agar, the PTEN-expressing cells lost their ability to grow in soft agar (Fig. 2B Upper). We also assayed these cells for their ability to form tumors in nude mice. In mice injected with vector control cells, 6/6 injected sites grew tumors with average tumor size 1,766 mm3 at 8 weeks after inoculation. But in mice injected with PTEN-expressing cells, 9 of 10 injected sites were tumor free when examined by dissection 15 weeks after inoculation, and 1 in 10 sites contained a very small tumor (tumor size <14 mm3). In contrast, in mice injected with PTEN(CS)-expressing cells, 7 out of 8 sites grew tumors with average tumor size 5,960 mm3 at 8-weeks. The more aggressive tumor growth was observed with the PTEN(CS)-expressing cells, suggesting that the catalytic inactive mutant phosphatase may have a growth promoting effect. A comparison of these mice at 8-weeks after inoculation is shown in Fig. 2B (Lower). These studies indicate that the expression of PTEN suppressed the anchorage-independent growth of U87MG cells in soft agar and their tumorigenecity in vivo and such function of PTEN is dependent on its phosphatase activity.
PTEN Expression Leads G1 Cell Cycle Arrest.
To examine the molecular mechanisms by which PTEN mediates growth suppression, we have examined the cell cycle effect after the transient PTEN-expression in U87MG glioblastoma cells. To improve the retrovirus gene delivery efficiency into human cells, we have established U87MG derivatives that stably express the murine ecotropic receptor (12). These cells, designated as U87-EcoR, could be infected with ecotropic retroviruses at >90% efficiency, as assayed using a control LacZ retrovirus (data not shown). Such an efficient gene delivery system allows us to analyze the effect of PTEN on the cell cycle in the total cell population.
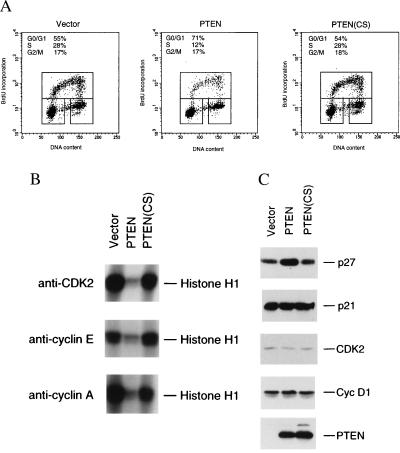
To examine the effect of PTEN expression on cell cycle progression, U87-EcoR cells were infected with ecotropic retroviruses expressing either PTEN, the catalytically-inactive PTEN(CS) mutant, or an empty vector control. Forty-eight hours postinfection, cells were pulsed-labeled with BrdUrd, a thymidine analog. The cells were fixed, stained with fluorescein-conjugated anti-BrdUrd antibody and propidium iodide, and examined for cell cycle profile by using the fluorescence-activated cell sorting analysis method. As shown in Fig. 3A, expression of PTEN led to >50% reduction in the S phase cell population and a substantial increase in the G0/G1 population, as compared with that of the vector control. The cell cycle arrest caused by PTEN expression also depended on its phosphatase activity, as very little effect on the cell cycle profile was observed when the catalytically-inactive PTEN(CS) mutant was expressed (Fig. 3A).
Figure 3.
