Abstract
Relatively little is known about the neural bases of the Big Five personality trait Openness/Intellect. This trait is composed of two related but separable aspects, Openness to Experience and Intellect. On the basis of previous behavioral research (DeYoung, Peterson, & Higgins, 2005), we hypothesized that brain activity supporting working memory (WM) would be related to Intellect but not Openness. To test this hypothesis we used fMRI to scan a sample of 104 healthy adults, as they performed a difficult WM task. Intellect (and not Openness) was found to correlate with WM accuracy and with accuracy-related brain activity, in left lateral anterior prefrontal cortex and posterior medial frontal cortex. Neural activity in these regions mediated the association between Intellect and WM performance, implicating these regions in the neural substrate of Intellect. Intellect was also correlated significantly with scores on tests of intelligence and working memory capacity, but the association of Intellect with brain activity could not be entirely explained by cognitive ability.
Keywords: Intellect, Openness, Prefrontal Cortex, Frontopolar Cortex, Working Memory, Intelligence
The five factor model or Big Five classifies personality traits into five broad domains: Extraversion, Agreeableness, Conscientiousness, Neuroticism, and Openness/Intellect. The compound label for the last of these traits reflects an old debate about how best to characterize the content of this domain, with some researchers preferring “Openness to Experience” (e.g., Costa & McCrae, 1992a) and others “Intellect” (e.g., Goldberg, 1993). This debate has been largely resolved conceptually by the observation that “Openness” and “Intellect” describe two related but separable aspects of the larger domain (Johnson, 1994; Saucier, 1992). Lexical studies make it clear that both aspects are well represented in natural language and that content related to both appears among the terms loading on a single Big Five factor (e.g., Goldberg, 1990; Saucier, 1992). There are a many terms in English that describe Intellect: intellectual, intelligent, philosophical, erudite, clever, etc.1 There are also many terms (though perhaps fewer) that describe Openness to Experience: artistic, perceptive, poetic, fantasy-prone, etc. Additionally, there are many terms that could characterize people high in Intellect or Openness or both: imaginative, original, complex, innovative, etc. (indeed, Saucier (1994) proposed that “Imagination” might be a better single label for the domain as a whole). Johnson (1994) demonstrated that, of the facets (sub-traits) in the Revised NEO Personality Inventory (NEO PI-R; Costa & McCrae, 1992b), the Ideas and Aesthetics facets were the purest markers of the lexical Openness/Intellect factor, and he suggested that interests in truth and beauty are complementary qualities at the heart of the Openness/Intellect domain. McCrae and Costa (1997) argued that this domain reflects the “depth, breadth, and permeability of consciousness.” Perhaps Openness reflects these qualities of consciousness in relation to sensory or perceptual information, whereas Intellect reflects them in relation to abstract or semantic information.
The identification of Openness and Intellect as the two major aspects of this domain of personality was recently given empirical support by a factor analysis of 15 scales measuring facets of Openness/Intellect (DeYoung, Quilty, & Peterson, 2007). The covariance of those facets indicated the presence of two correlated factors, clearly recognizable as Openness and Intellect. Each factor was strongly marked by six facets, suggesting their similarity in importance to the larger domain. The two factors were further characterized by examining their correlations with over 2000 items of the International Personality Item Pool (Goldberg, 1999). This analysis revealed that Intellect encompasses traits reflecting intellectual engagement and perceived intelligence (e.g. “Avoid philosophical discussions” (reversed); “Am quick to understand things”), whereas Openness encompasses traits reflecting artistic and contemplative qualities related to engagement in sensation and perception (e.g., “Believe in the importance of art”; “See beauty in things that others might not notice”) (DeYoung et al., 2007). Having established the existence of these two related but separable aspects of Openness/Intellect, one important concern is discriminant validity. How do these two aspects differ from each other in their associations with other variables?
Levels of personality organization below the Big Five are associated with unique genetic variance (Jang et al., 2002; Jang, McCrae, Angleitner, Riemann, & Livesley, 1998), suggesting that it may be possible to identify biological systems that differentiate Intellect from Openness. The present study tested the hypothesis that brain function associated with working memory (WM) performance would be related to Intellect but not Openness. Intellectual individuals seem more likely than those who are simply open to experience to utilize brain systems that allow for successful processing of information during WM tasks with a high cognitive demand. This hypothesis was derived in part from behavioral research relating facets of Openness/Intellect to a battery of tests of WM and other cognitive functions associated with prefrontal cortex (PFC) (DeYoung, Peterson, Higgins, 2005). That study, like the present one, measured the Big Five using the NEO PI-R (Costa & McCrae, 1992b), which divides each of the Big Five into six facets. Four of these facets (Fantasy, Aesthetics, Feelings, and Actions) clearly mark the Openness aspect of the domain, in factor analysis (DeYoung et al., 2007). The Ideas facet, however, is a good marker of Intellect (DeYoung et al., 2007) and was the facet most strongly associated with performance on the WM battery (DeYoung et al., 2005).2 The sixth facet, Values, does not mark either aspect strongly but was also found to be associated with WM.
The present study employed functional magnetic resonance imaging (fMRI) of individuals performing a difficult WM task, in order to test the hypothesis that Intellect (but not Openness) would be associated with WM performance and with brain activity supporting WM performance. Use of a large sample (by the standards of neuroimaging research) enabled the identification of brain regions in which individual variation in neural activity predicted WM performance. Because this analytic approach identifies regions with meaningful variability, it appears particularly promising for personality neuroscience (DeYoung & Gray, 2009), in contrast to approaches that identify regions consistently activated in the sample as a whole.
Two brain regions of particular interest in our analyses were the left lateral region of anterior PFC (aPFC; also called frontopolar cortex) and the region of posterior medial frontal cortex (pMFC) that encompasses the dorsal anterior cingulate cortex (ACC). Left aPFC appears to support the abstract integration of information from multiple cognitive operations (Gilbert et al., 2006; Green, Fugelsang, Kraemer, Shamosh, & Dunbar, 2006; Ramnani & Owen, 2004) and has been implicated in intelligence (Jung & Haier, 2007). Several neuroimaging studies have implicated left lateral aPFC in abstract integration, as distinct from more basic WM processes such as maintenance and manipulation of information (Reynolds, West, & Braver, 2008). For example, activity in left aPFC has been associated with abstract, relational integration in analogical reasoning (Green et al., 2006). Left aPFC appears similarly important for integration during mathematical problem solving (De Pisapia, Slomski, & Braver, 2007), matrix reasoning (Christoff et al., 2001), and episodic memory (Reynolds, McDermott, & Braver, 2006). In a previous study of the sample examined here (Shamosh et al., 2008), we found that activity in left lateral aPFC was associated with individual differences in WM, intelligence, and the tendency to prefer larger, delayed rewards over smaller, immediate rewards. The association of left lateral aPFC with WM and intelligence suggests the hypothesis that this brain region is associated with the trait of Intellect.
