Abstract
Objective:
To evaluate the efficacy and safety of eszopiclone 3 mg, a nonbenzodiazepine medication/hypnotic indicated for the treatment of insomnia with comorbid rheumatoid arthritis (RA).
Method:
This multicenter, double-blind, placebo-controlled pilot study was conducted in 153 patients aged 25–64 years with American College of Rheumatology–defined RA who met DSM-IV criteria for insomnia. The data were collected from February to November of 2004. Patients were randomly assigned to either eszopiclone or placebo nightly for 4 weeks, followed by a 2-week placebo run out. Efficacy was evaluated using patient reports of sleep (wake time after sleep onset [WASO], sleep latency [SL], and total sleep time [TST]), daytime function, pain, and RA assessments. Insomnia severity was evaluated using the Insomnia Severity Index. Safety was also evaluated.
Results:
Eszopiclone significantly improved all patient-reported sleep measures (WASO, SL, and TST), sleep quality, depth of sleep, and daytime function (P < .05 vs placebo). At week 4, 48% of eszopiclone-treated patients had no clinically meaningful insomnia as assessed by ISI score (versus 30% of placebo-treated patients, P = .03). Eszopiclone was significantly better than placebo on some RA-associated pain measures: (1) overall (P = .05), pain (P = .006), and pain and other symptoms (P = .02) scores of the Arthritis Self-Efficacy Scale, (2) tender joint counts (P = .03) and pain severity scores (P = .023), (3) the activities domain of the Health Assessment Questionnaire-Disability Index (P = .04), and (4) the role physical (P = .03) and bodily pain (P = .01) scales of the 36-item Medical Outcomes Study Short-Form General Health Survey. The most commonly reported adverse events with eszopiclone were unpleasant taste and transient increases in RA symptoms.
Conclusions:
In this pilot study of patients with insomnia comorbid with RA, eszopiclone 3 mg improved all assessed sleep and daytime function measures over the treatment period, as well as some measures of RA-associated pain, disability, and quality of life.
Trial Registration:
clinicaltrials.gov Identifier: NCT00367965
Rheumatoid arthritis (RA) is a painful, chronic, progressive inflammatory disease characterized by proliferative synovitis causing swelling and deformity of multiple joints. A variety of comorbid mood, anxiety, and sleep disorders, with an associated decline in health-related quality of life, are commonly found among adults with RA.1–3 Sleep in particular can be severely impaired in those with RA.4–8 Nearly 60% of RA patients report that their sleep is restless most of the time.9 Compared to healthy controls, individuals with RA have been found to have reduced sleep efficiency, fragmented sleep, and impaired sleep quality.10
The presence and severity of pain in RA and other chronic pain disorders has been found to be associated with sleep disruption.10,11 The exact nature of the connection between pain and sleep is unclear. Some studies suggest that chronic pain disrupts sleep,12,13 and other studies indicate that sleep deprivation can result in enhanced pain and hyperalgesia.14 Recent evidence in normal subjects has provided support to the hypothesis that sleep disruption can influence the experience of pain.15,16 The amount of both slow wave sleep and time awake during the night has been found to be correlated with morning stiffness, pain, and joint tenderness in RA patients.17 In a longitudinal investigation of RA patients, increased pain and morning stiffness were associated with increases in time spent in slow wave sleep, time spent awake, and time spent in non–rapid eye movement (REM) stage 2 sleep.5
Despite the evidence for an association between sleep and pain, little is known about the effects of treating insomnia in patients with RA or other chronic pain conditions. In 1 study, cognitive-behavioral therapy (n = 32), compared to a waitlist control group (n = 29), improved sleep-onset latency, wake time after sleep onset (WASO), sleep efficiency, and sleep quality but not pain in those individuals with chronic pain of musculoskeletal origin (excluding primary fibromyalgia).18 Nonsteroidal anti-inflammatory drugs (NSAIDs) reduced pain but did not influence sleep parameters (total sleep time [TST], sleep latency [SL], REM latency, sleep efficiency, number of awakenings, REM sleep, non-REM sleep, wake within sleep, and time in stages 1, 2, 3, and 4) in RA patients.19 Benzodiazepines are often prescribed for the treatment of insomnia and/or nonspecific pain complaints.20 In 1 small study,21 triazolam increased total sleep time but did not reduce SL or sleep fragmentation as measured by polysomnography. Interestingly, patients also reported shorter SL, fewer arousals, and longer sleep time, suggesting changes in patient perception of sleep. Improvements were noted in morning stiffness among RA patients treated with triazolam compared to placebo.21
Clinical Points
♦ Compared to healthy controls, individuals with rheumatoid arthritis have been found to have reduced sleep efficiency, fragmented sleep, and impaired sleep quality.
♦ In this pilot study of patients with insomnia comorbid with rheumatoid arthritis, eszopiclone 3 mg improved all assessed sleep and daytime function measures over the treatment period, as well as some measures of rheumatoid arthritis–associated pain, disability, and quality of life.
♦ The most commonly reported adverse events with eszopiclone were unpleasant taste and transient increases in rheumatoid arthritis symptoms.
