Dr Greg Rosenfeld
Gastric antral vascular ectasia (GAVE) and radiation proctitis are two vascular disorders of the gastrointestinal tract that typically present with recurrent gastrointestinal bleeding. Although the pathogenesis of either condition is not known, they are unlikely to be similar. GAVE appears to be related to autoimmune disorders or cirrhosis, while radiation proctitis is the result of pelvic irradiation, most commonly used for the treatment of pelvic malignancies. Medical therapies for both conditions are not typically effective, and surgical therapies are usually not required because endoscopic treatment, aimed at coagulation of the underlying vascular lesions, has evolved as the most effective therapy. There is limited evidence in the literature for the use of medical and surgical therapies, with most of the evidence coming from case reports involving small numbers of patients. In the present article, we review the evidence for the use of argon plasma photocoagulation (APC, the most commonly used endoscopic modality) in the treatment of GAVE and radiation proctitis.
GAVE
GAVE is a rare disorder, accounting for less than 4% of non-variceal upper gastrointestinal bleeding (1). Clinically, patients with this disorder most often present with chronic anemia and occult blood loss, but may also present with signs of acute bleeding such as melena or hematochezia (2). Most patients with GAVE are believed to have GAVE-related comorbid conditions. Cirrhosis is found in 30% of patients with GAVE. In noncirrhotic patients, autoimmune disorders are most common, with connective tissue diseases (particularly scleroderma or CREST [Calcinosis, Raynaud’s syndrome, Esophageal dysmotility, Sclerodactyly, Telangiesctasia] variants) forming the majority, with an incidence as high as 62% (3). The majority of noncirrhotic GAVE patients are women (71%), with a mean age of 73 years. Conversely, cirrhotic patients with GAVE are more often men (75%), with a mean age of 65 years (3).
Endoscopically, GAVE has a characteristic appearance, originally described by Jabbari et al (4) in 1984, which is commonly referred to as ‘watermelon stomach’. The original description of “longitudinal rugal folds traversing the antrum and converging on the pylorus, each containing a visible convoluted column of vessels, the aggregate resembling the stripes on a watermelon” – is pathognomonic for the condition. This striped appearance is more commonly encountered in association with autoimmune or connective tissue diseases. A second pattern, more commonly seen in association with portal hypertension and cirrhosis, is one of a diffuse pattern of small, punctate spots primarily in the antrum (5). Histological features include vascular ectasia within the mucosa, fibrin thrombi, fibrohyalinosis and spindle cell proliferation. However, biopsy specimens may miss the lesions because they are often focal; thus, the diagnosis is typically made based on the characteristic endoscopic appearance, making biopsy unnecessary.
The pathophysiology of GAVE remains unknown. Despite the high frequency of cirrhosis and GAVE, a causal link has never been proven. GAVE and portal hypertensive gastropathy appear to be unique entities, and can often be distinguished from one another on biopsy and usually by endoscopic appearance (6). GAVE does not respond to a reduction in portal pressure (1), lending further support to the uniqueness of these two entities and making an appropriate diagnosis essential for effective therapy. Within the cardia and extending just distal to the gastroesophageal junction, pathopneumonic diminutive vascular ectasias associated with GAVE are often seen. The body of the stomach is subsequently spared, with the characteristic lesions seen in the antrum.
Treatment for GAVE ranges from supportive therapy, medical therapy and endoscopic therapy to surgery. Surgical treatment typically involves gastric antrectomy, which, although very effective, is associated with significant morbidity and mortality and, therefore, has an extremely limited role (2). Medical therapy aimed at reducing portal hypertension has not been successful in reducing the incidence of bleeding episodes or transfusion requirements. Attempts at reducing portal pressure have included the use of beta-blockers and transhepatic portosystemic shunting procedures (1). There have been several case reports on the successful use of corticosteroids for treating GAVE; however, the use of steroids is limited by their side effects (7,8). There is also a case report (9) on the use of estrogen and progesterone to reduce the requirement for blood transfusion in patients with GAVE. However, estrogen and progesterone therapy also has significant side effects and has not been used widely for GAVE. For both hormonal and corticosteroid treatments, there was no improvement in the endoscopic appearance of the stomach, suggesting that bleeding from GAVE is likely to recur on cessation of treatment (7). Furthermore, there are case reports on the use of tranexamic acid (10), thalidomide, alpha-interferon, calcitonin and cyproheptadine, mostly in single or small case series (1). As a result, there is limited experience and evidence to support the use of these therapies.
