Abstract
Diverse kinds of gem- and vic-diborylated compounds are now readily available thanks to advances in gem-diborylation of lithium carbenoids as well as vic-diborylation of carbon–carbon multiple bonds with diboron compounds. These diborylated reagents lead to invention of polyborylated reagents and many novel and useful synthetic methods for supreme stereocontrol. This review summarizes preparative methods and synthetic reactions of di- and polyborylated reagents with the emphasis on multiple bond formation.
Keywords: boron, carbenoid, dimetalated reagents, organic synthesis
Introduction
Dimetalated organic compounds have recently emerged as versatile reagents for organic synthesis because such bifunctional compounds allow us to synthesize target organic frameworks very efficiently through multiple bond formations in a single operation or stepwise transformations in one-pot.1) Furthermore, organodimetallic compounds can serve as valuable precursors of polyfunctional organometallic reagents.2) Therefore, it is of great importance to develop efficient preparations as well as chemo-, regio-, stereo-, and enantioselective reactions of dimetalated compounds. Simultaneous incorporation of two metals into organic substrates with reagents having metal–metal linkage is a highly attractive approach as the preparative method in view of atom economy and straightforward strategy (Scheme 1). Indeed, transition-metal catalyzed cleavage of the metal–metal linkage such as B–B, B–Si, B–Sn, Mg–Si, Mg–Zn, Mg–Sn, Al–Si, Al–Sn, Si–Si, Si–Sn, Si–Zn, Sn–Sn, and Sn–Zn, followed by addition to carbon–carbon unsaturated bonds have been well-described as efficient synthesis of vic-dimetalated compounds.3)
Scheme 1.
In sharp contrast, gem-dimetalation using such metal–metal compounds has remained unexplored.4) In 1976, Kitatani, Hiyama, and Nozaki reported stereoselective one-pot dialkylation of gem-dihalocyclopropanes with diorganocuprate and alkyl halide. The reaction is considered to proceed through generation of an ate-type carbenoid by bromine-copper exchange at the less hindered site, consecutive alkyl 1,2-migration from the negatively charged copper to the carbenoid carbon with inversion of configuration, and the second alkylation of the resulting copper reagent with methyl iodide (Scheme 2).5) This type of transformation is disclosed now to be applicable to not only cyclopropylidene- but also alkylidene-type carbenoid reagents with a variety of metals.6) Worthy to note is that an SN2 type substitution reaction with inversion of configuration at the carbogenic center, which is otherwise inert to conventional nucleophilic substitution reaction.
Scheme 2.
Based on the novel concept, the authors designed gem-dimetalation of lithium carbenoids with such metal–metal compounds as B–B and B–Si (Scheme 3). Thus, they envisioned that gem-diboryl and gem-silylboryl products should result via a sequence of reactions of (1) borate formation and (2) 1,2-migration of a boron or silicon atom from the negatively charged borate centers to the carbenoid carbons in a 1,2-fashion with elimination of a halogen atom.7) Actually the designed sequence did work well.8) Consequently, repertoire of gem-silylboryl and gem-diboryl compounds are largely expanded, and organic synthesis utilizing those bifunctional reagents has dramatically advanced. This review focuses on not only gem-diboryl but also vic-diboryl compounds illustrated in Figure 1 and summarizes the preparations and selective transformations for organic synthesis.9)
Scheme 3.
Fig. 1.
Preparation of gem- and vic-diboryl compounds
Double hydroboration of 1-alkynes with such borane reagents as diborane, dicyclohexylborane, and 9-BBN is a classical way for the preparation of 1,1-diborylalkanes.10) In view of yield and selectivity of 1,1- versus 1,2-double boration, 9-BBN is the reagent of choice. For example, 1-hexyne reacts with two molar amounts of 9-BBN to give 1-hexanol quantitatively after oxidative workup as illustrated in Scheme 4.
Scheme 4.
Insertion of diazoalkanes into bis(pinacolato)diboron (1, abbreviated as Bpin-Bpin) is catalyzed by a platinum catalyst to produce disubstituted diborylmethanes eq. [1].11) Tetrakis(dimethoxyboryl)methane, prepared from carbon tetrachloride by treatment with lithium and dimethoxyboryl chloride (Scheme 5),12) undergoes transesterification with pinacol or 1,3-propanediol to give the corresponding tetraborylmethanes, respectively.13)
Scheme 5.
Convenient synthesis of 1,1-diborylated cyclopropanes is achieved by gem-diborylation of cyclopropylidene lithium carbenoids with 1 (Scheme 6).14) Thus, the carbenoids generated by treatment of dibromocyclopropanes with BuLi in THF/Et2O at −110°C react with co-existing 1 to give gem-diborylcyclopropanes in good to high yields. The method is applicable to the preparation of not only tri- and tetrasubstituted cyclopropanes but also fused and hexasubstituted cyclopropanes.