Eszopiclone is approved for the treatment of insomnia. It is a novel, single-isomer, nonbenzodiazepine hypnotic that interacts with γ-aminobutyric acid-A receptor subtypes containing the α1, 2, 3, and 5 subunits. Nightly administration improved sleep initiation, maintenance, duration, and quality, as well as daytime symptoms of insomnia including fatigue, daytime alertness, and ability to function for up to 6 months in patients with chronic primary insomnia.22 Eszopiclone has also demonstrated sleep efficacy in patients with chronic insomnia and comorbid major depressive disorder,23 generalized anxiety disorder,24 and perimenopausal transition.25 In these populations, improvements following eszopiclone treatment were also seen in assessments of the comorbid condition when compared with placebo.
The current pilot study assessed the efficacy and safety of eszopiclone 3 mg in adults with insomnia comorbid with RA. The effects of eszopiclone 3 mg on RA-related pain, joint stiffness, ability to function during the day, and health-related quality of life endpoints were also evaluated.
METHOD
Institutional review boards of participating institutions approved the protocol, and all patients provided written, informed consent before screening. The study was conducted in accordance with the standards of good clinical practice and followed guidelines and regulations established by the Declaration of Helsinki (1989). The data were collected from February to November of 2004.
Patients
All study participants were informed about the study procedures before giving their signed consent. Patients were aged 25 to 64 years (inclusive) with a diagnosis of RA according to American College of Rheumatology (ACR) criteria and on stable doses of chronic RA medications for a minimum of 90 days prior to random assignment. Patients were required to meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for insomnia and had a WASO ≥ 45 minutes and a TST < 6.5 hours at least 3 times per week for the previous month. The diagnosis of RA must have predated the onset of insomnia symptoms.
Patients taking disease-modifying medications (such as methotrexate, plaquenil hydrochloroquine and or sulf asalazine, gold salts, and d-penicillamine, azathioprine, and anti–tumor necrosis factor agents) at screening were allowed to continue those medications (with monitoring) during the study. Patients with a history of fibromyalgia or juvenile RA were excluded. Patients with insomnia related to known medical diagnosis (eg, sleep apnea) and diagnosed and untreated restless legs syndrome or periodic leg movement syndrome were excluded. Patients with undiagnosed or untreated cases of restless legs syndrome or periodic leg movement syndrome were not enrolled based on an actimeter assessment of periodic leg movements for 1 week during the single-blind run-in period of the trial.
Patients were excluded if they were taking any of the following medications within 7–30 days of the start of baseline: >10 mg prednisone (or equivalent); monoamine oxidase inhibitors; tricyclic antidepressants; serotonin-norepinephrine reuptake inhibitors or trazadone, nefazodone, bupropion, mirtazapine, tramadol, gabapentin or other anticonvulsant medications; benzodiazepines; any medication or herbal supplement known to affect sleep; or any narcotics or central nervous system active pain medications other than those listed as approved rescue medications. Selective serotonin reuptake inhibitors were allowed as long as the dose was stable for at least 30 days.
In the event of an exacerbation of RA symptoms (postbaseline), the use of selected medications was allowed (disease-modifying medications; NSAIDs; any non narcotic pain medications including tricyclic antidepressants, tramadol, gabapentin, venlafaxine, or duloxetine; glucocorticoids; local steroid injections; over-the-counter analgesic medications; and topical ointments). The following narcotic medications were permitted to treat an exacerbation of RA symptoms: acetaminophen with codeine, hydrocodone, or oxycodone; aspirin with codeine; and aspirin and caffeine with propoxyphene. The use of narcotic pain medications was not to exceed 2 days per week, and the drugs had to be taken after completing the morning interactive voice response system (IVRS) assessments, could not be taken after 2 pm, and could not exceed 2 doses in 1 day or participants were discontinued from the study.
Study Design
This was a multicenter (43 US sites), randomized, double-blind, placebo-controlled, parallel-group study. Patients were screened for eligibility, and 5–14 days later, eligible patients were trained on the use of an IVRS. Throughout the study, the IVRS was used each morning to capture sleep patterns, daytime functioning, and RA symptoms. Patients first entered a 3- to 7-day, single-blind, placebo run-in period to establish baseline values and ensure compliance. In addition, an actimeter was used to identify undiagnosed or untreated periodic leg movement syndrome. After the run-in period, patients were randomly assigned to treatment with eszopiclone 3 mg or placebo at bedtime for 4 weeks. Patients returned to the clinic for an interim visit at week 2 and at week 4. After 4 weeks of treatment, patients completed a 2-week, single-blind, placebo discontinuation phase to assess possible rebound effects.
Study Endpoints
The following sleep endpoints were assessed via daily, morning IVRS: patient self-report of SL (defined as the number of minutes taken to fall asleep after bedtime), WASO (defined as the total number of minutes of wake time after initial sleep onset), TST (defined as the total number of minutes of sleep during the night), depth and quality of sleep (each rated on a scale of 0 [poor] to 10 [excellent]), and daytime function (daytime alertness, ability to concentrate, physical well-being, and ability to function, each scored on a 0- to 10-point scale with higher scores indicating better functioning). The severity of insomnia and its effect on daytime function was also determined with the Insomnia Severity Index (ISI),26 a subjective assessment of the severity of nighttime and daytime insomnia symptoms administered at the end of the run-in period (baseline) and at week 2, week 4, and the end of the single-blind run-out period.