The pMFC region is of interest because it is reliably engaged in WM tasks (Cabeza & Nyberg, 2000; Owen, McMillan, Laird, & Bullmore, 2005) and appears to be involved in the cognitive functions of monitoring performance during goal-directed activity (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004) and detecting the likelihood of error (Brown & Braver, 2005). Monitoring one’s performance during cognitive tasks seems likely to be associated with Intellect, especially given the degree to which Intellect reflects the tendency to be motivated and engaged by intellectual activities. We therefore hypothesized that this region might be among the neural correlates of Intellect. Finally, other parts of the canonical WM network (Cabeza & Nyberg, 2000; Owen et al., 2005; Wager & Smith, 2003), such as the dorsolateral PFC and parietal cortex, which have also been associated with tests of intelligence (Gray & Thompson, 2004), may also be associated with Intellect, though our predictions regarding these regions were less specific than those for left aPFC and pMFC.
A key question in testing our hypotheses is whether any association of Intellect with brain function is due to the association of both Intellect and WM with intelligence. The Ideas facet of the NEO PI-R, our marker for Intellect, is the facet most consistently associated with scores on intelligence tests (DeYoung et al., 2005; Furnham, Dissou, Sloan, & Chamorro-Premuzic, 2007; McCrae, 1993; Moutafi, Furnham, & Crump, 2003, 2006). Although WM is not identical to intelligence, the two are strongly related (Conway, Kane, & Engle, 2003), suggesting that WM forms a key part of the cognitive substrate of intelligence. Additionally, the neural substrates of intelligence and WM are at least partially overlapping (Gray, Chabris, & Braver, 2003; Gray & Thompson, 2004; Kane & Engle, 2002).
If Intellect, like intelligence, is associated with the neural substrates of WM, what will that finding add to our knowledge? We addressed this question empirically, using ability tests of intelligence. As noted above, measures of Intellect reflect perceived intelligence as well as intellectual engagement. Neither perceived intelligence nor intellectual engagement can be considered identical, or even strongly related, to intelligence as measured by ability tests (correlations are typically in the range of .2 to .3; e.g., Ackerman & Heggestad, 1997; Paulhus, Lysy, & Yik, 1998). Intellectual engagement reflects motivation, interest, and enjoyment in intellectual pursuits, without necessarily reflecting cognitive ability (though ability could encourage engagement and vice versa). The items of the Ideas facet were selected to reflect intellectual engagement rather than perceived intelligence (Costa & McCrae, 1992b), and Ideas is the NEO PI-R facet most strongly related to measures of Typical Intellectual Engagement, r = .77 (Ackerman & Goff, 1994) and the similar construct Need for Cognition, r = .78 (Cacioppo, Petty, Feinstein, & Jarvis, 1996). Nonetheless, the fact that both intellectual engagement and perceived intelligence tend to be positively associated with tests of ability makes it important to test whether any association with Intellect is due to intelligence measured as an ability.
There are two possibilities for the outcome of an analysis of the relations among Intellect, intelligence, and brain activity supporting WM performance (assuming the correctness of our initial hypothesis that Intellect is associated with WM-related brain activity). First, any association between Intellect and WM-related brain activity may be eliminated by controlling for intelligence, which would mean that self-rated Intellect is associated with WM-related brain activity only because it reflects intelligence with some limited degree of accuracy. Second, the association may be independent of intelligence, which would indicate that the brain activity in question is probably associated with intellectual engagement, rather than, or in addition to, intelligence. We used mediation tests to examine the relations among these constructs. As an even more specific test of the degree to which Intellect is associated with WM-related brain activity for reasons other than cognitive ability, we included measures of WM capacity, in addition to tests of intelligence.
Method
Participants
Right-handed participants were recruited from Washington University and the surrounding community, in St. Louis, Missouri, to participate in a neuroimaging study. (There is no overlap with the sample described by Gray et al. (2003); there is substantial overlap with the samples described by Shamosh et al. (2008), and Fales et al. (2008).) The experimental protocol was approved by the Washington University Medical Center Human Subjects Committee. All participants gave informed consent and were screened for history of neurological or psychiatric disorders and use of psychoactive drugs. Participants (N = 107) were selected for the present study if they had completed the NEO PI-R and had complete imaging data. One of these participants was excluded because performance on the WM task was not significantly better than chance (d' = 0.31, 3.19 SDs below the mean). Another participant was excluded for omitting more than 20 responses in multiple blocks of the task. Finally, the participant with the highest WM score was excluded as an outlier (d' = 3.80, 3.24 SDs above mean, the next highest score being only 2.12 SDs above the mean). This left 104 participants (59 female), ranging in age from 18 to 40 years (M = 22.67, SD = 5.12), who were included in all analyses reported below. There were no significant gender differences for any of the variables examined in this study.
Measures
Personality
Personality was assessed using the NEO PI-R, a standard instrument for measuring the Big Five, with excellent reliability and validity (Costa & McCrae, 1992b). Cronbach’s Alphas for the Big Five domains were: N = .92, = .87, O = .89, A = .91, C = .91. Cronbach’s Alphas for the six facets of Openness/Intellect were: Fantasy = .84, Aesthetics = .81, Feelings = .67, Actions = .66, Ideas = .84, Values = .70.
Working memory
WM was assessed during fMRI using a 3-back WM task. Participants were required to press one button if the item presented on the screen was identical to that presented 3 trials previously, and another button if the item was different. The task was made additionally difficult by the inclusion of lure trials, in which the stimulus matched one seen previously (and hence was familiar in the context of the task) but did not match the one 3 back. Trial proportions were 31% targets, 19% lures, and 50% non-lure/non-targets. Participants performed this task in six functional scanning runs, each comprising two blocks of 32 trials (64 total trials per functional run) lasting 2 seconds each. The first 3 trials of each block were discarded because no match was possible, leaving 58 trials per run. Runs alternated between using faces and concrete nouns as stimuli, with order counterbalanced across participants; all face stimuli displayed a mildly positive (smiling) expression, and all nouns were emotionally neutral. Every run was preceded by a short video; two of these videos involved positive emotion inductions, two involved negative emotion inductions, and two were emotionally neutral. The order of video presentation was counterbalanced, and we do not focus on this variable in our analyses. (All significant effects reported in Table 1 below remained significant as main effects when controlling for stimulus type and emotion condition in a series of 2 × 3 repeated measures ANCOVA, run post hoc.) 3-back performance was assessed by the signal detection measure of accuracy, d', averaged across runs. Cronbach’s Alpha for d' across the six runs was .84. Blocks of the 3-back task in which participants omitted responses to more than 15 trials were excluded from all analyses. For 6 participants, 3-back accuracy (and associated brain activity) was therefore computed based on 5 blocks. Accuracy was not significantly correlated with reaction times on the task, nor was reaction time correlated with any of the other variables examined in the study.