Transient expression of PTEN leads to G1 cell cycle arrest and causes changes in the activities of cell cycle regulators. (A) Flow cytometry analysis of cell cycle distribution. U87-EcoR cells were infected with retroviruses carrying either the empty vector, PTEN or PTEN(CS). Infected cells were harvested 48 hr later. Before harvesting, cells were pulse-labeled with BrdUrd for 3 hr. BrdUrd incorporation was analyzed by fluorescein-conjugated anti-BrdUrd antibody (vertical axis) and DNA-content was measured by propidium iodide staining (horizontal axis). The percentage of cells in S phase (upper boxes), G0/G1 phase (lower-left boxes), G2/M phase (lower-right boxes) are indicated. Similar results were obtained from five independent experiments and one of them is shown here. (B) Histone H1 kinase activity assay. Cells were infected with either vector, PTEN or PTEN(CS)-expressing retroviruses and harvested 48 hr later. Immunoprecipitation was carried out by using either anti-CDK2, anti-cyclin E, or anti-cyclin A antibody from each cell lysate (350 μg), respectively. The immunoprecipitated CDKs were assayed for kinase activity by using histone H1 as the substrate. Similar results were obtained from the duplicates of the immunoprecipitation and kinase assay (one set of the duplicates is presented). (C) Western blot analysis. Cell lysates (50 μg each) from cells infected with either vector, PTEN, or PTEN(CS)-expressing retroviruses were subjected to Western blot analysis with specific antibodies against p27KIP1, p21CIP1/WAF1, CDK2, cyclin D1, or PTEN, respectively.
PTEN Expression Causes Inhibition of the G1 CDKs.
In mammalian cells, cell cycle progression is controlled by the sequential activation of CDKs (14). To examine whether the observed G0/G1 cell cycle arrest by PTEN expression is accompanied by changes in the activities of the cell cycle regulatory machinery, we have examined the G1 CDK activities. The cells were infected with either the vector control or PTEN retroviruses. Forty-eight hours after infection, CDKs were isolated from cell lysates by immunoprecipitation by using specific CDK or cyclin antibodies. The isolated CDKs were assayed for their kinase activities in vitro by using histone H1 as a substrate. As shown in Fig. 3B, in cells expressing PTEN, there was a significant decrease in the CDK2 kinase activity, as compared with the vector control cells. Because CDK2 is normally associated with cyclin E and cyclin A, which regulate the G1/S transition, we also assayed for the kinase activity associated with cyclin E and cyclin A. Both cyclin E- and cyclin A-associated kinase activities were also significantly reduced by the expression of PTEN (Fig. 3B). In contrast, in cells expressing the catalytically inactive PTEN(CS) mutant, no reduction in CDK2, cyclin E/CDK2, or cyclin A/CDK2 kinase activities was observed (Fig. 3B). These studies indicate that PTEN expression leads to inhibition of the G1 cyclin-dependent kinase activities, and such inhibition depends on the phosphatase activity of PTEN. Due to technical difficulties (15), we have not been able to assay the cyclin D/CDK4 or cyclin D/CDK6-associated kinase activities in these cells.
PTEN Expression Induces Accumulation of p27KIP1.
The functions of several tumor suppressors have been shown to be mediated in part through the regulation of the CDK inhibitors (14). To investigate whether CDK2 inhibition observed in PTEN-expressing cells were associated with alteration in the levels of CDK inhibitors, we examined the levels of p21CIP1/WAF1 or p27KIP1 in cells infected with retroviruses either carrying no insert (vector control), PTEN, or its mutant derivative PTEN(CS). As shown in Fig. 3C, expression of PTEN led to a significant elevation of p27, as revealed by Western blot analysis with anti-p27 antibody. The accumulation of p27 also depended on the phosphatase activity of PTEN, because the catalytically inactive PTEN(CS) mutant did not cause p27 accumulation. In these experiments, the increase of p27 could be observed in either direct Western blot analysis of the cell lysates (Fig. 3C) or immunoprecipitation followed by Western blot analysis with anti-p27 antibody (data not shown). We have consistently observed increase of p27 level after PTEN expression in five independent experiments, with an average increase of ≈3-fold. In parallel, we have also examined the level of p21CIP1/WAF1 in these cells by Western blot analysis with anti-p21 antibody. In contrast with the p27 results, there was very little change in the p21 protein level in cells expressing PTEN or its PTEN(CS) mutant, as compared with vector control (Fig. 3C). In addition, PTEN expression had little effect on the protein levels of CDK2 or cyclin D1 (Fig. 3C). Because the cells infected with PTEN-retroviruses contain the similar levels of cyclin D1 as the control virus-infected cells and because cyclin D1 is known to be expressed at high levels at G1 but very low in G0 phase cells, the PTEN-expressing cells are likely to be arrested in the G1 phase of the cell cycle. The expression of PTEN and PTEN(CS) proteins were confirmed by Western blot analysis with anti-PTEN antibody (Fig. 3C).