Endoscopic treatments have included sclerotherapy, heater probe, cryotherapy, band ligation, laser photocoagulation and APC. Sclerotherapy has been used successfully but with evidence limited to a few case reports (11). Contact coagulation, such as heater probe and bipolar coagulation, has also been used successfully in the treatment of GAVE. Unfortunately, it tends to induce oozing and some bleeding, which obscures the field. It is considered inferior to laser and APC because of its inability to cover large areas, thereby creating the need for multiple treatment sessions (7).
A recent pilot study (12) reported on the use of endoscopic cryotherapy using carbon dioxide to successfully treat GAVE lesions in 12 patients. Patients received three sessions of cryotherapy at three- to six-week intervals, with 50% of the patients having a complete response and 50% having a partial response. Complete response was defined as a significant improvement in the endoscopic appearance and an increase in hemoglobin level, with no requirement for blood transfusion. A partial response was defined as the incomplete resolution of endoscopic GAVE appearance, with a stable hemoglobin level and reduced transfusion requirements. There were no immediate treatment complications, and 25% of patients developed minor scarring and ulceration of the gastric antrum, with no strictures or gastric outlet obstructions. Although this was a small pilot study with a short follow-up period (three months), it showed promising results.
Endoscopic band ligation has been documented to have been used successfully to treat GAVE-associated bleeding in two different case reports (13). In both cases, two treatment sessions were required with only minor side effects. Laser coagulation with a neodymium:yttrium-aluminum-garnet (Nd:YAG) laser has been used in several studies (3,14). Nd:YAG laser treatment has been shown to resolve the disease endoscopically, reduce the need for transfusion and prevent the need for surgery, with only one treatment session in the majority of patients (7). However, despite their effectiveness, the use of Nd:YAG lasers have been limited by their cost, inconvenience and complications including bleeding from induced ulceration, gastric perforation and antral stenosis (2).
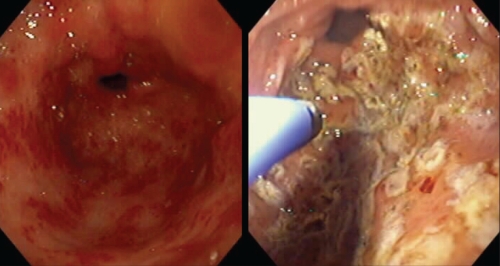
APC has been the most widely studied endoscopic treatment and is the most frequently used for GAVE-related bleeding (15) (Figure 1). In a study of 26 patients over a 16-month period (16), APC was used to avoid the requirements for transfusion in 77% of patients. APC has been successfully used in the treatment of both the striped and diffuse patterns of GAVE (17). The APC unit is less expensive, more portable and has shown results similar to laser therapy, with fewer side effects. As a result, it has largely replaced laser treatment as the first-line treatment for GAVE. Treatments are usually required on at least two occasions separated by six to eight weeks.
Figure 1).
Gastric antral vascular ectasia. Marked disease treated with argon plasma coagulation
Previously, the APC probes were reusable; however, over time (as with much of the endoscopic equipment), they have become single use. Therefore, the cost has escalated, particularly because multiple treatments are required.
Overzealous cautery may result in hypertrophic inflammatory polyps, which can be yet another source of bleeding and are endoscopically impressive to view. For some patients, bleeding may seem to be controlled with one or two endoscopic sessions; however, many will have further episodes a year or more later that typically respond to repeat therapy. Often, therapy can be directed by the clinical response as opposed to complete eradication of all vascular lesions. Long-term studies have been lacking because most acute problems with bleeding can be controlled with the intital therapy; however, many investigators have noticed that most patients will return for necessary subsequent treatments within five years.
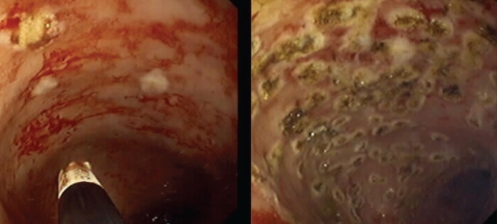
APC is also a first-line treatment for chronic radiation proctitis (18) (Figure 2). Radiation proctitis and GAVE are similar in that they are both vascular lesions of the digestive mucosa. They both tend to present with bleeding, and treatment is aimed at controlling the bleeding via reduction or coagulation of the superficial vessels. However, their main similarity (and the reason they are reviewed together here) is that the preferred method of treatment for both conditions is APC.
Figure 2).