Table 1.
Correlations of working memory (d' and WMC), intelligence (g), the Big Five, and facets of Openness/Intellect with brain activity in four ROIs (with MNI and Talairach coordinates).
| d' | g | WMC | R SPC | L aPFC | R aPFC | pMFC | |
|---|---|---|---|---|---|---|---|
| MNI | 12, −69, 66 | −24, 66, 6 | 24, 66, 18 | −3, 6, 66 | |||
| Talairach | 10, −70, 54 | −22, 61, 8 | 20, 61, 19 | −3, 3, 58 | |||
| d' | – | .58** | .51** | .43** a | .37** a | .34** a | .31** a |
| g | .58** | – | .60** | .27* | .21* | .20* | .21* |
| WMC | .51** | .60** | – | .30** | .19* | .09 | .27* |
| Fantasy | .03 | −.09 | −.11 | −.06 | .16 | .10 | .03 |
| Aesthetics | .03 | .06 | −.04 | .01 | .08 | .01 | −.07 |
| Feelings | .00 | −.06 | −.09 | −.06 | .08 | −.02 | .03 |
| Actions | .05 | −.01 | −.04 | −.04 | .12 | −.01 | .11 |
| Ideas | .23* | .27** | .19* | .14 | .21* | .06 | .27** |
| Values | .23* | .33** | .12 | .07 | .15 | .10 | .08 |
| E | −.08 | −.13 | −.03 | −.11 | .11 | −.05 | −.01 |
| A | .01 | .11 | .09 | .18 | .05 | .03 | .15 |
| C | −.15 | −.10 | .06 | −.01 | .02 | .10 | −.02 |
| N | −.05 | .09 | −.05 | .00 | −.09 | .01 | −.09 |
| O/I | .14 | .09 | .01 | .02 | .25* | .10 | .10 |
p < .05
p < .01
These correlations are not independent of the initial statistical test that identified the ROIs. Their designation as significant therefore reflects that initial test.
Note. N = 104. E = Extraversion, A = Agreeableness, C = Conscientiousness, N = Neuroticism, O/I = Openness/Intellect, WMC = working memory capacity, SPC = superior parietal cortex, aPFC = anterior prefrontal cortex, pMFC = posterior medial frontal cortex, L = left, R = right.
In addition, participants completed four WM span tasks outside of the scanner. These tasks required participants to keep information in mind despite interference (Conway et al., 2003). Two were verbal and two were spatial: Operation span required keeping several words in mind over a short delay while doing math problems. Reading span required keeping several letters in mind while reading sentences out loud and judging their meaningfulness. Symmetry span required keeping several spatial locations in mind while making symmetry judgments. Rotation span required keeping the size and direction of several arrows in mind while making unrelated judgments that required mental rotation. For all four tasks, keeping more items in mind resulted in higher scores, on a metric ranging from 0.00 to 1.00. Factor analysis showed that all four tasks loaded strongly on a single factor accounting for 70% of total variance (loadings ranged from .81 to .86). WM capacity (WMC) was defined as the average score across all four measures (α = .86).
Intelligence
Participants completed four standard measures of intelligence: Raven’s Advanced Progressive Matrices, Set II (APM; Raven et al., 1998), the Cattell Culture Fair Intelligence Test (Cattell, 1973), the Vocabulary subscale of the WAIS-R (Wechsler, 1997) and the National Adult Reading Test - Revised (J. R. Blair & Spreen, 1989). Intelligence was assessed as general cognitive ability (g), computed as the average of participants’ standardized scores on the four psychometric tasks (α = .88). Common factor analysis revealed that a single factor explained 74% of the shared variance in these measures, the scree plot indicated no second factor, and all variables loaded strongly and approximately equally on the first unrotated factor (range: .79 – .84). Operationalizing g using scores derived from the first unrotated factor did not change the results appreciably and has the disadvantage of capitalizing on sampling variability.
fMRI Data Acquisition
Whole-brain images were collected on a 3 Tesla Allegra System (Siemens, Erlangen, Germany), including T1-weighted MP-RAGE structural images (FOV = 256 mm; 256 × 256 matrix; 1.25 mm thick axial slices) and T2* BOLD functional images (asymmetric spin-echo echo-planar sequence; TR = 2360 ms; TE = 25 ms; FOV = 256 mm; flip angle = 90°; matrix = 64 × 64; 4 mm thick axial slices). Each functional run comprised 149 sequential whole-brain volumes (32 contiguous slices, 4 × 4 mm in-plane resolution). During each functional run, the inter-trial intervals were jittered across a range of 0 to 4720 msec (0 to 2 TRs) in steps of 2360 msec (1 TR). Each task block was preceded and followed by a resting fixation block of 35 seconds, during which participants were instructed to watch a simple dash that remained at the center of the screen. Each scanning run began with an unanalyzed 4 TR fixation period that allowed the scanner to reach steady state.
fMRI Data Analysis
Data were analyzed using Statistical Parametric Mapping 2 (SPM2) software (http://www.fil.ion.ucl.ac.uk/spm). Each functional run was preprocessed prior to analysis. Data were realigned using INRIAlign (http://wwwsop.inria.fr/epidaure/Collaborations/IRMf/INRIAlign.html) to correct for movement. Images were normalized to Montreal Neurological Institute (MNI) stereotaxic space using a 12-parameter affine transformation followed by nonlinear warping using basis functions, resampled into 3 mm isotropic voxels, and smoothed using an 8 mm full-width at half-maximum Gaussian kernel.
For each participant, a basic contrast, task > fixation, was computed across all six functional runs.3 Each 32-trial block of 3-back performance was modeled as a boxcar function convolved with a canonical hemodynamic response function. The magnitude of neural activity at each voxel was estimated using the general linear model. This contrast produced statistical parametric maps of the t statistic at each voxel for each subject. These maps of the brain, indicating the difference in neural activity at each voxel between when participants were engaged in the WM task and when they were simply focusing on the fixation point, were used in all subsequent analyses.