Scheme 6.
Generation of triborylmethyllithium from tetraborylmethanes with methyllithium followed by condensation with aldehydes or ketones produces 1,1-diboryl-1-alkenes (Scheme 7).15) Such functional groups as chloro, ethoxycarbonyl, and amino groups tolerate the conditions.
Scheme 7.
Diborylation of alkylidene-type lithium carbenoids with 1 also serves as an efficient preparative method for 1,1-diboryl-1-alkenes (Scheme 8).16) Various types of gem-diborylalkenes are easily prepared starting from the corresponding 1-halo- or 1,1-dihaloalkenes with the aid of butyllithium or a base.
Scheme 8.
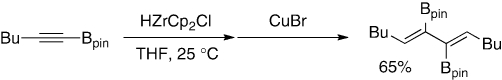
The methodology is readily extended to synthesis of 2,3-bis(pinacolatoboryl)-1,3-butadiene (2) (Scheme 9).17) Thus, when 1 is treated with 1-bromo-1-lithioethene in excess generated from vinyl bromide with LiTMP, 2 is produced in high yield. Formation of 2 is ascribed to the borate formation between the initial product, 1,1-diborylethene, and another 1-bromo-1-lithioethene, followed by 1,2-migration of a 1-borylethenyl group. Meanwhile, 1,4-disubstituted 2,3-diboryl-1,3-butadienes are prepared by regioselective hydrozirconation of alkynylboronates with HZrCp2Cl followed by dimerization with CuBr (Scheme 10).18)
Scheme 9.
Scheme 10.
Various vic-diborylated compounds are readily available through transition metal-catalyzed 1,2-diboration of carbon–carbon multiple bonds with diborons.19) Platinum complexes such as Pt(PPh3)4, Pt(norbornene)/PPh2(o-tolyl), and Pt(PCy3)(η2-C2H4)2 catalyze cis-addition of diborons to both terminal and internal alkynes to give 1,2-diborylated acyclic alkenes stereoselectively (Scheme 11).20) Terminal and strained cyclic alkenes are smoothly diborylated in the presence of phosphine-free Pt catalyst to provide 1,2-diborylalkanes.21) β-Borylallyllic boranes are prepared by Pt-catalyzed diboration of allenes.22) A catalyst system consisting of phosphine-free Pd complex and an aryl/alkenyl iodide or iodine is effective for diboration of a terminal C=C bond in 1-substituted and 1,1-disubstituted allenes,23) whereas the allenes are vic-diborylated at the internal C=C bonds with the aid of Pd2(dba)3/optically active phosphoramidite catalyst to give the corresponding 2,3-diboryl-1-alkenes in good yields with high enantioselectivity.24)
Scheme 11.
Synthetic transformation of gem-diborylalkanes
Reactions of gem-diborylalkanes with electrophiles are facilitated by borate formation with alkyllithiums. For example, when 1,1-diborylhexane and 1,1-diboryl-2-phenylethane are treated with two molar amounts of butyllithium and then carbon dioxide, the corresponding malonic acids are obtained eq. [2].25) Boron enolates are also prepared from 1,1-diborylalkanes via methyllithium-mediated borate formation and reaction with methyl benzoate (Scheme 12).26)
Scheme 12.
 |
gem-Diborylalkanes containing a halogen atom or tosyloxy group at 3- or 4-position undergo intramolecular cyclization upon treatment with methyllithium, giving rise to the corresponding cyclopropyl or cyclobutylboranes, which are readily transformed into the corresponding alcohols by oxidative workup with alkaline hydrogen peroxide (Scheme 13).27)
Scheme 13.
Synthetic transformation of 1,1-diborylcyclopropanes
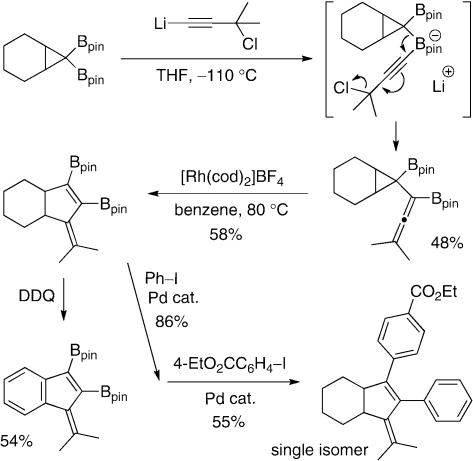
Since a variety of transition metal-catalyzed reactions using cyclopropanes are available, such transformations, when applied to diborylcyclopropanes, can provide us with novel diborylated building blocks. Diborylcyclopropanes, upon treatment with 3-chloro-1-lithio-3-methyl-1-butyne, give diborylated allenylcyclopropanes (Scheme 14).14) The formation can be explained by 1,2-migration of the cyclopropyl moiety in the borate intermediate with release of a chloride ion in an SN2' fashion. The allenylcyclopropanes undergo ring-expansion with the aid of a rhodium catalyst upon heating to afford 1,2-diboryl-3-methylenecyclopentenes, which are difficult to prepare via conventional methods. The diborylcyclopentenes can be easily transformed into polysubstituted fulvenes and cyclopentenes through oxidation and regiospecific cross-coupling reaction, respectively, as demonstrated in Scheme 14.