Radiation proctitis. Treatment with argon plasma coagulation
RADIATION PROCTITIS
Radiation therapy is frequently used to treat pelvic malignancies such as cervical and prostate cancer, as well as local therapy for rectal or anal cancers. Up to 15% of patients who receive such radiation treatments develop chronic radiation proctosigmoiditis (19). The symptoms include diarrhea, rectal bleeding, urgency, tenesmus, rectal strictures, proctalgia and fecal incontinence. The etiology of this condition is unknown, but believed to be related to radiation-induced fibrosis, scarring and mucosal ischemia (20). Symptom onset is typically within two years after radiation therapy.
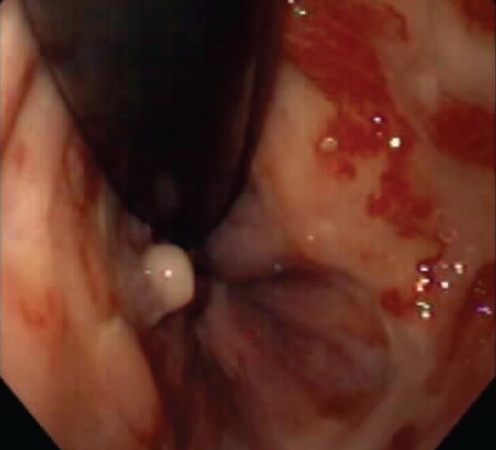
Radiation proctitis produces a characteristic pattern of mucosal telangiectasias, which are usually located in the distal 5 cm of the rectum (21) (Figure 3). As a result, diagnosis can usually be made based on endoscopic appearance, making biopsy unnecessary. Hematochezia, sometimes requiring repeated blood transfusions, is a common reason for presentation in patients with chronic radiation proctitis.
Figure 3).
Radiation proctitis. Ocasionally, the vascular lesions are very close to the anal verge, making it difficult to cauterize in an antegrade fashion. Additionally, when cautery is applied close to the dentate line, sensitivity results
Several different modalities have been used in attempts to treat radiation proctitis. Medical and pharmacological therapies have included topical corticosteroids, sulfasalazine and sulcralfate enemas – all being generally ineffective (22). Surgery is associated with high rates of morbidity in patients with previous radiation therapy and, therefore, usually avoided (23).
Endoscopic therapy using heater and bipolar probes, and laser therapy using the Nd:YAG and titanyl phosphate lasers have been used with some success. Several studies have reported good efficacy with the Nd:YAG laser; however, the side effects have been significant. These include transmural necrosis, fibrosis, stricture formation and rectovaginal fistulae. As in cases of GAVE, the use of Nd:YAG lasers to treat radiation proctitis has declined because of the associated costs and technical difficulties.
Topical formalin applied through the endoscope has also been successful. A dilute 4% formalin solution is applied either by instillation through a rigid proctoscope, via direct application with a gauze soaked in formalin (22), or through a ‘spray catheter’ and standard endoscope. In the intial study by Rubenstein et al (24), patients underwent rectal irrigation with formalin, which improved the bleeding and resolved symptoms over a 14-month follow-up period. Subsequent studies have also shown topical 4% formalin to be effective but not without risks (23). The risks include anal stenosis, fissures, fecal incontinence and mucosal ulceration (25).
A recent study compared the safety and efficacy of topical formalin application with APC in the treatment of chronic radiation proctopathy (22). Of the 22 patients studied, 11 received APC alone, eight received formalin alone and three patients received both. Response was defined as a 10% or greater increase in baseline hemoglobin level without transfusion. Seventy-nine per cent of patients receiving APC responded, while only 27% of patients receiving formalin alone responded (P=0.017). There were more side effects in the formalin group, including nausea and/or vomiting, flushing and rectal pain and/or fever. As illustrated in this study, the high efficacy and favourable side effect profile of APC has led to its emergence as the preferred method of treatment for radiation proctitis.
APC
APC was developed by ERBE Medical (Tubingen, Germany). This technique, which can be applied through a flexible endoscope, involves the passing of inert argon gas through a probe at a preset flow rate. The gas is then ionized by a high-voltage current flowing through a tungsten filament at the end of the probe. The tip of the probe is held above and not in contact with the mucosa and the ‘plasma’ of ionized gas grounds via the nearest mucosal surface. The circuit is completed via a return plate placed on the patient’s buttock or thigh. The thermal energy uniformly heats the surface to a depth of 0.5 mm to 3 mm, coagulating the superficial blood vessels (19). Deeper coagulation may be achieved with higher power current, continued treatment, or contact between the monofilament and the mucosa during treatment (2). The probes are available in several different forms.