PTEN Expression Inhibits the Akt/PKB Signaling Pathway.
Down-regulation of p27KIP1 in the G1 phase has been shown in certain cells to involve the function of PI 3-kinases (16, 17). PI 3-kinases regulate the levels of inositol phospholipids, which act as second messengers (18). One of the best characterized downstream targets activated by PI 3-kinase signaling is Akt/PKB, a serine/threonine kinase encoded by a protooncogene (19). To investigate if PI 3-kinase downstream signaling is involved in PTEN-mediated growth suppression, we examined the status of Akt in cells infected with retroviruses carrying PTEN, PTEN(CS), or the vector control. As shown in Fig. 4A, expression of PTEN, but not its catalytically-inactive mutant PTEN(CS), led to a substantial reduction in the amount of the phosphorylated, active forms of Akt, as determined by using an antibody that specifically recognizes the Ser-473-phosphorylated Akt (Fig. 4A). The reduction in Akt phosphorylation signal is not due to the decrease in protein level, because the total protein level of Akt was even slightly elevated by PTEN expression, as revealed by Western blot analysis using antibody that recognized both phosphorylated and unphosphorylated forms of Akt (Fig. 4A). Dephosphorylation of Akt was also accompanied by shift of its electrophoretic mobility to faster migrating species, which could be resolved under certain electrophoresis condition (Fig. 4A). In contrast, the active and phosphorylated forms of both p42 and p44 MAPKs were not detectably altered by PTEN expression, as examined by using an antibody that recognizes the Thr-202/Tyr-204 phosphorylated forms of p42 and p44 MAPKs (Fig. 4A). As control, the level of p42 MAPK was also not affected by PTEN expression (Fig. 4A).
We further examined the phosphorylation status of Akt and MAPKs in U87MG derived cell lines that either stably express PTEN, its catalytically inactive mutant PTEN(CS), or the vector control. Western blot analysis of these cell lysates revealed that cells stably expressing PTEN, but not cells stably expressing the PTEN(CS) mutant, had greatly reduced phosphorylation of Akt, as compared with the vector control cells (Fig. 4B). Consistent with the transient expression assays, the phosphorylated forms of MAPKs were not affected in cell lines that stably express either PTEN or PTEN(CS) (Fig. 4B). PTEN and PTEN(CS) were expressed at the similar level in these cells (Fig. 4B), which were comparable to the endogenous PTEN protein levels in several cell lines such as DLD1 or MvLu (see Fig. 1C).
In addition, we have examined the tyrosine phosphorylation status of PI 3-kinase in these cells. The best-characterized PI 3-kinase, p85/p110 heterodimer, is known to be activated by tyrosine phosphorylation of p85 regulatory subunit, which leads to conformation change and thus activation of the p110 catalytic subunit (18). We had found that there was no change in the tyrosine phosphorylation level of the p85 subunit of PI 3-kinase in U87MG derivatives either transiently or stably expressing PTEN or PTEN(CS) (data not shown). Therefore, our studies indicate that PTEN expression leads to the selective inhibition of Akt signaling pathway and the inhibition of Akt phosphorylation is likely to occur downstream of PI 3-kinase.
Inhibition of PI 3-Kinase by Pharmacological Reagents Leads to Up-Regulation of p27KIP1 and Cell Cycle Arrest.