Scheme 14.
Cross-coupling reaction of 1,1- and 1,2-diboryl-1-alkenes with organic halides
Since tetrasubstituted ethenes with four different carbonaceous groups are often found in biologically active natural products as well as functional organic materials, stereocontrolled synthesis of those constitutes a significant and challenging issue in organic synthesis.28) Palladium-catalyzed cross-coupling reaction of 1,1-diboryl-1-alkenes is one of the efficient solutions for the synthetic problem (Scheme 15).
Scheme 15.
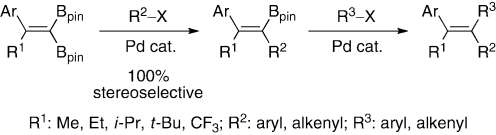
When 2-aryl-1,1-bis(pinacolatoboryl)-1-alkenes are coupled with aryl iodides with the aid of a Pd catalyst and a base, (E)-alkenylboronates are obtained as single stereoisomers in good to high yields with no trace of di-coupled products (Scheme 16).29) Irrespective of substituent R1, the stereochemical outcome is uniform. The following coupling reaction with other aryl iodides allows us to synthesize diverse stereocontrolled triarylethenes. The advantages of this methodology are that both stereoisomers of the tetrasubstituted ethenes can be prepared simply by changing the order of employed electrophiles, and the whole transformation can be achieved in one-pot. The synthetic value is demonstrated by one-pot synthesis of (Z)-tamoxifen that is currently used for treatment of breast cancer. The stereocontrol can be extended to the reactions with alkenyl halides and allows us to prepare stereodefined polysubstituted [3] dendralenes.
Scheme 16.
Aryl and alkenyl-substituted diborylethenes also react stereoselectively with aryl and alkenyl iodides, providing stereocontrolled route to polyfunctional 1,3,5-hexatrienes.30) Thus, 2,4-diaryl-1,1-diboryl-1,3-butadienes couple with aryl iodides in the presence of a Pd catalyst at the boryl group cis to the C(3)=C(4) group and the corresponding mono-coupled products are obtained as a single stereoisomer as illustrated in Scheme 17. Subsequent coupling reaction of the boronates with (E)-alkenyl iodides gives 1,3,4,6-tetraaryl-1E,3E,5E-hexatrienes that exhibit aggregation-induced emission upon photo-excitation and thus may find application to light-emitting materials. Palladium-catalyzed coupling reaction of (E)-1,2-bis(pinacolatoboryl)hex-1-ene with aryl, benzyl, alkenyl, and allylic halides proceeds selectively at the terminal boryl group to give 1,2-disubsituted 2-alkenylboronates as a major product along with di-coupled products (5–10%).31) Stereodefined trisubstituted ethenes are obtained by further coupling reaction of monoboronates as illustrated in Scheme 18.
Scheme 17.
Scheme 18.
Meanwhile, when multi-substituted unsymmetrical 1,2-diborylethenes are coupled with aryl halides in the presence of a Pd catalyst and a base, mono-coupled products are produced as a mixture of two possible regioisomers (Scheme 19).32) Ratio of the regioisomers varys depending on the substituents on the aryl groups of aryl halides. The second coupling reaction with an aryl iodide connected to solid support allows us to achieve combinatorial synthesis of triarylated 1-butenes, an important class of nonsteroidal anti-estrogen agents.
Scheme 19.
Cross-coupling reaction of 2,3-diboryl-1,3-butadiene with organic halides
Diborylbutadiene 2 is useful for straightforward synthesis of 1,3-butadiene-2,3-diyl moiety-containing organic molecules.33) For example, the coupling reaction of 2 with 4-acetoxyphenylmethyl chloride followed by hydrolysis of the acetate groups gives anolignan B, isolated from Anogeissus acuminata and shown to be an active inhibitory constituent of HIV-1 reverse transcriptase in the plant (Scheme 20). Facile synthesis of [4]- and [6] dendralenes, which constitute a class of cross-conjugated polyenes, were achieved by double coupling reaction of 2 with 2 molar amounts of alkenyl and dienyl iodides, respectively. Unsymmetrical dendralenes were prepared from 2 by stepwise coupling reactions with two different electrophiles.
Scheme 20.