Benefits of APC
APC has many advantages over other endoscopic therapies. The unit is mobile, compact, easy to maintain and requires a lower initial capital expenditure than other therapies. The noncontact technique allows for the treatment of larger areas more rapidly than the use of the heater probe or bipolar cautery. APC has a predictable depth of penetration when used without mucosal contact and allows tangential arcing to lesions that are not directly in view or are behind folds (16). Few complications have been reported with the use of APC, with a quoted rate of 2.5% compared with 5% to 15% for laser (16).
Limitations of APC
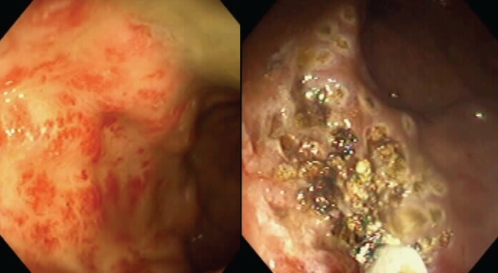
Most patients require multiple treatments with APC (two to four) for initial control of bleeding and the lesions tend to recur. As a result, maintenance treatments and follow-up endoscopies may be required (2) (Figure 4). One case series reported three colonic explosions resulting in one bowel perforation associated with APC (26). The authors report that this effect can be prevented by adequate colonic cleansing before treatment (ie, all patients should undergo an appropriate preparation, although typically, the procedure can be performed without sedation). Despite the lower initial capital cost of APC, there is a significant per-treatment cost for the APC probes. Although the therapy can be delivered safely, those not experienced with the device will often hold the probe too far from the surface, thereby inflating the lumen with excessive gas, resulting in discomfort. A careful, methodical treatment can result in a bloodless field of cautery at the completion of treatment.
Figure 4).
Radiation proctitis, with subsequent treatment
CONCLUSION
With respect to the treatment of radiation proctitis and GAVE, the limitations of APC are outweighed by its benefits. APC targets the abnormal telangiectasias and ectatic vessels in these two conditions to coagulate the vessels and alleviate the bleeding that is so problematic for patients. Although the evidence to support the use of APC is somewhat limited, the other available medical, surgical and endoscopic therapies are all inferior to APC because of its safety, efficacy and ease of use. Potential alternative therapies, such as cryotherapy for GAVE and topical formalin for radiation proctitis, require further study to surpass APC as the preferred first-line treatment for these conditions.
REFERENCES
- 1.Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): An update on clinical presentation, pathophysiology and treatment. Digestion. 2008;77:131–7. doi: 10.1159/000124339. [DOI] [PubMed] [Google Scholar]
- 2.Jensen DM, Chaves DM, Grund KE. Endoscopic diagnosis and treatment of watermelon stomach. Endoscopy. 2004;36:640–7. doi: 10.1055/s-2004-814525. [DOI] [PubMed] [Google Scholar]
- 3.Gostout CJ, Viggiano TR, Ahlquist DA, Wang KK, Larson MV, Balm R. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol. 1992;15:256. doi: 10.1097/00004836-199210000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Jabbari M, Cherry R, Lough JO, Daly DS, Kinnear DG, Goresky CA. Gastric antral vascular ectasia: The watermelon stomach. Gastroenterology. 1984;87:1165–70. [PubMed] [Google Scholar]
- 5.Ito M, Uchida Y, Kamano S, Kawabata H, Nishioka M. Clinical comparisons between two subsets of gastric antral vascular ectasia. Gastrointest Endosc. 2001;53:764–70. doi: 10.1067/mge.2001.113922. [DOI] [PubMed] [Google Scholar]
- 6.Payen JL, Calès P, Voigt JJ, et al. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138–44. doi: 10.1016/0016-5085(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 7.Sebastian S, O’Morain CA, Buckley MJ. Review article: Current therapeutic options for gastric antral vascular ectasia. Aliment Pharmacol Ther. 2003;18:157–65. doi: 10.1046/j.1365-2036.2003.01617.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhowmick B. Watermelon stomach treated with oral corticosteroid. J R Soc Med. 1993;86:52. [PMC free article] [PubMed] [Google Scholar]
- 9.Tran A, Villeneuve JP, Bilodeau M, et al. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: An open pilot study. Am J Gastroenterol. 1999;94:2909–11. doi: 10.1111/j.1572-0241.1999.01436.x. [DOI] [PubMed] [Google Scholar]