As potential neural correlates of Intellect, we identified candidate regions of interest (ROIs) in which brain activity during the WM task covaried with 3-back accuracy between subjects, using a group-level random-effects analysis. ROIs were selected if they comprised 15 or more contiguous voxels in which the task > fixation contrast values correlated with d' at p < .001, uncorrected. The MarsBar toolbox (http://marsbar.sourceforge.net) was used to define these ROIs and to extract average percent signal change values from each ROI (computed as the mean B value across all voxels in the ROI divided by the global signal, or mean across all voxels in the brain). Percent signal change in each ROI was then averaged across all six functional runs (mean α across all ROIs = .83), and this index of brain activity was examined for correlation with personality.
Mediation Tests
Mediation tests indicate whether the association between two variables is due to another variable or set of variables. In an imaging context, mediation analyses can be used to test whether activity in a given brain region can plausibly account for the covariation between two behavioral variables, thereby implicating the region’s function in that association (Gray et al., 2003). In the present study, a significant mediation by WM-related brain activity of the association between Intellect and WM accuracy in the 3-back task would indicate that this brain activity is responsible, at least in part, for the relation between Intellect and WM. Mediation tests were also used to assess the role of cognitive ability in the associations of interest. Mediation tests were computed using path analysis in Amos 7.0 (Arbuckle, 2006), using maximum likelihood estimation and the bootstrap method to test the significance of indirect effects of personality on WM performance through brain activity (bootstrap N = 2000). (The bootstrap method replaces the inferior Sobel test; Shrout & Bolger, 2002.) Additionally, the independence of indirect effects in multiple mediation analysis was tested using the SPSS multiple mediation macro by Preacher and Hayes (2008).
Results
Before discussing the ROIs associated with d', we present an image of the basic contrast of task > fixation (Figure 1A), thresholded at p < .001. This figure illustrates the pattern of neural activity, for the sample as a whole, when participants engaged in the WM task as opposed to when they simply focused on a fixation cross. The key point is that the canonical WM brain network (Cabeza & Nyberg, 2000; Owen et al., 2005; Wager & Smith, 2003) was engaged by the task; group level activations were apparent in lateral PFC, parietal cortex, and regions in and adjacent to dorsal ACC.
Figure 1.
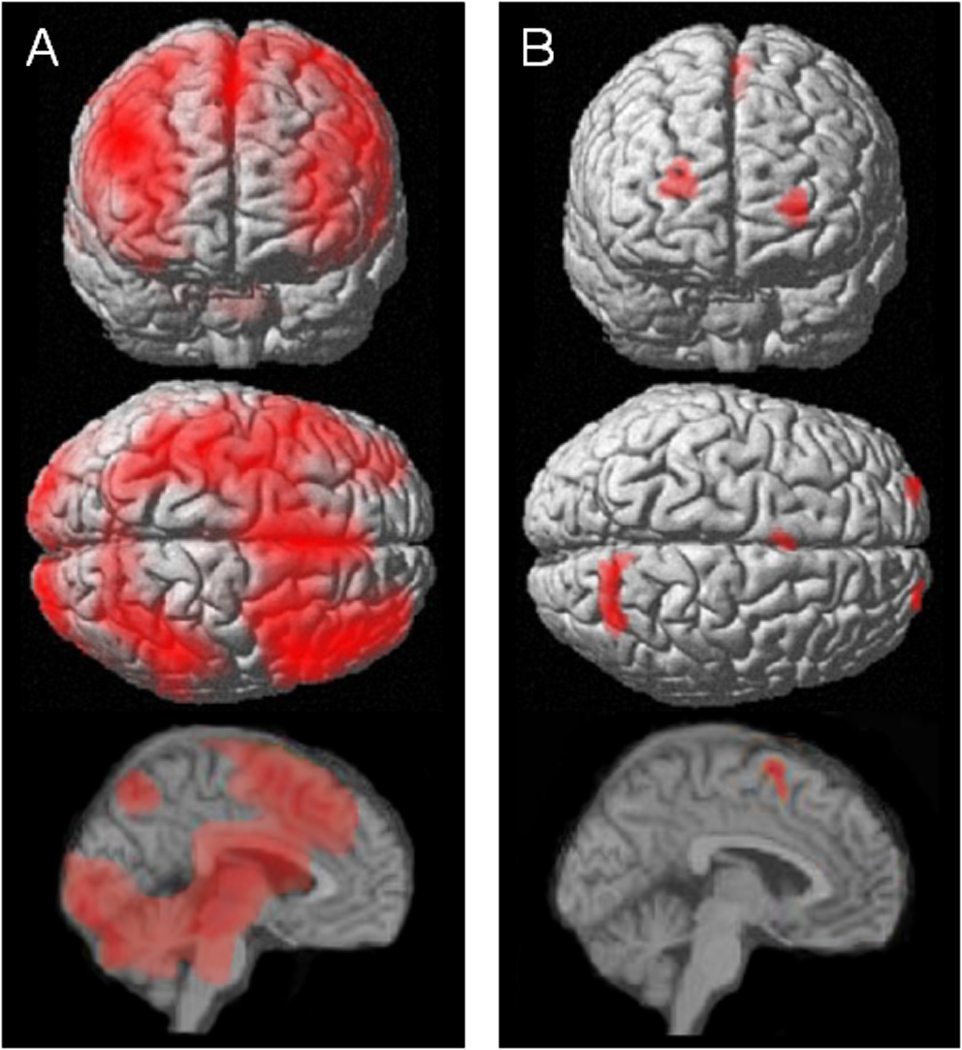
Coronal (from the front), axial (from above), and mid-sagital views of (A) neural activity associated with performing a working memory task, relative to focusing on a fixation point, for the sample as a whole (N = 104), and (B) four regions where neural activity was associated with working memory accuracy, in between-subjects analysis.
The t values for the contrast of task > fixation at each voxel across the whole brain were then used as input for a second step of the analysis that identified ROIs for which there were a positive correlation between activation level and between-subjects variation in d' – in other words, brain regions where individual differences in neural activity predicted task performance. Three ROIs were identified based on correlations with d', one ROI of 62 voxels in right superior parietal cortex (SPC, Brodmann area 7), and ROIs of 20 voxels each in left and right lateral aPFC (Brodmann area 10).4 Because we had an a priori hypothesis that activity in pMFC would also be related to Intellect, and because there were strong activations in this region for the sample as a whole (Figure 1A), we examined whether voxels in this brain region also showed correlations with performance that were reliable but did not meet our stringent statistical thresholds (which were employed to protect against false positives in whole-brain analyses). Indeed, when the statistical threshold was lowered to p < .005, we identified a 21-voxel ROI in pMFC (Brodmann area 6) superior to dorsal ACC. This ROI is shown with the other three in Figure 1B, and coordinates of the point of strongest correlation within each ROI are given in Table 1. Scatterplots of the correlations of d' with average activation in each ROI are shown in Figure 2. Importantly, the tests that identified these ROIs are statistically independent of our primary test of interest, which is the association of activation in the ROIs with facets of Openness/Intellect.