Transformations of 2,3-diboryl-1,3-butadiene into 1,2-diborylcyclohexene and tetraboryl-2-butene
Since 1,3-butadienes are valuable substrates in a variety of organic reactions such as cycloadditions and transition metal-catalyzed addition reactions of unsaturated bonds, diborylbutadienes can be converted into novel polyborylated reagents by utilizing such transformations.34) Diels-Alder reactions of 2 with electron-deficient alkenes and dimethyl acetylenedicarboxylate proceed smoothly upon heating, giving rise to the corresponding 1,2-diborylcyclohexenes and -1,4-cyclohexadiene that can serve as building blocks for polysubstituted cyclohexenes and benzenes, respectively, in conjunction with Pd-catalyzed cross-coupling reaction and oxidation using DDQ (Scheme 21).35)
Scheme 21.
Platinum-catalyzed 1,4-diboration of 1,3-diene 2 with 1 produces (Z)-1,2,3,4-tetraboryl-2-butene in quantitative yield as a single stereoisomer (Scheme 22). The resulting tetraboryl-2-butene undergoes triple aldehyde addition in a one-pot manner to give 2,3-bis(alkylidene)-1,5-anti-diols as a single stereoisomer.36) The cascade reaction involves sequential conversion of four C–B bonds into two C–C bonds and one C=C bond with perfect stereocontrol. One-pot preparation–triple carbonyl addition starting with 2 is also possible. These features clearly demonstrate the versatility of polymetalated compounds as reagents for organic synthesis with high efficiency.
Scheme 22.
Allylation of aldehydes and imines with β-borylallyllic boranes
Enantioenriched β-borylallylic boranes, prepared in situ by Pd-catalyzed diboration of allenes, react with aldehydes and imines to give boryl-substituted homoallylic alcohols and amines, respectively, with high enanitoselectivity. The adducts are readily transformed into optically active β-hydroxy and -amino ketones via oxidative workup (Scheme 23).37)
Scheme 23.
Preparation and oxidation of optically active 1,2-diborylalkanes
Enantioselective diboration of trans-disubstituted alkenes with bis(catecholato)diboron proceeds diastereo- and enantioselectively, using a Rh(acac)(nbd)/(S)-quinap catalyst system. The resulting vic-diborylalkanes are led to the corresponding syn-1,2-diols with high diastereo- and enantioselectivities upon oxidation with alkaline hydrogen peroxide (Scheme 24).38) Under the same conditions, diboration of styrene, cis-1,2-, and 1,1-disubstituted alkenes results in moderate enantioselectivities. Alternatively, rhodium-catalyzed hydrogenation of 2-substituted 1,2-diborylethenes with optically active phosphine such as walphos allows to prepare 1,2-diborylalkanes with high enantiomeric excess.39)
Scheme 24.
Conclusion
Recent progress on preparation and synthetic reactions of gem- and vic-diborylated compounds are reviewed. Diverse diborylated compounds are now readily available in a stereodefined form owing greatly to the development of diborylation utilizing diborons. Efficient and straightforward synthetic methods for polyfunctional multi-substituted and cross-conjugated olefins as well as enantio- and diastereocontrolled alcohols and amines have been developed based on the chemistry of gem- and vic-diborylated compounds.
Biographies
Profile
Tamejiro Hiyama was born in Osaka, 1946. He started his academic career in 1972 as Research Associate at Kyoto University, received the Doctor of Engineering degree in 1975 (Kyoto University, Mentor Hitosi Nozaki), and spent a postdoctoral year (Harvard University, Yoshito Kishi). In 1981, he joined Sagami Chemical Research Center as Research Fellow to start his own research team. There he was promoted to Senior Research Fellow and then to Executive Research Fellow before he moved in 1992 to Research Laboratory of Resources Utilization, Tokyo Institute of Technology, as Professor of Division of Newer Metal Resources. Since 1997, he is Professor of Organic Materials at Kyoto University. He studied and invented many new reactions including, the highly selective carbon-carbon bond formation with Cr reagents (Nozaki-Hiyama-Kishi reaction), the reaction of nitriles with magnesium ester enolates (Hiyama reaction), the cross-coupling reaction with organosilicon reagents (Hiyama coupling), the oxidative desulfurization-fluorination for synthesis of organofluorine compounds, the carbostannylation and carbocyanation of alkynes, novel polysilacage compounds for electronic materials, novel liquid crystalline compounds for display in addition to carbenoid reagens for organic synthesis. He received Young Chemist Award of the Chemical Society of Japan in 1980, Japan Liquid Crystal Society Award in 2004, Synthetic Organic Chemistry Award in 2007, and is going to receive the CSJ Award, 2008. His current research interest extends to new organometallic reagents for selective organic synthesis, organofluorine and organosilicon chemistry, synthesis of biologically active substances, design and synthesis of novel functionality molecules and materials.