Assessments of RA disease state included duration of morning stiffness; current and previous day pain severity (rated on a 0- to 5-point scale: 0 = no pain, 5 = excruciating); the Arthritis Self-Efficacy Scale (ASES),27 an assessment of patients’ perceived ability to cope with the consequences of arthritis (rated on a scale of 0–10: 10 being best function and least pain) at baseline, week 4, and the end of the single-blind run-out period; ACR response criteria,28 including limited 28-count joint assessments of swollen and tender joints; patient and physician global assessment; Subjective Pain Severity Assessment scale29 based on an 11-point (0–10) Lichert scale at baseline and week 4; calculation of the percentage of patients with an increase in dose or a new prescription of pain medications; and calculation of the percentage of patients with an increase in dose or a new prescription of disease-modifying medication.
The 36-item Medical Outcomes Study Short-Form SF-36 General Health Survey,30 a subjective assessment of 8 dimensions of health-related quality of life, was administered at baseline, week 4, and the end of the single-blind run-out period. The Health Assessment Questionnaire-Disability Index (HAQDI),31 a subjective assessment of the subject's ability to perform and participate in selected activities of daily living over the last week, was administered at baseline and week 4.
Safety variables included spontaneously reported adverse events (AEs), 12-lead echocardiograms, clinical lab oratory assessments, vital signs, and physical examinations. Potential withdrawal effects were evaluated by examining the prevalence of new or worsening AEs and any worsening of sleep relative to baseline values (rebound insomnia) that occurred during the single-blind placebo run-out period.
Statistical Analysis
The study was originally planned to include 440 subjects, but as a result of slow subject accrual, was changed to a pilot study, and the target sample size was reduced to 150. All significance tests for efficacy were changed to 1-sided tests, consistent with the exploratory nature of a pilot study.32 This was the only change in analytic plan and was amended prior to patient unblinding, and all patients were treated according to the a priori study protocol. The study had approximately a 65% chance to detect a treatment difference of 0.185 between the 2 treatment groups on the log scale for WASO, assuming a standard deviation of 0.534 (based on previous studies33), using a 1-sided test (α = .05). This difference corresponds to a 20% greater reduction in mean WASO for eszopiclone compared to placebo.
The analyses of all efficacy and safety endpoints were performed using the intent-to-treat (ITT) population. The ITT population included all subjects who were randomized and received at least 1 dose of double-blind study medication. The single-blind washout population included all randomized subjects who completed the double-blind treatment period and received at least 1 dose of single-blind study medication during the washout period. All analyses of rebound and withdrawal were performed using the single-blind washout population.
The primary sleep endpoint was subjective WASO during week 1, defined as the average of the daily WASO values obtained via IVRS during the first week of double-blind treatment. No imputation methods were used for missing data. The primary analysis utilized an analysis of variance based on the ranked data, including terms for treatment and site as fixed effects. Supportive secondary sleep efficacy endpoints included WASO (except the week 1 average, which was the primary endpoint), TST, SL, depth of sleep, quality of sleep, daytime alertness, ability to concentrate, physical well-being, and ability to function. These secondary endpoints were defined in the same manner as for WASO, and the double-blind average was defined as the average of all values obtained during the double-blind treatment period.
In addition, the change from baseline to week 1, 2, 3, and 4 and the double-blind average were also analyzed for each of the subjective sleep and daytime efficacy endpoints using an analysis of covariance (ANCOVA) model with treatment and site as fixed effects and baseline as the covariate. Values for WASO and SL were log transformed prior to the analysis, and medians are reported because they best represent central tendency of log-transformed data.
Secondary endpoints including ISI, ASES, SF-36, physician clinical global impressions of treatment, and ACR response criteria (number of swollen joints, number of tender joints, subject global assessment, physician global assessment, subject pain severity assessment, HAQDI, and C-reactive protein) were analyzed using ANCOVA for change from baseline. Because these secondary endpoints were intended to provide supportive information for this pilot study, no adjustment for multiplicity was applied, and the results should be interpreted accordingly.
During the discontinuation phase, the occurrence of rebound insomnia was examined. A Wilcoxon-signed rank test was performed for each treatment group to assess whether the distribution of the change from baseline was centered at zero. Between-group comparisons were performed on these change-from-baseline variables using the same method as for the primary analysis. Between-group comparisons were also performed using an ANCOVA model.
RESULTS
Patient Disposition
A total of 210 patients were screened for study inclusion, and 153 patients were randomly assigned to treatment and received double-blind study drug; 142 patients (92.8%) completed the study. There were 7 discontinuations in the placebo group (3 due to AEs, 1 due to protocol violation, 1 due to voluntary withdrawal, and 2 due to lack of efficacy) and 4 in the eszopiclone group (2 due to adverse events and 2 due to voluntary withdrawal). Demographic characteristics and disease severity (including disease-modifying medication use) at baseline were not significantly different between the 2 treatment groups, although both the percentage of males (18.4% vs 7.8%, P = .054) and the mean duration of morning stiffness were higher in the placebo group than in the eszopiclone group (110.8 min vs 82.9 min, respectively, P = .074) (Table 1).