Figure 2.

Scatterplots showing the correlation of brain activity (percent signal change for task versus fixation averaged across all voxels in each ROI) during the 3-back working memory task with accuracy (d') in the task. (See Table 1 for significance.)
Table 1 shows the correlations of the Big Five and the facets of Openness/Intellect with d', intelligence, WMC, and brain activity in the four ROIs. Confirming our hypothesis, Intellect, as represented by the Ideas facet, was significantly correlated with d', g, WMC, and with activity in left lateral aPFC and pMFC. Additional regressions showed that these associations were not moderated by gender or age. However, in one of the other two ROIs, an association with Intellect was moderated by gender (β for Ideas × Gender = .36, p < .05), such that Intellect was associated with activity in right SPC but only for females. For females, r = .33, p < .05, whereas for males, r = −.15, p = .33. (Because this moderated association was not predicted, we do not focus on this ROI in additional analyses.)
No other NEO PI-R variable was associated with both cognitive performance and brain activity. The Values facet was significantly correlated with d' and g, but was not correlated with WMC or any of the ROIs. The full Openness/Intellect domain score was correlated with activity in left lateral aPFC but was not correlated with WM or any other ROI. None of the other NEO PI-R traits were significantly correlated with WM, g, or activity in the four ROIs.5 As expected, based on the literature showing their strong behavioral and neural overlap, WM (measured by both d' and WMC) and g were strongly correlated with each other, and g was significantly correlated with neural activity in all four ROIs. WMC was correlated with all ROIs except the one in right lateral aPFC.
Because the Ideas facet was significantly correlated with both d' and brain activity in two ROIs, we conducted a multiple mediation test to determine whether the association between Intellect (represented by Ideas) and d' was due to activity in these ROIs (Figure 3). The indirect effect of Intellect on d' was significant, β = .12 (SE = .047), p < .01, indicating significant mediation by the two ROIs. Additionally, the indirect effect through each ROI was significant even when controlling for the other, p < .05 for both (and this remained true when controlling for both gender and age). Thus, there are at least two independent neural mechanisms by which Intellect is associated with WM accuracy, one in left lateral aPFC and one in pMFC. The extent of this mediation can be quantified by noting that there was a 77% decrease in variance in WM accuracy explained directly by Intellect, after partialling out activity in the two ROIs.
Figure 3.
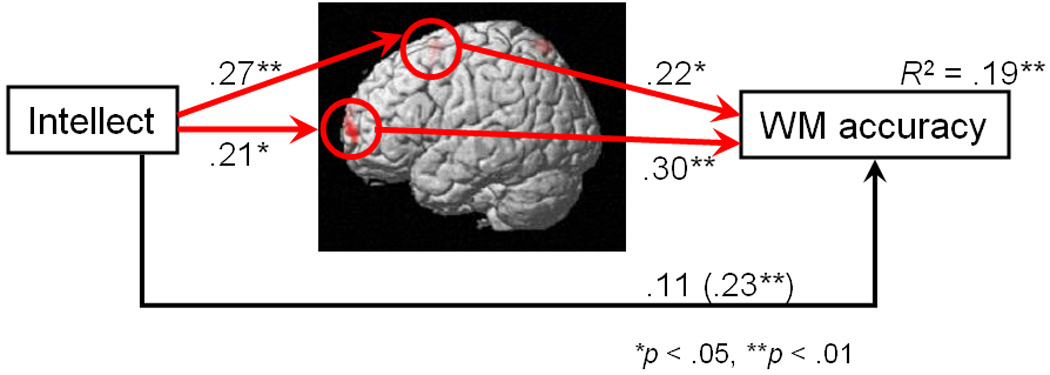
Path analysis demonstrating that working memory-related neural activity in two brain regions (left lateral anterior prefrontal cortex and posterior medial frontal cortex) mediates the association of Intellect (the Ideas facet of the NEO PI-R) with working memory accuracy (d'). Each brain region is an independent mediator at p < .05. The zero-order correlation between Ideas and d' appears in parentheses. Red paths involve neural variables, black paths do not.
Additional mediation models were run to test whether intelligence or WM mediate the associations of Intellect with brain activity. Intelligence (g), WMC, and d' were all tested individually as mediators. Finally, all three were used simultaneously to provide an even more stringent test of whether cognitive ability accounted for the association between Intellect and brain activity. Note that there is a serious conceptual difficulty in using d' as a mediator here, because d' represents performance on the task in which brain activity was assessed and this performance was caused by that brain activity. In a causal model, therefore, the arrow must run from brain activity to d', not the other way around. Nonetheless, if one is willing to consider d' simply as another indicator of a trait of working memory ability, it can be used as an additional potential mediator, and doing so provides a particularly stringent test of whether the association of Intellect with brain activity is independent of ability because of the fact that association with d' was used to select the ROIs in the first place.
Results of these mediation tests are presented in Table 2 and reveal that, whereas controlling for cognitive ability did reduce the association of Intellect with activity in left aPFC below significance, it did not reduce the association of Intellect with activity in pMFC below significance. Thus, we have evidence that the association between Intellect and activity in pMFC during a difficult cognitive task is not simply due to the fact that Intellect is associated with cognitive ability.
Table 2.
Tests of mediation of the associations between Intellect (I) and brain activity by cognitive ability (intelligence (g) and working memory).
| Mediation model | Paths | Indirect effect | |
|---|---|---|---|
| (ROI and mediator) | I to ROI | Ability to ROI | |
| aPFC | |||
| g | .17 | .16 | .045 |
| WMC | .18 | .16 | .030 |
| d' | .13 | .34** | .077** |
| All 3 | .14 | – | .072 |
| pMFC | |||
| g | .23* | .14 | .039 |
| WMC | .22* | .23* | .044* |
| d' | .20* | .26* | .060** |
| All 3 | .20* | – | .062 |
p < .05
p < .01
Note. N = 104. For path weights from Intellect to abilities, see Table 1. WMC = working memory capacity; All 3 = g, WMC, and d' used as simultaneous predictors.
Neither g nor WMC could be demonstrated to mediate the association between Intellect and activity in aPFC (d' did mediate this association, but, as noted above, this is a special case because d' was used to select the ROI and was causally dependent on the brain activity in question). In contrast, WMC (and d' but not g) did partially mediate the association between Intellect and activity in MFC. This means there was not only a significant direct path from Intellect to MFC, but also a significant and independent indirect path, through working memory ability.