Profile
Masaki Shimizu was born in 1965 in Tokyo, Japan. He received his Ph.D. from Tokyo Institute of Technology in 1994 under the supervision of Professors Takeshi Nakai and Koichi Mikami. After working at Mitsubishi Chemical Co. Ltd. as a research associate, he joined Research Laboratory of Resources Utilization, Tokyo Institute of Technology as a research associate in 1995 to work with Professor Tamejiro Hiyama. In 1998, he moved to Kyoto University as Assistant Professor to continue collaboration with Professor Hiyama. He spent one year at Massachusetts Institute of Technology as a postdoctoral fellow (Professor S. L. Buchwald) and was promoted to Associate Professor of Kyoto University in 2003. His research interest focuses on development of novel synthetic methods of organofluorine compounds and synthetic methodology utilizing organometallic reagents, and creation of functional organic materials.

References
- 1).Reviews on dimetalated compounds: Matsubara, S. (2005) In Handbook of Functionalized Organometallics (ed. Knochel, P.). Wiley-VCH, Weinheim, Vol. 2, pp. 347–377; Foubelo, F. and Yus, M. (2005) Curr. Org. Chem. 9, 459–490; Langer, P. and Freiberg, W. (2004) Chem. Rev. 104, 4125,–4150; Xi, Z. (2004) Eur. J. Org. Chem., 2773–2781; Matsubara, S. and Oshima, K. (2003) Proc. Jpn. Acad., Ser B 79, 71,–77; Dembitsky, V.M. and Srebnik, M. (2002) In Titanium and Zirconium in Organic Synthesis (ed. Marek, I.). Wiley-VCH, Weinheim, pp. 230–281; Normant, J.F. (2001) Acc. Chem. Res. 34, 640,–644; Matsubara, S., Oshima, K. and Utimoto, K. (2001) J. Organomet. Chem. 617–618, 39–46; Marek, I. (2000) Chem. Rev. 100, 2887,–2900; Marek, I. and Normant, J.F. (1999) In Organozinc Reagents–A Practical Approach (eds. Knochel, P. and Jones, P.). Oxford University Press, Oxford, pp. 119–137; Marek, I. and Normant, J.F. (1996) Chem. Rev. 96, 3241,–3267; Cannon, K.C. and Krow, R.R. (1996) In Handbook of Grignard Reagents (eds. Silverman, G.S. and Rakita, P.E.). Marcel Dekker, Inc., New York, pp. 497–526; Rieke, R.D. and Sell, M.S. (1996) In Handbook of Grignard Reagents (eds. Silverman, G.S. and Rakita, P.E.). Marcel Dekker, Inc., New York, pp. 527–538; Rieke, R.D. and Sell, M.S. (1996) In Handbook of Grignard Reagents (eds. Silverman, G.S. and Rakita, P.E.). Marcel Dekker, Inc., New York, pp. 539–555; Knochel, P. (1996) In Handbook of Grignard Reagents (eds. Silverman, G. S. and Rakita, P. E.). Marcel Dekker, Inc., New York, pp. 633–643; Thomson, C.M. (1994) Dianion Chemistry in Organic Synthesis. CRC Press, Boca Raton; Thompson, C.M. and Green, D.L.C. (1991) Tetrahedron 47, 4223,–4285; Bubnov, Y.N. (1991) Pure Appl. Chem. 63, 361,–364; Bickelhaupt, F. (1987) Angew. Chem. Int. Ed. Engl. 26, 990–1005 [Google Scholar]
- 2).Reviews on functionalized organometallics: Knochel, P. (ed.) (2005) Handbook of Functionalized Organometallics. Wiley-VCH, Weinheim; Ila, H., Baron, O., Wagner, A.J. and Knochel, P. (2006) Chem. Commun., 583–593; Chinchilla, R., Najera, C. and Yus, M. (2005) Tetrahedron 61, 3139–3176; Knochel, P., Dohle, W., Gommermann, N., Kneisel, F.F., Kopp, F., Korn, T., Sapountzis, I. and Vu, V.A. (2003) Angew. Chem. Int. Ed. 42, 4302,–4320; Najera, C., Sansano, J.M. and Yus, M. (2003) Tetrahedron 59, 9255,–9303; Boudier, A., Bromm, L.O., Lotz, M. and Knochel, P. (2000) Angew. Chem. Int. Ed. 39, 4414,–4435; Knochel, P. and Singer, R.D. (1993) Chem. Rev. 93, 2117–2188 [Google Scholar]