Table 1.
Baseline Demographics and Rheumatoid Arthritis (RA) Parameters
| Characteristic | Placebo (n = 76) | Eszopiclone (n = 77) | P Value |
| Gender, n (%) | |||
| Male | 14 (18.4) | 6 (7.8) | .054 |
| Female | 62 (81.6) | 71 (92.2) | |
| Race, n (%) | |||
| White | 63 (82.9) | 65 (84.4) | .97 |
| Black | 9 (11.8) | 9 (11.7) | |
| Hispanic | 3 (3.9) | 2 (2.6) | |
| Age, mean (SD), y | 51.9 (9.5) | 52.3 (8.1) | .79 |
| BMI, mean (SD) (kg/m2), male | 30.8 (4.9) | 27.4 (3.6) | .14 |
| BMI, mean (SE) (kg/m2), female | 30.7 (6.6) | 31.0 (9.6) | .83 |
| RA parameters, mean (SD) | |||
| ASES scorea | |||
| Overall | 6.2 (1.9) | 6.0 (2.0) | .51 |
| Pain | 5.3 (1.9) | 5.2 (2.3) | .73 |
| Function | 6.7 (2.4) | 6.4 (2.5) | .43 |
| Other symptoms | 6.1 (2.1) | 6.0 (2.3) | .72 |
| Pain and other symptoms | 5.8 (1.9) | 5.7 (2.0) | .69 |
| ACR response criteria | |||
| Swollen joint count | 6.9 (6.6) | 6.6 (5.9) | .76 |
| Tender joint count | 9.2 (8.3) | 8.0 (7.3) | .29 |
| Subject pain severity | 4.9 (2.5) | 5.1 (2.3) | .54 |
| IVRS measures | |||
| Pain severity (previous day)b | 1.8 (0.9) | 1.8 (0.9) | .99 |
| Pain severity (current day)b | 1.7 (0.9) | 1.6 (0.9) | .50 |
| Duration of morning stiffness | 110.8 (105.0) | 82.9 (82.4) | .074 |
ASES scoring on a 0–10 scale (10 = best function and least pain).
0 = none, 1 = mild, 2 = discomforting, 3 = distressing, 4 = horrible, and 5 = excruciating.
Abbreviations: ACR = American College of Rheumatology, ASES = Arthritis Self-Efficacy Scale, BMI = body mass index, IVRS = interactive voice response system.
Sleep Outcomes
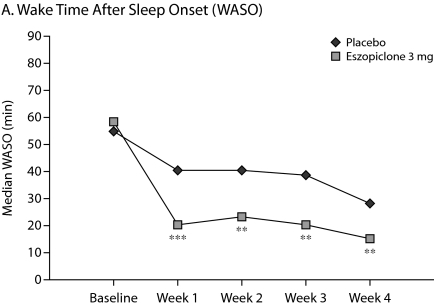
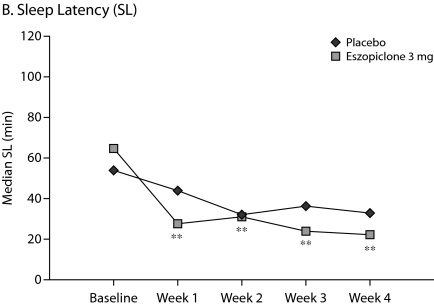
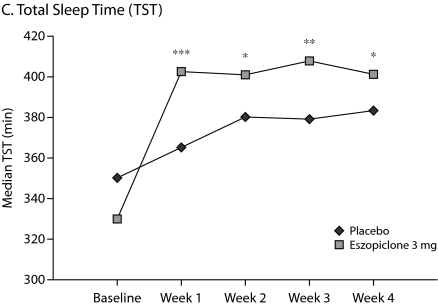
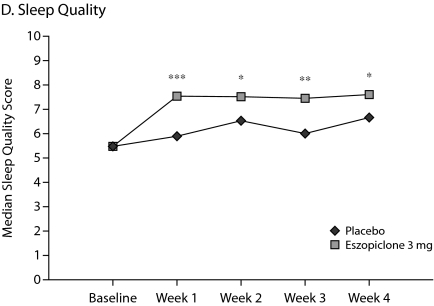
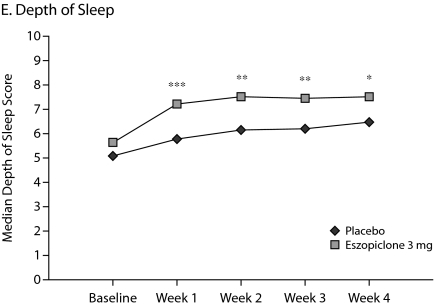
The primary outcome measure, WASO, was significantly lower in the eszopiclone group when compared with placebo (medians: 20.0 min vs 40.0 min with placebo, P < .0001, Figure 1A) for the first week of treatment. The results were similar for SL during week 1 (medians: 27.0 min vs 43.8 min with placebo, P = .0003, Figure 1B), and TST was significantly higher relative to placebo (medians: 402.0 min vs 364.7 min with placebo, P < .0001, Figure 1C) in the eszopiclone group during the first week. Significantly better improvement in the eszopiclone group when compared with placebo for sleep quality (medians: 7.5 vs 5.9 with placebo, P < .0001, Figure 1D) and depth of sleep (medians: 7.3 vs 5.8 with placebo, P < .0001, Figure 1E) was also observed during the first week in this study.