Discussion
As hypothesized, the Intellect aspect of the Openness/Intellect trait domain (represented by the Ideas facet of the NEO PI-R) was associated both with performance on a difficult WM task and with brain activity in two brain regions that supported accuracy in WM. Neural activity in both left lateral aPFC and pMFC mediated the association of Intellect with WM, suggesting that one reason why people who describe themselves as more intellectual show better WM performance is that they tend to engage these brain regions more strongly during difficult cognitive operations.
The associations of Intellect with WM performance and brain activity were in the range of r = .2 to .3, which should be considered moderate, based on empirical guidelines for interpreting effect sizes (Hemphill, 2003; Richard, Bond, & Stokes-Zoota, 2003). Associations of intelligence (g) and working memory capacity (WMC) with brain activity were in the same range. Observe that the correlations of brain activity with WM accuracy, during the WM task itself, were only in the range of .3 to .4 (Table 1). Notably, neural activity in the two PFC regions accounted for over three quarters of the variance in WM accuracy associated with Intellect.
Mediation tests were used to determine whether the association of Intellect with brain activity was independent of cognitive ability, measured both by intelligence tests and by tests of working memory capacity. The association of Intellect with pMFC activity was indeed independent of cognitive ability, whereas the association of Intellect with left aPFC activity was not. By the standards of formal mediation tests, intelligence did not mediate the association between Intellect and either brain region (and WMC only partially mediated the association of Intellect with pMFC). Nonetheless, with g or WMC in the model, neither Intellect nor g or WMC was significantly associated with neural activity in left aPFC. The fact that the effects of both Intellect and cognitive ability on left aPFC were suppressed relative to their zero-order magnitude suggests that the shared variance of Intellect and cognitive ability is associated with activity in this region. This interpretation is sensible when one considers that Intellect subsumes perceived intelligence as well as intellectual engagement (DeYoung et al., 2007), and to some limited extent perceived intelligence accurately reflects intelligence as measured by ability tests (Paulhus et al., 1998). Thus, one plausible source of both Intellect and cognitive ability is the function of left lateral aPFC, which has been characterized as integrating information from multiple cognitive operations, across a variety of different cognitive tasks (Gilbert et al., 2006; Green et al., 2006; Ramnani & Owen, 2004).
In contrast to left aPFC, neural activity in pMFC remained significantly associated with Intellect even after partialling out variance associated with g and working memory. This suggests that intellectual engagement plays a major role in the association of Intellect with activity in pMFC. The ROI identified in pMFC lies in a region that is involved in monitoring goal-directed performance (Ridderinkhof et al., 2004) and is sensitive to the likelihood of error (Brown & Braver, 2005). This performance monitoring function aids in the resolution of uncertainty during decisions and in resolving response conflict (Brown & Braver, 2005; Ridderinkhof et al., 2004). Both uncertainty and response conflict should be frequent in the 3-back WM task, as participants attempt to decide whether the present stimulus matches the one exactly three previously and to inhibit the urge to respond to lures. The tendency to monitor cognitive performance closely and accurately is another plausible source of Intellect as a trait and one that may reflect not just intelligence, but also one’s motivation and interest in intellectual activities (i.e., one’s intellectual engagement).
In relation to this conclusion, it is important to note that the function served by pMFC is unlikely to be specific to difficult working memory operations or even complex cognitive tasks. This brain region is similarly active during much simpler cognitive tasks that involve response conflict and/or uncertainty, such as Stroop tasks (Ridderinkhof et al., 2004). (Of course, the mere fact that the same brain region is active during different tasks does not guarantee that it is performing similar functions during different tasks, but many cognitive tasks clearly require the kind of performance monitoring that this region subserves.) In order to distinguish between brain activity related exclusively to working memory and brain activity that would support performance on simpler cognitive tasks as well, we would have needed to include a simple cognitive task as a control condition. However, our concern was to detect neural correlates of the personality trait Intellect, not to pin down the specific neural correlates of working memory, and we were, therefore, interested broadly in neural processes that support cognition. Given that the variance responsible for most of the association between Intellect and pMFC activity appeared to be related to intellectual engagement, rather than cognitive ability, it seems quite likely that the Intellect-pMFC association reflects a tendency toward monitoring cognitive performance that would be heightened across a wide variety of cognitive tasks for those high in Intellect. This brain region may be modulated by motivation to perform any cognitive task vigilantly and accurately.
One additional region that was identified as supportive of WM accuracy was associated with Intellect only in females. For females, but not males, Intellect was associated with activity in right SPC. We did not hypothesize any such interaction with gender. However, this brain region was strongly associated with working memory performance and is part of the canonical working memory network (Cabeza & Nyberg, 2000; Owen et al., 2005; Wager & Smith, 2003), so the finding is reasonably consistent with our hypotheses. Future research might investigate gender differences in the relation of personality to cognitive and brain function, building on research showing gender differences in brain function (e.g., Canli, Desmond, Zhao, & Gabrieli, 2002) and in various forms of cognitive ability, including working memory (e.g., Kaufman, 2007).
The one ROI identified that was entirely unrelated to Intellect was in right lateral aPFC, and this ROI may not be reliable because it did not appear when using the same technique to identify ROIs in a largely overlapping sample (Shamosh et al., 2008). The left (but not the right) aPFC ROI is consistent with previous studies of abstract relational integration (Bunge, Helskoga, & Wendelken, in press; Green et al., 2006) and with a review suggesting that left rather than right aPFC is predominantly involved in intelligence (Jung & Haier, 2007). Although research on WM suggests that lateralization in PFC is influenced somewhat by whether visual or verbal stimuli are used (Wager & Smith, 2003), our WM task employed both visual and verbal stimuli, in order to identify brain activity involved in WM regardless of modality. Our results are consistent with previous research, but additional research will be necessary to determine the significance of the left lateralization of activity in aPFC in relation to complex cognition.
Although Ideas was the only facet of Openness/Intellect associated with brain activity and performance on all cognitive tasks, the Values facet was associated with g and one WM performance variable; these associations are consistent with previous research, reporting similar effect sizes (DeYoung et al., 2005). The finding that the Values facet was associated with cognitive variables but not brain activity suggests that, compared to Ideas, Values has a less direct link to the brain activity that supports WM. Nonetheless, the behavioral associations are worth noting, given related research. Because Values reflects a liberal worldview and its alternative label is “Liberalism” (Goldberg, 1999), our finding may be relevant to the characterization of cognitive processes associated with political attitudes. They are consistent with findings that liberalism, as a political orientation, is associated with higher IQ, relative to conservatism (Block & Block, 2006; Deary, Batty, & Gale, 2008). Further, one recent study reported that liberalism was associated with better performance and increased brain activity in MFC, during a cognitive control task (Amodio, Jost, Master, & Yee, 2007). The Values facet is not a particularly good marker of Intellect, but it is nonetheless moderately related to Intellect as well as to Openness (DeYoung et al., 2007), and it may be fruitful to continue investigating this politically-relevant dimension of personality in future research on the cognitive and neural correlates of personality.