- 3).Reviews on element–element additions to alkenes and alkynes: Suginome, M., Matsuda, T., Ohmura, T., Seki, A. and Murakami, M. (2007) In Comprehensive Organometallic Chemistry III (eds. Crabtree, R.H. and Mingos, D.M.P.). Elsevier, Oxford, pp. 725–787; Burks, H.E. and Morken, J.P. (2007) Chem. Commun., 4717–4725; Rossi, R.A. and Martin, S.E. (2006) Coord. Chem. Rev. 250, 575–601; Beletskaya, I. and Moberg, C. (2006) Chem. Rev. 106, 2320,–2354; Suginome, M. and Ito, Y. (2000) Chem. Rev. 100, 3221,–3256; Ishiyama, T. and Miyaura, N. (2000) J. Organomet. Chem. 611, 392,–402; Beletskaya, I. and Moberg, C. (1999) Chem. Rev. 99, 3435,–3461; Han, L.-B. and Tanaka, M. (1999) Chem. Commun., 395–402; Marder, T.B. and Norman, N.C. (1998) Top. Catal. 5, 63,–73; Casson, S. and Kocienski, P. (1995) Contemporary Organic Synthesis 2, 19–34 [Google Scholar]
- 4).Buynak, J.D. and Geng, B. (1995) Organometallics 14, 3112–3115; Suginome, M., Fukuda, T., Nakamura, H. and Ito, Y. (2000) Organometallics 19, 719,–721; Ali, H.A., Goldberg, I. and Srebnik, M. (2001) Organometallics 20, 3962,–3965; Ali, H.A., Goldberg, I., Kaufmann, D., Burmeister, C. and Srebnik, M. (2002) Organometallics 21, 1870,–1876; Coapes, R.B., Souza, F.E.S., Thomas, R.L., Hall, J.J. and Marder, T.B. (2003) Chem. Commun. 9, 614–615 [Google Scholar]
- 5).Kitatani, K., Hiyama, T. and Nozaki, H. (1976) J. Am. Chem. Soc. 98, 2362–2364; Kitatani, K., Hiyama, T. and Nozaki, H. (1977) Bull. Chem. Soc. Jpn. 50, 1600–1607 [Google Scholar]
- 6).Posner, G.H., Loomis, G.L. and Sawaya, H.S. (1975) Tetrahedron Lett., 1373–1376; Matteson, D.S. and Majumdar, D. (1980) J. Am. Chem. Soc. 102, 7588–7590; Duraisamy, M. and Walborsky, H.M. (1984) J. Am. Chem. Soc. 106, 5035,–5037; Danheiser, R.L. and Savoca, A.C. (1985) J. Org. Chem. 50, 2401,–2403; Negishi, E.I. and Akiyoshi, K. (1988) J. Am. Chem. Soc. 110, 646,–647; Harada, T., Hara, D., Hattori, K. and Oku, A. (1988) Tetrahedron Lett. 29, 3821,–3824; Miller, J.A. (1989) J. Org. Chem. 54, 998,–1000; Kocienski, P., Wadman, S. and Cooper, K. (1989) J. Am. Chem. Soc. 111, 2363,–2365; Negishi, E.I., Akiyoshi, K., O’Connor, B., Takagi, K. and Wu, G. (1989) J. Am. Chem. Soc. 111, 3089,–3091; Knochel, P., Jeong, N., Rozema, M.J. and Yeh, M.C.P. (1989) J. Am. Chem. Soc. 111, 6474,–6476; Harada, T., Katsuhira, T., Hattori, K. and Oku, A. (1989) Tetrahedron Lett. 30, 6039,–6040; Alexakis, A., Hanaizi, J., Jachiet, D. and Normant, J.F. (1990) Tetrahedron Lett. 31, 1271,–1274; Kocienski, P. and Barber, C. (1990) Pure Appl. Chem. 62, 1933,–1940; Knochel, P. and Achyutha Rao, S. (1990) J. Am. Chem. Soc. 112, 6146,–6148; Harada, T., Kotani, Y., Katsuhira, T. and Oku, A. (1991) Tetrahedron Lett. 32, 1573,–1576; De Lima, C., Julia, M. and Verpeaux, J.N. (1992) Synlett, 133–134; Harada, T., Katsuhira, T., Hara, D., Kotani, Y., Maejima, K., Kaji, R. and Oku, A. (1993) J. Org. Chem. 58, 4897,–4907; Matsumoto, K., Aoki, Y., Oshima, K., Utimoto, K. and Rahman, N.A. (1993) Tetrahedron 