Eszopiclone treatment also significantly (P < .05) improved the change from baseline in WASO, TST, SL, depth of sleep, and quality of sleep for every week relative to placebo. In addition, at week 4, eszopiclone treatment significantly improved the change from baseline in WASO (P < .01), TST (P < .0001), SL (P < .01), depth of sleep (P < .0001), and quality of sleep (P < .0001) compared to placebo.
Figure 1.
Median at Baseline and Weeks 1, 2, 3, and 4a
aAnalysis of covariance change from baseline vs. placebo.
*P < .05.
**P < .01.
***P < .0001.
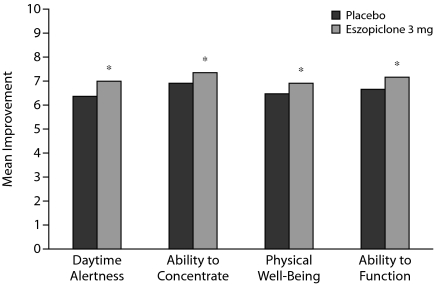
For all daytime functioning measures, mean scores with eszopiclone were significantly higher (better functioning) compared with placebo for the double-blind average (all P values < .05) (Figure 2). Treatment with eszopiclone also resulted in significantly higher scores for mean daytime alertness and ability to function at every time point compared with placebo (all P values < .05, data not shown). Similarly, results for mean change from baseline showed significant treatment group differences for ability to function and daytime alertness at all assessed time points and the double-blind average, for physical well-being at week 2, and for ability to concentrate at weeks 2 and 3 and the double-blind average (all P values < .05, data not shown).
Figure 2.
Daytime Functioning: Mean Double-Blind Averagesa
aThe analysis used an analysis of variance on rank-transformed data with treatment and site as fixed effects.
*P < .05.
Treatment with eszopiclone significantly improved the ISI total score compared with placebo at both week 2 (means: 8.5 for eszopiclone vs 12.5 for placebo) and week 4 (means: 8.5 vs 11.9) (all P values < .05). At week 4, there were significantly more eszopiclone-treated patients (47.9%) with no clinically significant insomnia (ISI total score ≤ 7) than placebo-treated subjects (30.4%, P = .033). Additionally, ISI “extended” items of sleep quality (mean change for eszopiclone = 1.30, placebo =0.43, P < .0001), feeling refreshed/rested (0.79, 0.42, P = .0013), daytime fatigue (–1.08, –0.46, P = .0002), relationship enjoyment (–0.74, –0.38, P = .02), and nights per week sleep difficulties (–2.49, –1.16, P = .0008) were significantly improved with eszopiclone compared to placebo.
Quality of Life and RA-Related Outcomes
At week 4, the eszopiclone group showed significantly greater improvement in 2 of the 8 domains of the SF-36 (role physical and bodily pain) compared with the placebo group (P < .05, Table 2). No significant differences between treatments were observed in the other SF-36 domains. One of the 8 domains of the HAQDI (activities) showed significantly greater improvement with eszopiclone compared with placebo (P < .05, Table 2). No significant differences between treatments were observed in the other HAQDI domains.
Table 2.
Week 4 Health Assessment Questionnaire-Disability Index, Pain, and Rheumatoid Arthritis (RA) Assessments for Patients Taking Placebo or Eszopiclonea
| Placebo |
Eszopiclone |
||||
| Outcome Measure | Observed Value | Change From Baseline | Observed Value | Change From Baseline | P Valueb |
| SF-36 score | |||||
| General health | 52.27 (21.57) | 0.84(11.94) | 54.05 (22.57) | 2.41 (12.94) | .2 |
| Health transition | 2.93 (0.96) | 0.05 (0.84) | 2.71 (1.02) | −0.26 (0.78) | .99 |
| Physical functioning | 48.47 (26.67) | −0.92(11.24) | 51.62(26.88) | 1.01 (13.27) | .1 |
| Role physical | 37.16(39.96) | −9.80(41.75) | 44.16(39.09) | 2.6 (35.49) | .03 |
| Role emotional | 69.82 (41.72) | 1.80(38.58) | 64.50 (39.86) | −4.33 (42.01) | .8 |
| Social functioning | 69.67 (24.59) | 0.17(21.31) | 74.84 (26.24) | 3.57 (20.86) | .1 |