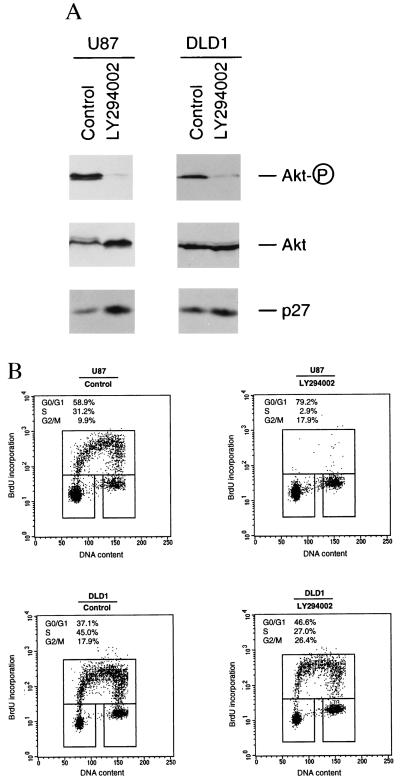
The inhibition of phosphorylation of Akt in PTEN-expressing cells suggests that PTEN may be involved in the PI 3-kinase signaling pathway. In addition, our studies suggest that PTEN expression causes the accumulation of p27KIP1, raising the possibility that the PI 3-kinase signaling pathway may regulate the level of p27. To confirm that the PTEN-induced increase of p27 is caused by down-regulation of the PI 3-kinase signaling pathway, we have examined whether p27 accumulation can be mimicked by inhibition of PI 3-kinase pathway by using pharmacological agents. Log-phase growing U87-EcoR cells were treated with LY294002, an inhibitor that selectively inhibits PI 3-kinases. As shown in Fig. 5A, such treatment potently inhibited the phosphorylation of Akt and also led to a significant increase of p27 level. Similar to the LY294002 effect, treatment of cells with wortmannin, another inhibitor for PI 3-kinase, also led to reduction of Akt phosphorylation and up-regulation of p27 (data not shown). In contrast, treatment of cells with PD98059, a specific inhibitor of MEK (MAPK kinase), had very little effect on the p27KIP1 protein level, whereas it readily inhibited the phosphorylation of p42 and p44 MAPKs (data not shown).
Figure 5.
Effect of LY294002 on Akt, p27KIP1, and cell cycle profile. (A) Western blot analysis of drug-treated cells. Actively growing U87 cells (Left) or DLD1 cells (Right) were treated with either dimethyl sulfoxide (control) or LY294002 (20 μM) for 24 hr. Cell lysates (50 μg each) were examined by Western blot analysis with phosphorylation status specific or regular antibodies against Akt, respectively. A duplicated blot was also examined by Western blot analysis with anti-p27 antibody. (B) Fluorescence-activated cell sorting analysis of cell cycle profile of drug-treated cells. Actively growing U87 cells (Upper) or DLD1 cells (Lower) were treated with either dimethyl sulfoxide (control) or LY294002 (20 μM) for 24 hr. Cells were harvested and examined by fluorescence-activated cell sorting analysis for cell cycle distribution as described in the legend of Fig. 3A.
We further examined whether PTEN-mediated cell cycle arrest can be mimicked by the drug LY294002. As shown in Fig. 5B, treatment of U87-EcoR cells with LY294002 nearly abolished the S-phase population (>90% reduction of S phase), with a concomitant increase of G0/G1-phase population and a modest increase in the G2/M-phase population. These studies further suggest that PTEN-mediated cell cycle arrest in U87 cells involves the inhibition of PI 3-kinase signaling pathway and one critical target for this signaling pathway is p27KIP1.