49, 8487,–8502; Sidduri, A., Rozema, M.J. and Knochel, P. (1993) J. Org. Chem. 58, 2694,–2713; Creton, I., Marek, I., Brasseur, D., Jestin, J.L. and Normant, J.F. (1994) Tetrahedron Lett. 35, 6873,–6876; Inoue, R., Shinokubo, H. and Oshima, K. (1996) Tetrahedron Lett. 37, 5377,–5380; Kakiya, H., Inoue, R., Shinokubo, H. and Oshima, K. (1997) Tetrahedron Lett. 38, 3275,–3278; Kasatkin, A. and Whitby, R.J. (1999) J. Am. Chem. Soc. 121, 7039,–7049; Shibli, A., Varghese, J.P., Knochel, P. and Marek, I. (2001) Synlett, 818–820; Kondo, J., Inoue, A., Shinokubo, H. and Oshima, K. (2001) Angew. Chem. Int. Ed. 40, 2085,–2087; Vu, V.A., Marek, I. and Knochel, P. (2003) Synthesis, 1797–1802; Kondo, J., Inoue, A., Ito, Y., Shinokubo, H. and Oshima, K. (2005) Tetrahedron 61, 3361,–3369; Abramovitch, A., Varghese, J.P. and Marek, I. (2004) Org. Lett. 6, 621,–623; Dixon, S., Fillery, S.M., Kasatkin, A., Norton, D., Thomas, E. and Whitby, R.J. (2004) Tetrahedron 60, 1401,–1416; Kondo, J., Ito, Y., Shinokubo, H. and Oshima, K. (2004) Angew. Chem. Int. Ed. 43, 106–108 [Google Scholar]
- 7).Matteson, D.S. (1989) Chem. Rev. 89, 1535–1551 [Google Scholar]
- 8).Hata, T., Kitagawa, H., Masai, H., Kurahashi, T., Shimizu, M. and Hiyama, T. (2001) Angew. Chem. Int. Ed. 40, 790–792; Shimizu, M., Kitagawa, H., Kurahashi, T. and Hiyama, T. (2001) Angew. Chem. Int. Ed. 40, 4283,–4286; Shimizu, M., Kurahashi, T. and Hiyama, T. (2001) Yuki Gosei Kagaku Kyokai Shi 59, 1062,–1069; Kurahashi, T., Hata, T., Masai, H., Kitagawa, H., Shimizu, M. and Hiyama, T. (2002) Tetrahedron 58, 6381,–6395; Shimizu, M., Kurahashi, T., Kitagawa, H. and Hiyama, T. (2003) Org. Lett. 5, 225,–227; Shimizu, M., Kurahashi, T., Kitagawa, H., Shimono, K. and Hiyama, T. (2003) J. Organomet. Chem. 686, 286,–293; Shimizu, M., Schelper, M., Nagao, I., Shimono, K., Kurahashi, T. and Hiyama, T. (2006) Chem. Lett. 35, 1222–1223 [DOI] [PubMed] [Google Scholar]
- 9).Dembitsky, V.M., Ali, H.A. and Srebnik, M. (2003) Appl. Organomet. Chem. 17, 327–345 [Google Scholar]
- 10).Zweifel, G. and Arzoumanian, H. (1967) J. Am. Chem. Soc. 89, 291–295; Brown, H.C., Scouten, C.G. and Liotta, R. (1979) J. Am. Chem. Soc. 101, 96–99 [Google Scholar]
- 11).Ali, H.A., Goldberg, I., Kaufmann, D., Burmeister, C. and Srebnik, M. (2002) Organometallics 21, 1870–1876 [Google Scholar]
- 12).Castle, R.B. and Matteson, D.S. (1968) J. Am. Chem. Soc. 90, 2194; Castle, R.B. and Matteson, D.S. (1969) J. Organomet. Chem. 20, 19–28 [Google Scholar]
- 13).Matteson, D.S., Davis, R.A. and Hagelee, L.A. (1974) J. Organomet. Chem. 69, 45–51 [Google Scholar]
- 14).Shimizu, M., Schelper, M., Nagao, I., Shimono, K., Kurahashi, T. and Hiyama, T. (2006) Chem. Lett. 35, 1222–1223 [Google Scholar]
- 15).Matteson, D.S. and Tripathy, P.B. (1974) J. Organomet. Chem. 69, 53–62; Matteson, D.S. (1975) Synthesis, 147–158. [Google Scholar]
- 16).Hata, T., Kitagawa, H., Masai, H., Kurahashi, T., Shimizu, M. and Hiyama, T. (2001) Angew. Chem. Int. Ed. 40, 790–792; Kurahashi, T., Hata, T., Masai, H., Kitagawa, H., Shimizu, M. and Hiyama, T. (2002) Tetrahedron 58, 6381–6395 [DOI] [PubMed] [Google Scholar]
- 17).Shimizu, M., Kurahashi, T. and Hiyama, T. (2001) Synlett, 1006–1008.