A question for future research is what brain functions might contribute specifically to Openness as opposed to Intellect. This might be revealed through fMRI by using a task more directly relevant to the artistic and contemplative qualities that characterize Openness. Additionally, future research on Openness and Intellect could benefit from using personality measures designed specifically to distinguish these two aspects (e.g., the Big Five Aspect Scales; DeYoung et al., 2007).
Conclusion
Intellect and Openness are separable aspects of one larger domain of personality, which raises the question of their discriminant validity. To our knowledge, the present study provides the first assessment of the neural correlates of either aspect. Intellect subsumes traits reflecting intellectual engagement and perceived intelligence. Consistent with this characterization, we found that the Ideas facet of the NEO PI-R (a good marker of Intellect) was associated with measures of intelligence and WM, whereas the facets that strongly mark Openness were not. This study went beyond previous research (DeYoung et al., 2005) by investigating the biological sources of the link between Intellect and WM accuracy, and found two regions of PFC that mediated this association, one in the left anterior PFC, or frontal pole, and the other in posterior medial frontal cortex. The region in pMFC, which has been implicated in performance monitoring, was significantly associated with Intellect even after controlling for intelligence and working memory ability. Neither region was associated with any other Openness/Intellect facets. These findings demonstrate the possibility of distinguishing two aspects of a single Big Five domain in terms of neural correlates and suggest that the functions of pMFC may be an important substrate of Intellect that is distinct from cognitive ability and is perhaps driven by the motivation to engage with intellectual activities
Acknowledgments
This research was supported by grants from the National Institute of Mental Health to J.R.G. (MH R01 66088) and C.G.D. (F32 MH077382). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/psp
Similar collections of terms have defined the Openness/Intellect factor in other languages, except when terms reflecting intellectual ability have been intentionally omitted (e.g., Dutch, Italian), in which case the factor tends to tilt more toward content related to unconventionality (John, Naumann, & Soto, 2008).
The fact that the NEO PI-R contains only one clear Intellect facet and four clear Openness facets is due to the inventory’s history and does not constitute evidence that Intellect is peripheral to the larger Openness/Intellect domain. The facets of the NEO PI-R were derived rationally; those for Openness to Experience were developed prior to McCrae and Costa’s (1985) attempt to harmonize the NEO with the lexical Big Five; and McCrae and Costa have consistently resisted the idea that Intellect might be a valid interpretation of content in this domain (e.g., McCrae & Costa, 1997). As noted above, however, considerable evidence in both lexical and questionnaire research indicates that Intellect is just as central to the larger domain as Openness.
A previous study utilizing the same task in a completely different sample, in order to investigate neural correlates of fluid intelligence, focused on neural activity associated specifically with lure trials, as a phasic departure from sustained activity (Gray et al., 2003). Additionally, they identified ROIs on the basis of correlations with fluid intelligence rather than WM accuracy. These differences may explain their identification of a different set of ROIs. For the present purposes, we were interested in sustained activity throughout the WM task, rather than phasic activity in lure trials, because the n-back task requires continual maintenance, monitoring, updating, and integration of information, all of which are likely to be relevant to Intellect.
Because our previous study of these data (Shamosh et al., 2008) used a slightly different subsample, based on the availability of different measures, we there reported a partially different set of ROIs based on the same selection criteria. As well as nearly identical ROIs in right superior parietal cortex and left aPFC, that study also reported four additional ROIs and did not report an ROI in right aPFC. We examined those four additional ROIs in the present sample and found that none of them were correlated with any NEO PI-R variables.
Two previous studies of different samples (Gray & Braver, 2002; Gray, Burgess, Schaefer, Yarkoni, Larsen, & Braver, 2005), have examined the association of WM accuracy (d') and brain activity in the same task with personality scales measuring sensitivity of the behavioral inhibition and behavioral activation systems (BIS/BAS scales; Carver & White, 1994). The first found a significant correlation of BAS with d' (r = .18, p < .05), when examining the first time participants performed the task. The second replicated this finding for the first administration, but found that the association was not significant when averaging over repeated administrations of the task (r = −.03). The BIS/BAS scales were administered the current sample; correlations of d' with BIS and BAS were .06 and −.04, respectively, in the first block, and .08, and −.03 across all blocks (all p > .40). There were no significant correlations between BIS or BAS and the four ROIs.