| Bodily pain | 47.20 (20.72) | −0.88 (15.76) | 53.35 (20.92) | 4.81 (17.53) | .01 |
| Vitality | 40.81 (21.45) | 5.56(19.58) | 46.93 (24.30) | 6.55 (20.53) | .2 |
| HAQDI scorec | |||||
| Overall | 0.93 (0.73) | −0.09 (0.46) | 1.06(0.71) | −0.06 (0.48) | .7 |
| Dressing/grooming | 0.87 (0.93) | −0.13 (0.82) | 1.05 (0.97) | −0.09 (0.89) | .8 |
| Arising | 0.92 (0.92) | −0.26 (0.75) | 1.23 (0.89) | 1.23 (0.89) | .99 |
| Eating | 0.92 (0.92) | −0.14(0.74) | 1.19(0.95) | −0.06 (0.86) | .9 |
| Walking | 1.00(0.91) | −0.05 (0.66) | 1.13 (0.95) | 0.03 (0.73) | .8 |
| Hygiene | 1.16(1.18) | −0.04 (0.79) | 1.12(1.06) | −0.04 (0.64) | .5 |
| Reach | 0.86 (0.83) | −0.11 (0.59) | 0.85 (0.82) | −0.14(0.62) | .4 |
| Grip | 0.64(0.71) | −0.11 (0.57) | 0.81 (0.69) | −0.06 (0.58) | .8 |
| Activities | 1.11 (0.87) | 0.14(0.75) | 1.04(0.85) | −0.14(0.68) | .04 |
| Pain severity score (previous day)d | 1.71 (0.98) | −0.07 (0.71) | 1.54(0.87) | −0.22 (0.68) | .09 |
| Pain severity score (current day)d | 1.60 (1.00) | −0.11 (0.66) | 1.43 (0.93) | −0.17(0.65) | .3 |
| Duration of morning stiffness (min) | 93.99 (98.87) | −17.91 (69.11) | 90.10(122.44) | −0.26 (92.22) | .8 |
| ACR RA joint assessment | |||||
| Swollen joint count | 6.25 (6.57) | −0.97 (5.87) | 5.61 (5.40) | −0.97 (5.87) | .2 |
| Tender joint count | 8.96 (8.86) | −0.37 (6.59) | 6.43 (6.51) | −1.53 (4.35) | .03 |
| Subjective Pain Severity | 5.08 (2.73) | 0.47 (2.58) | 4.61 (2.69) | −0.47 (2.58) | .023 |
| Assessment scale score | |||||
| ASES scoree | |||||
| Overall | 6.23 (2.02) | 0.17(1.53) | 6.54(1.93) | 0.61 (1.41) | .05 |
| Pain | 5.22(1.96) | −0.09 (2.06) | 5.87 (1.98) | 0.67 (2.09) | .006 |
| Function | 6.76 (2.65) | 0.26(1.63) | 6.88 (2.49) | 0.57 (1.73) | .2 |
| Other symptoms | 6.27 (2.19) | 0.21 (1.91) | 6.55(2.11) | 0.5 (1.75) | .1 |
| Pain and other symptoms | 5.79 (1.88) | 0.09 (1.75) | 6.24(1.93) | 0.62(1.50) | .02 |
Data are presented as mean (SD).
P values from 1-sided analysis of covariance with change from baseline as the dependent variable, treatment and site as fixed effects, and baseline as the covariate.
Negative changes in HAQDI scales are improvements.
0 = none, 1 = mild, 2 = discomforting, 3 = distressing, 4 = horrible, and 5 = excruciating.
Higher scores are better.
Abbreviations: ACR = American College of Rheumatology, ASES = Arthritis Self-Efficacy Scale, HAQDI = Health Assessment Questionnaire-Disability Index, SF-36 = 36-item Medical Outcomes Study Short-Form SF-36 General Health Survey.
There was significantly (P = .05) greater improvement in the overall ASES change from baseline at week 4 with eszopiclone compared to placebo. This finding was driven primarily by significantly greater improvement in the pain-related scores (Table 2). The Subjective Pain Severity Assessment scale also showed significantly greater improvement from baseline to week 4 with eszopiclone compared to placebo (P < .05). There were significantly greater reductions from baseline in tender joint counts at week 4 with eszopiclone relative to placebo (P = .03). However, swollen joint counts were not significantly different between the treatment groups.
Eszopiclone-treated patients did not have a larger decrease in morning stiffness compared with placebo-treated patients (P = .8). Nor were there significant dif ferences between eszopiclone and placebo for the subject global assessment score, ACR-based physician global assessment score, C-reactive protein level, or overall HAQDI score at week 4. No significant differences in the percentages of patients with an increase in dose or with a new prescription for either pain or disease-modifying medications were observed between treatment groups.