- 18).Desurmont, G., Klein, R., Uhlenbrock, S., Laloe, E., Deloux, L., Giolando, D.M., Kim, Y.W., Pereira, S. and Srebnik, M. (1996) Organometallics 15, 3323–3328 [Google Scholar]
- 19).Marder, T.B. and Norman, N.C. (1998) Top. Catal. 5, 63–73; Ishiyama, T. and Miyaura, N. (2000) J. Organomet. Chem. 611, 392,–402; Ishiyama, T. and Miyaura, N. (2004) Chem. Rec. 3, 271–280; Burks, H.E. and Morken, J.P. (2007) Chem. Commun., 4717–4725. [DOI] [PubMed] [Google Scholar]
- 20).Ishiyama, T., Matsuda, N., Miyaura, N. and Suzuki, A. (1993) J. Am. Chem. Soc. 115, 11018–11019; Ishiyama, T., Matsuda, N., Murata, M., Ozawa, F., Suzuki, A. and Miyaura, N. (1996) Organometallics 15, 713,–720; Lesley, G., Nguyen, P., Taylor, N.J., Marder, T.B., Scott, A.J., Clegg, W. and Norman, N.C. (1996) Organometallics 15, 5137,–5154; Iverson, C.N. and Smith, M.R. (1996) Organometallics 15, 5155–5165; Thomas, R.L., Souza, F.E.S. and Marder, T.B. (2001) J. Chem. Soc., Dalton Trans., 1650–1656. [Google Scholar]
- 21).Ishiyama, T., Yamamoto, M. and Miyaura, N. (1997) Chem. Commun., 689–690; Iverson, C.N. and Smith, M.R. (1997) Organometallics 16, 2757–2759 [Google Scholar]
- 22).Ishiyama, T., Kitano, T. and Miyaura, N. (1998) Tetrahedron Lett. 39, 2357–2360 [Google Scholar]
- 23).Yang, F.Y. and Cheng, C.H. (2001) J. Am. Chem. Soc. 123, 761–762 [DOI] [PubMed] [Google Scholar]
- 24).Pelz, N.F., Woodward, A.R., Burks, H.E., Sieber, J.D. and Morken, J.P. (2004) J. Am. Chem. Soc. 126, 16328–16329 [DOI] [PubMed] [Google Scholar]
- 25).Cainelli, G., Dal Bello, G. and Zubiani, G. (1965) Tetrahedron Lett. 6, 3429–3432 [Google Scholar]
- 26).Mukaiyama, T., Murakami, M., Oriyama, T. and Yamaguchi, M. (1981) Chem. Lett., 1193–1196.
- 27).Brown, H.C. and Rhodes, S.P. (1969) J. Am. Chem. Soc. 91, 4306–4307 [Google Scholar]
- 28).Flynn, A.B. and Ogilvie, W.W. (2007) Chem. Rev. 107, 4698–4745 [DOI] [PubMed] [Google Scholar]
- 29).Shimizu, M., Nakamaki, C., Shimono, K., Schelper, M., Kurahashi, T. and Hiyama, T. (2005) J. Am. Chem. Soc. 127, 12506–12507 [DOI] [PubMed] [Google Scholar]
- 30).Shimizu, M., Shimono, K., Schelper, M. and Hiyama, T. (2007) Synlett, 1969–1971.
- 31).Ishiyama, T., Yamamoto, M. and Miyaura, N. (1996) Chem. Lett., 1117–1118.
- 32).Brown, S.D. and Armstrong, R.W. (1997) J. Org. Chem. 62, 7076–7077; Brown, S.D. and Armstrong, R.W. (1996) J. Am. Chem. Soc. 118, 6331–6332 [DOI] [PubMed] [Google Scholar]
- 33).Shimizu, M., Tanaka, K., Kurahashi, T., Shimono, K. and Hiyama, T. (2004) Chem. Lett. 33, 1066–1067; Shimizu, M., Kurahashi, T., Shimono, K., Tanaka, K., Nagao, I., Kiyomoto, S.-i. and Hiyama, T. (2007) Chem. Asian J. 2, 1400–1408 [Google Scholar]
- 34).Hilt, G. and Bolze, P. (2005) Synthesis, 2091–2115.
- 35).Shimizu, M., Shimono, K., Kurahashi, T., Kiyomoto, S.-i., Nagao, I. and Hiyama, T. (2008) Bull. Chem. Soc. Jpn. (in press). [DOI] [PubMed]
- 36).Shimizu, M., Shimono, K. and Hiyama, T. (2006) Chem. Lett. 35, 838–839; Shimizu, M., Shimono, K. and Hiyama, T. (2007) Chem. Asian J. 2, 1142–1149 [Google Scholar]
- 37).Woodward, A.R., Burks, H.E., Chan, L.M. and Morken, J.P. (2005) Org. Lett. 7, 5505–5507; Sieber, J.D. and Morken, J.P. (2006) J. Am. Chem. Soc. 128, 74,–75; Burks, H.E., Liu, S. and Morken, J.P. (2007) J. Am. Chem. Soc. 129, 8766–8773 [DOI] [PubMed] [Google Scholar]
- 38).Morgan, J.B., Miller, S.P. and Morken, J.P. (2003) J. Am. Chem. Soc. 125, 8702–8703; Trudeau, S. and Morgan, J.B., Shrestha, M. and Morken, J.P. (2005) J. Org. Chem. 70, 9538–9544 [DOI] [PubMed] [Google Scholar]
- 39).Morgan, J.B. and Morken, J.P. (2004) J. Am. Chem. Soc. 126, 15338–15339 [DOI] [PubMed] [Google Scholar]