We also examined the response in DLD1 cells toward LY294002 treatment. As discussed above, DLD1 appeared to be relatively resistant toward growth inhibition mediated by PTEN-expressing retroviruses (Fig. 1A). As shown in Fig. 5A, although LY294002 treatment of DLD1 cells readily inhibited the phosphorylation of Akt, however, it only modestly affected the level of p27KIP1. It is worth pointing out that the steady-state level of phosphorylated Akt in DLD1 was severalfold lower than that in U87 cells when they were compared side by side (data not shown). In addition, LY294002 treatment led to a partial reduction of S phase population (≈40%) in DLD1 cells (Fig. 5B). These results showed that U87 and DLD1 cells exhibited different sensitivity toward inhibition of PI 3-kinase. One possible explanation for such different cell responses toward ectopic PTEN-expression or LY294002 treatment is that the relative contribution of PI 3-kinase signaling toward cell cycle progression is different in these two types of cells. Although PI 3-kinase signaling may be a rate-limiting step for p27KIP1 down-regulation and cell cycle progression in U87 cells, additional pathways could be involved in DLD1 cells for these processes.
DISCUSSION
Our studies have shown that expression of PTEN/MMAC1/TEP1 potently suppresses the growth and tumorigenicity of human glioblastoma U87MG cells in vitro and in vivo. This growth suppression activity is dependent on the intrinsic phosphatase activity of the enzyme. Our results are consistent with earlier minichromosome transfer experiments showing that introduction of human chromosome 10q into glioblastoma cells suppressed the ability of these tumor cells to grow in soft agar or form tumors in nude mice (20). Very recently, it has also been shown that adenovirus-mediated PTEN gene delivery, which expressed PTEN at very high level, also suppressed the growth of these cells in soft agar and in nude mice (21). Our retrovirus system has shown that PTEN can suppress tumorigenicity in U87MG cells at the expression level that is comparable to the endogenous PTEN protein level observed in many cell lines.
Our results strongly suggest that the growth suppression effect of PTEN in U87MG cells is mediated by its ability to block cell cycle progression at G1 phase. The PTEN-induced cell cycle arrest is correlated with a significant accumulation of the cell cycle kinase inhibitor p27KIP1. The importance of down-regulation of p27KIP1 for G1 cell cycle progression has been suggested. It has been reported that expression of a dominant-negative form of Ras, likely through inhibition of PI 3-kinase pathway, blocked the down-regulation of p27KIP1 in NIH 3T3 cells at mid- or late-G1 stage (16). In aortic smooth muscle cells, wortmannin treatment blocked cell cycle progression in late G1, which was accompanied by failure to down-regulate p27KIP1 (17). In U87MG glioblastoma cells, we have observed that transient expression of PTEN led to significant up-regulation of p27KIP1 and G1 cell cycle arrest. Both PTEN-induced G1 cell cycle arrest and p27KIP1 accumulation can be mimicked by treatment of cells with pharmacologic reagents that inhibited PI 3-kinases, suggesting that PTEN can negatively regulates the PI 3-kinase pathway. We have also shown that expression of PTEN leads to inhibition of Akt/PKB, a serine–threonine kinase activated by PI 3-kinase signaling pathway, and such inhibition appears to occur downstream of activation of PI 3-kinase. Very recently, it has been reported that the PTEN protein possesses a phosphatase activity toward phosphatidylinositol 3,4,5-trisphosphate (8). It is likely that in our system, the inhibition of Akt by PTEN expression is mediated by a reduction of the inositol phospholipid levels that are required for activation of this kinase.
PI 3-kinase activity has been shown to be required for G1 to S progression in NIH 3T3 cells, as microinjection of neutralizing antibodies against PI 3-kinase inhibited growth factor induced DNA synthesis (22). Akt/PKB has been shown to be a critical downstream target of PI 3-kinase (19). Akt is an essential mediator for prevention of apoptosis in neuronal cells or lymphocytes after growth factor withdrawal (19). We have observed inhibition of Akt phosphorylation during PTEN-mediated cell cycle arrest. It is possible that Akt itself, or other molecules activated by PI 3-kinase signaling process, serves an essential function for G1 cell cycle progression in U87MG glioblastoma cells. Such a process involves the down-regulation of CDK inhibitor p27KIP1 during the G1 progression.