Contributor Information
Colin G. DeYoung, Department of Psychology, University of Minnesota
Noah A. Shamosh, Department of Psychology, Yale University
Adam E. Green, Department of Psychology, Yale University
Todd S. Braver, Department of Psychology, Washington University
Jeremy R. Gray, Department of Psychology and Interdepartmental Neuroscience Program, Yale University
References
- Ackerman PL, Goff M. Typical intellectual engagement and personality: Reply to Rocklin (1994) Journal of Educational Psychology. 1994;86:150–153. [Google Scholar]
- Ackerman PL, Heggestad ED. Intelligence, personality, and interests: Evidence for overlapping traits. Psychological Bulletin. 1997;121:219–245. doi: 10.1037/0033-2909.121.2.219. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Jost JT, Master SL, Yee CM. Neurocognitive correlates of liberalism and conservatism. Nature Neuroscience. 2007;10:1246–1247. doi: 10.1038/nn1979. [DOI] [PubMed] [Google Scholar]
- Arbuckle JL. Amos 7.0 (Build 1140) Amos Development Corporation; 2006
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. The Clinical Neuropsychologist. 1989;3(22):129–136. [Google Scholar]
- Block J, Block JH. Nursery school personality and political orientation two decades later. Journal of Research in Personality. 2006;40:734–749. [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Helskoga EH, Wendelken C. Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. NeuroImage. doi: 10.1016/j.neuroimage.2009.01.064. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cacioppo, Petty, Feinstein, Jarvis Dispositional differences in cognitive motivation: The life and times of individuals differing in need for cognition. Psychological Bulletin. 1996;119:197–253. [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JDE. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioural inhibition, behavioural activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Cattell RB. Measuring intelligence with the culture fair tests. Champaign, IL: The Institute for Personality and Ability Testing; 1973. [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JDE. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences. 2003;7(12):547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Four ways five factors are basic. Personality and Individual Differences. 1992a;13:653–665. [Google Scholar]
- Costa PT, Jr, McCrae RR. NEO PI-R Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992b. [Google Scholar]
- De Pisapia N, Slomski JA, Braver TS. Functional specializations in lateral prefrontal cortex associated with the integration and segregation of information in working memory. Cerebral Cortex. 2007;17:993–1006. doi: 10.1093/cercor/bhl010. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Batty GD, Gale CR. Bright children become enlightened adults. Psychological Science. 2008;19:1–6. doi: 10.1111/j.1467-9280.2008.02036.x. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Gray JR. Personality neuroscience: Explaining individual differences in affect, behavior, and cognition. In: Corr PJ, Matthews G, editors. The Cambridge handbook of personality psychology. Cambridge University Press: 2009. p. New York. [Google Scholar]
- DeYoung CG, Peterson JB, Higgins DM. Sources of Openness/Intellect: Cognitive and neuropsychological correlates of the fifth factor of personality. Journal of Personality. 2005;73:825–858. doi: 10.1111/j.1467-6494.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Quilty LC, Peterson JB. Between facets and domains: Ten aspects of the Big Five. Journal of Personality and Social Psychology. 2007;93:880–896. doi: 10.1037/0022-3514.93.5.880. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. Personality and intelligence: Psychometric and experimental approaches. In: Sternberg RJ, Ruzgis P, editors. Personality and Intelligence. New York: Cambridge University Press; 1994. pp. 3–31. [Google Scholar]
- Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Braver TS, Gray JR. Anxiety and cognitive efficiency: Differential modulation of transient and sustained neural activity during a working memory task. Cognitive, Affective, and Behavioral Neuroscience. 2008;8:239–253. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- Furnham A, Dissou G, Sloan P, Chamorro-Premuzic T. Personality and intelligence in business people: A study of two personality and two intelligence measures. Journal of Business and Psychology. 2007;22:99–109. [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. Journal of Cognitive Neuroscience. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. The development of markers for the big-five factor structure. Psychological Assessment. 1992;4:26–42. [Google Scholar]
- Goldberg LR. The structure of phenotypic personality traits. American Psychologist. 1993;48:26–34. doi: 10.1037//0003-066x.48.1.26. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models. In: Mervielde I, Deary I, De Fruyt F, Ostendorf F, editors. Personality psychology in Europe. Vol. 7. Tilburg: The Netherlands: Tilburg University Press; 1999. pp. 7–28. [Google Scholar]
- Gray JR, Braver TS. Personality predicts working-memory-related activation in the caudal anterior cingulate cortex. Cognitive Affective & Behavioral Neuroscience. 2002;2:64–75. doi: 10.3758/cabn.2.1.64. [DOI] [PubMed] [Google Scholar]
- Gray JR, Burgess GC, Schaefer A, Yarkoni T, Larsen RJ, Braver TS. Affective personality differences in neural processing efficiency confirmed using fMRI. Cognitive Affective & Behavioral Neuroscience. 2005;5:182–190. doi: 10.3758/cabn.5.2.182. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Gray JR, Thompson PM. Neurobiology of intelligence: Science and ethics. Nature Reviews Neuroscience. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Shamosh NA, Dunbar KN. Frontopolar cortex mediates abstract integration in analogy. Brain Research. 2006;1096:125–137. doi: 10.1016/j.brainres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Hemphill JF. Interpreting the magnitudes of correlation coefficients. American Psychologist. 2003;58:78–80. doi: 10.1037/0003-066x.58.1.78. [DOI] [PubMed] [Google Scholar]
- Jang KL, Hu S, Livesley WJ, Angleitner A, Riemann R, Vernon PA. Genetic and environmental influences on the covariance of facets defining the domains of the five-factor model of personality. Personality and Individual Differences. 2002;33:83–101. [Google Scholar]
- Jang KL, McCrae RR, Angleitner A, Riemann R, Livesley WJ. Heritability of facet-level traits in a cross-cultural twin sample: Support for a hierarchical model of personality. Journal of Personality and Social Psychology. 1998;74:1556–1565. doi: 10.1037//0022-3514.74.6.1556. [DOI] [PubMed] [Google Scholar]
- Johnson JA. Clarification of factor five with the help of the AB5C model. European Journal of Personality. 1994;8:311–334. [Google Scholar]
- John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative Big Five trait taxonomy: History, measurement, and conceptual issues. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: Theory and research. New York: Guilford Press; 2008. [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences. 2007;30:135–187. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin and Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kaufman SB. Sex differences in mental rotation and spatial visualization ability: Can they be accounted for by differences in working memory capacity? Intelligence. 2007;35:211–223. [Google Scholar]
- McCrae RR. Openness to Experience as a basic dimension of personality. Imagination, Cognition, and Personality. 1993;13:39–55. [Google Scholar]
- McCrae RR, Costa PT. Updating Norman's “adequate taxonomy”: Intelligence and personality dimensions in natural language and in questionnaires. Journal of Personality and Social Psychology. 1985;49:710–721. doi: 10.1037//0022-3514.49.3.710. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Conceptions and correlates of Openness to Experience. In: Hogan R, Johnson J, Briggs S, editors. Handbook of personality psychology. Boston: Academic Press; 1997. [Google Scholar]
- Moutafi J, Furnham A, Crump J. Demographic and personality predictors of intelligence: A study using the NEO Personality Inventory and the Myers-Brigg Type Indicator. European Journal of Personality. 2003;17:79–94. [Google Scholar]
- Moutafi J, Furnham A, Crump J. What facets of openness and conscientiousness predict fluid intelligence score? Learning and Individual Differences. 2006;16:31–42. [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulhus DL, Lysy DC, Yik MSM. Self-report measures of intelligence: Are they useful as proxy IQ tests? Journal of Personality. 1998;66:525–554. [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven progressive matrices and vocabulary scales. Oxford: Oxford Psychologists Press; 1998. [Google Scholar]
- Reynolds JR, McDermott KB, Braver TS. A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cerebral Cortex. 2006;16:519–528. doi: 10.1093/cercor/bhi131. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, West R, Braver TS. Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn164. Advance Access: doi:10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard FD, Bond CF, Jr, Stokes-Zoota JJ. One hundred years of social psychology quantitatively described. Review of General Psychology. 2003;7:331–363. [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Saucier G. Openness versus intellect: Much ado about nothing? European Journal of Personality. 1992;6:381–386. [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway ARA, Engle RW, Braver TS, Gray JR. Individual differences in delay discounting: Relation to intelligence, working memory, and frontopolar cortex. Manuscript submitted for publication. Psychological Science. 2008 doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wais-III administration and scoring manual. San Antonio, TX: Harcourt Brace & Company; 1997. [Google Scholar]