Single-Blind Placebo Run-Out Period
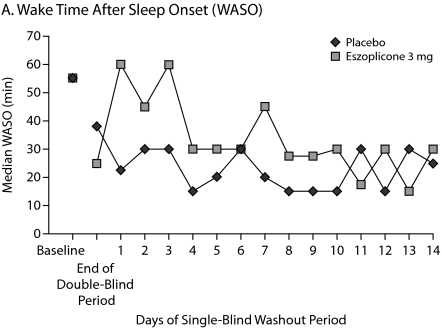
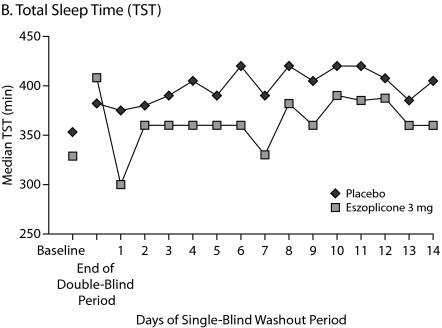
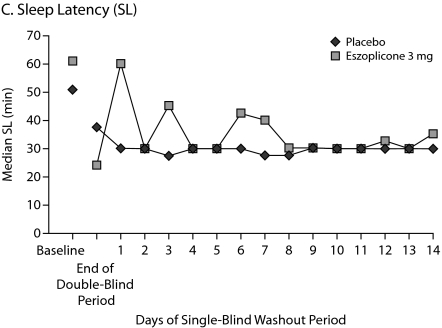
Patients were studied for an additional 2 weeks after discontinuation of treatment to assess potential rebound effects. There was a small, transient increase in WASO on days 1 and 3 of the run-out period, but these increases were not significantly greater than baseline. WASO values in the eszopiclone group were below baseline from day 4 to the end of the study (Figure 3A). Similarly, after a transient decrease in TST on day 1 of the run-out period, which was significantly lower than baseline (P = .01), TST values in the eszopiclone group were at or above baseline thereafter (Figure 3B). Sleep latency was significantly below baseline throughout the run-out period (P < .05: week 3, week 4, and double-blind average) (Figure 3C). Adverse events occurring after eszopiclone discontinuation included 3 patients with increased symptoms of RA (3.9%) and 2 patients with tremor (2.6%); no other events occurred in more than 1 patient.
Figure 3.
Median Minutes at Baseline, End of Double-Blind Phase, and Each of 14 Days During the Single-Blind Placebo Washout Period
Safety Outcomes
Overall AE rates (Table 3) were 67.5% in the eszopiclone group and 60.5% in the placebo group. The most frequently reported AEs in eszopiclone-treated patients were unpleasant taste (27.3%) and increased symptoms of RA (18.2%) versus 0% and 9.2% in placebo-treated patients, respectively. The proportion of subjects with AEs assessed by the investigator as related to treatment was similar between treatment groups with the exception of headache (7.8% in the eszopiclone group and 3.9% in the placebo group), pharyngitis (5.2% and 0%), and unpleasant taste (27.3% and 0%). The incidence of other clinically relevant central nervous system AEs was low in both the eszopiclone and placebo groups (Table 3).
Table 3.
Adverse Events (all causalities) During the Double-Blind Placebo Treatment Perioda
| Adverse Event | Placebo (n = 76) | Eszopiclone (n = 77) |
| Overall | 46 (60.5) | 52 (67.5) |
| Occurrence > 3% in either group | ||
| Unpleasant taste | 0 (0) | 21 (27.3) |
| Headache | 6 (7.9) | 8 (10.4) |
| Somnolence | 2 (2.6) | 3 (3.9) |
| Asthenia | 2 (2.6) | 5 (6.5) |
| Viral infection | 2 (2.6) | 5 (6.5) |
| Respiratory infection | 7 (9.2) | 3 (3.9) |
| Pharyngitis | 2 (2.6) | 8 (10.4) |
| Cough increased | 1 (1.3) | 3 (3.9) |
| Accidental injury | 4 (5.3) | 1 (1.3) |
| Rheumatoid arthritis | 7 (9.2) | 14 (18.2) |
| Back pain | 3 (3.9) | 1 (1.3) |
| Other clinically relevant central nervous system adverse events | ||
| Overall | 8 (10.5) | 10 (13.0) |
| Somnolence | 2 (2.6) | 3 (3.9) |
| Dizziness | 2 (2.6) | 1 (1.3) |
| Nervousness | 1 (1.3) | 1 (1.3) |
| Abnormal dreams | 0 (0) | 1 (1.3) |
| Neuralgia | 0 (0) | 1 (1.3) |
| Abnormal thinking | 0 (0) | 2 (2.6) |
Data are presented as n (%).
There were 2 serious AEs during the trial, 1 in each treatment group, that were judged by the investigator as not related to study drug. An event of severe chest pain occurred in an eszopiclone-treated patient 3 days after completing the double-blind treatment period (during the single-blind placebo run-out period). After the patient was admitted to a hospital, cardiac causes were ruled out, and the chest pain was judged to be musculoskeletal in etiology. There was no medication change, and the patient completed the study. One placebo-treated patient was diagnosed with papillary transitional cell carcinoma of the bladder 5 days after starting double-blind placebo treatment. Three placebo-treated patients (3.9%) had AEs leading to discontinuation (lupus erythematosus, bladder carcinoma, and heart palpitation). One eszopiclone-treated patient (1.3%) discontinued because of unpleasant taste.
DISCUSSION
The results of this preliminary study suggest that nightly treatment with eszopiclone 3 mg in patients with insomnia comorbid with RA resulted in significant improvements in self-reported measures of sleep induction, maintenance, duration, depth, and quality and daytime insomnia symptoms, whether measured by telephone diaries or by the validated ISI in the clinic, compared to placebo.
While there were no treatment differences in most of the SF-36 domains, patients treated with eszopiclone reported significant improvement in the role physical and bodily pain domains relative to patients treated with placebo. While these 2 SF-36 domains have previously been found to be sensitive to change in patients with RA,34 the difference observed in this study could have been due to an unexplained deterioration in the role physical items in the placebo group, rather than improvements in the eszopiclone group.