In summary, our studies suggest that PTEN functions to down-regulate PI 3-kinase signaling pathway that normally plays an essential role in G1 cell cycle progression. In the absence of PTEN tumor suppressor protein, the increased PI 3-kinase signaling may promote uncoordinated G1 cell cycle progression, allowing cells to bypass the normal signaling processes regulated by growth factors and cell anchorage, leading to tumorigenesis.
Acknowledgments
We thank Dr. Hui Zhang for helpful discussions, Dr. Dan DiMaio for critical reading of the manuscript, Drs. Doug Conklin and David Beach at Cold Spring Harbor Laboratory for kindly providing the pWZL-Neo-EcoR plasmid, and Rocco Carbone at the Yale Comprehensive Cancer Center for help with the flow cytometry analysis. D.-M.L. was a recipient of the Leslie H. Warner Fellowship in Cancer Research. H.S. is a Pew Scholar in the Biomedical Sciences. This work was supported by Pew Charitable Trust and the Department of the Army (DAMD 17-98-1-8271).
ABBREVIATIONS
- BrdUrd
bromodeoxyuridine
- CDK
cyclin-dependent kinase
- MAPK
mitogen-activated protein kinase
- PI 3-kinase
phosphatidylinositol 3-kinase
- PKB
protein kinase B
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Deimling A, Louis D N, Weiestler O D. Glia. 1995;15:328–338. doi: 10.1002/glia.440150312. [DOI] [PubMed] [Google Scholar]
- 2.Steck P A, Ligon A H, Cheong P, Yung W K, Pershouse M A. Genes Chromosomes Cancer. 1995;12:255–261. doi: 10.1002/gcc.2870120404. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 4.Steck P A, Pershouse M A, Jasser S A, Lin H, Yung W K A, Ligon A H, Langford L A, Baumgard M L, Hattier T, et al. Nat Genet. 1997;15:356–363. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 5.Li D-M, Sun H. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 6.Eng C. Intl J Oncol. 1998;12:701–710. doi: 10.3892/ijo.12.3.701. [DOI] [PubMed] [Google Scholar]
- 7.Myers M P, Stolarov J P, Eng C, Li J, Wang S I, Wigler M H, Parsons R, Tonks N K. Proc Natl Acad Sci USA. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maehama T, Dixon J E. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 9.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada K M. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Kobayashi R, Galaktionov K, Beach D. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 11.Pear W S, Scott M L, Nolan G P. In: Methods in Molecular Medicine, Gene Therapy Protocols. Robbins P, editor. Totowa, NJ: Humana; 1997. pp. 41–57. [DOI] [PubMed] [Google Scholar]
- 12.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 13.Furnari F B, Lin H, Huang H J S, Cavenee W K. Proc Natl Acad Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter T, Pines J. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 15.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 16.Takuwa N, Takuwa Y. Mol Cell Biol. 1997;17:5348–5358. doi: 10.1128/mcb.17.9.5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacqueville D, Casagrande F, Perret B, Chap H, Darbon J M, Breton-Douillon M. Biochem Biophys Res Commun. 1998;244:630–636. doi: 10.1006/bbrc.1997.7885. [DOI] [PubMed] [Google Scholar]
- 18.Toker A, Cantley L C. Nature (London) 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 19.Downward J. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 20.Pershouse M A, Stubblefield E, Hadi A, Killary A M, Yung W K, Steck P A. Cancer Res. 1993;53:5043–5050. [PubMed] [Google Scholar]
- 21.Cheney I W, Johnson D E, Vaillancourt M T, Avanzini J, Morimoto A, Demers G W, Wills K N, Shabram P W, Bolen J B, Tavtigian S V, et al. Cancer Res. 1998;58:2331–2334. [PubMed] [Google Scholar]
- 22.Roche S, Koegl M, Courtneidge S A. Proc Natl Acad Sci USA. 1994;91:9185–9189. doi: 10.1073/pnas.91.19.9185. [DOI] [PMC free article] [PubMed] [Google Scholar]