- 10.Park R, Danesh B, Upadhyay R, Howatson A, Lee F, Russell R. Gastric antral vascular ectasia (watermelon stomach) – therapeutic options. Postgrad Med J. 1990;66:720. doi: 10.1136/pgmj.66.779.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cugia L, Carta M, Dore MP, Realdi G, Massarelli G. The watermelon stomach: Successful treatment by monopolar electrocoagulation and endoscopic injection of polidocanol. J Clin Gastroenterol. 2000;31:93–4. doi: 10.1097/00004836-200007000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Cho S, Zanati S, Yong E, et al. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895–902. doi: 10.1016/j.gie.2008.03.1109. [DOI] [PubMed] [Google Scholar]
- 13.Zepeda-Gómez S, Marcon NE. Endoscopic band ligation for nonvariceal bleeding: A review. Can J Gastroenterol. 2008;22:748–52. doi: 10.1155/2008/165264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberski S, McGarrity T, Hartle R, Varano V, Reynolds D. The watermelon stomach: Long-term outcome in patients treated with Nd:YAG laser therapy. Gastrointest Endosc. 1994;40:584–7. doi: 10.1016/s0016-5107(94)70258-6. [DOI] [PubMed] [Google Scholar]
- 15.Fuccio L, Zagari RM, Serrani M, et al. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia-related bleeding in patients with liver cirrhosis. Digestion. 2009;79:143–50. doi: 10.1159/000210087. [DOI] [PubMed] [Google Scholar]
- 16.Kwan V, Bourke MJ, Williams SJ, et al. Argon plasma coagulation in the management of symptomatic gastrointestinal vascular lesions: Experience in 100 consecutive patients with long-term follow-up. Am J Gastroenterol. 2006;101:58–63. doi: 10.1111/j.1572-0241.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 17.Chaves DM, Sakai P, Oliveira CV, Cheng S, Ishioka S. Watermelon stomach: Clinical aspects and treatment with argon plasma coagulation. Arch Gastroenterol. 2006;43:191–5. doi: 10.1590/s0004-28032006000300007. [DOI] [PubMed] [Google Scholar]
- 18.Sebastian S, O’Connor H, O’Morain C, Buckley M. Argon plasma coagulation as first-line treatment for chronic radiation proctopathy. J Gastroenterol Hepatol. 2004;19:1169–73. doi: 10.1111/j.1440-1746.2004.03448.x. [DOI] [PubMed] [Google Scholar]
- 19.Postgate A, Saunders B, Tjandra J, Vargo J. Argon plasma coagulation in chronic radiation proctitis. Endoscopy. 2007;39:361–5. doi: 10.1055/s-2007-966284. [DOI] [PubMed] [Google Scholar]
- 20.Hasleton P, Carr N, Schofield P. Vascular changes in radiation bowel disease. Histopathology. 1985;9:517–34. doi: 10.1111/j.1365-2559.1985.tb02833.x. [DOI] [PubMed] [Google Scholar]
- 21.Dees J, Meijssen MA, Kuipers EJ. Argon plasma coagulation for radiation proctitis. Scand J Gastroenterol Suppl. 2006:175–8. doi: 10.1080/00365520600664300. [DOI] [PubMed] [Google Scholar]
- 22.Alfadhli A, Alazmi W, Ponich T, et al. Efficacy of argon plasma coagulation compared with topical formalin application for chronic radiation proctopathy. Can J Gastroenterol. 2008;22:129. doi: 10.1155/2008/964912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson SA, Rex DK. Endoscopic treatment of chronic radiation proctopathy. Curr Opin Gastroenterol. 2006;22:536. doi: 10.1097/01.mog.0000239869.27328.54. [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein E, Ibsen T, Rasmussen RB, Reimer E, Sorensen B. Formalin treatment of radiation–induced hemorrhagic proctitis. Am J Gastroenterol. 1986;81:44–5. [PubMed] [Google Scholar]
- 25.de Parades V, Etienney I, Bauer P, et al. Formalin application in the treatment of chronic radiation-induced hemorrhagic proctitis – an effective but not risk-free procedure: A prospective study of 33 patients. Dis Colon Rect. 2005;48:1535–41. doi: 10.1007/s10350-005-0030-z. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Soussan E, Antonietti M, Savoye G, Herve S, Ducrotte P, Lerebours E. Argon plasma coagulation in the treatment of hemorrhagic radiation proctitis is efficient but requires a perfect colonic cleansing to be safe. Eur J Gastroenterol Hepatol. 2004;16:1315–8. doi: 10.1097/00042737-200412000-00013. [DOI] [PubMed] [Google Scholar]