In a 6-month study of nightly eszopiclone in patients with primary chronic insomnia who did not have RA, sleep and quality of life were consistently improved, but the SF-36 domains of vitality and social functioning showed the greatest improvements.35 These results suggest that improvements in sleep perceptions may affect different aspects of quality of life depending on whether RA and chronic insomnia are both present or only chronic insomnia (without RA) is present.
Treatment group differences were found in the activities domain of the HAQDI and the pain and pain and other symptoms domains of the ASES. These results along with the improvements in SF-36 domains of bodily pain and role physical and the significant reduction in tender joint counts with eszopiclone suggest that improved perception of sleep with 4 weeks of eszopiclone treatment was associated with reduced perception of pain as measured using a variety of validated assessments. However, caution must be used when interpreting these secondary endpoints, as no adjustments were made for multiple comparisons.
Previous studies of the treatment of sleep problems in RA patients have yielded mostly discouraging findings. Nonsteroidal anti-inflammatory drugs (tenoxicam, diclofenac) helped with pain-related RA symptoms but did not improve sleep in RA patients.19 Some early small pilot studies suggested the combination of a NSAID and benzodiazepine might be useful for both the pain and sleep disturbance in RA,36,37 but these results have not been confirmed in larger studies.
In a small study, a benzodiazepine receptor agonist showed improvements in sleep but did not show improvements in pain or other RA-related symptoms.37 Moreover, conventional sleep assessments showed only small changes during treatment. Thus, the current findings are more encouraging based on improvements relative to placebo on both self-reported sleep parameters and pain-related measures (perception of pain, tender joint count, and ability to function).
Unlike previously published studies of eszopiclone in patients with primary insomnia35,38 or insomnia comorbid with depression,23 generalized anxiety disorder,24 or perimenopausal transition,25 there was some evidence of rebound insomnia in the current study on the first night after discontinuation with respect to TST but not for SL or WASO. Further investigations of rebound insomnia in patients with RA are needed to determine whether these patients are differentially affected by eszopiclone discontinuation.
There was a higher incidence of AEs coded to “increased symptoms of rheumatoid arthritis” with eszopiclone treatment relative to placebo (18.2% vs 9.2%), which may be clinically relevant. In the current study, eszopiclone did not worsen patients’ RA symptoms over 4 weeks of treatment, as the majority of AEs coded as increased symptoms of RA lasted < 48 hours, and all but 2 of the 21 events were assessed by the investigator as unrelated to treatment. Moreover, only 2 AEs required a change in treatment (1 in each treatment group), and none led to study drug discontinuation.
A number of limitations of this study are important to consider in interpreting the findings. First, this was a pilot study in which 1-sided significance levels were used, and there were no adjustments made for multiple comparisons for secondary endpoints. Second, the restrictions on use of concomitant medications may limit the generalizability of the findings to the full population of patients with RA. Third, the study duration was only 4 weeks; it remains to be seen whether a longer duration may yield larger benefit. Finally, no objective assessments of sleep or RA were conducted.
In summary, eszopiclone treatment compared to placebo resulted in statistically significant improvements in self-report measures of perceived sleep. Eszopiclone was also associated with statistically significant reductions in some pain parameters and measures of health-related quality of life (bodily pain and role physical), physical activities, and ability to function compared to placebo in patients with insomnia comorbid with RA. On the basis of these findings, future studies of eszopiclone in RA and other pain disorders with comorbid insomnia are warranted.
Drug names: azathioprine (Azasan, Imuran, and others), bupropion (Aplenzin, Wellbutrin, and others), diclofenac (Cambia, Cataflam, and others), duloxetine (Cymbalta), eszopiclone (Lunesta), gabapentin (Neurontin and others), methotrexate (Trexall and others), mirtazapine (Remeron and others), oxycodone (Oxycontin, Roxicodone, and others), propoxyphene (Dolene, Darvon, and others), sulfasalazine (Azulfidine and others), tramadol (Ultram, Ryzolt, and others), triazolam (Halcion and others), venlafaxine (Effexor and others).
Potential conflicts of interest: Dr Roth has served as a consultant to Abbott, Accadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis AVER, Bristol-Myers Squibb, BTG, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Impax, Intec, Intra-Cellular, Jazz, Johnson & Johnson, King, Ludbeck, McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Proctor & Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering-Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport; has received grant/research support from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport; and has served on the speakers’ bureaus of Cephalon, Sanofi, and Takeda. Dr Price is an employee of Sepracor. Drs Amato, Rubens, and Roach are former employees of Sepracor. Drs Price, Amato, and Rubens are stock shareholders in Sepracor. Dr Schnitzer reports no financial or other affiliations relevant to the subject of this article.
Funding/support: Financial support for this study was provided by Sepracor Inc, Marlborough, Massachusetts.
Previous presentation: These data were presented at the American College of Rheumatology Annual Scientific Meeting, November 12–17, 2005, San Diego, California.
Acknowledgments
The authors wish to acknowledge Paul Crits-Cristoph, PhD, of Paladin Consulting Group, Westchester, Pennsylvania, and Amy Wilson, PhD, and Jacqueline Zummo, MS, of Sepracor Inc, Marlborough, Massachusetts, for their assistance in the preparation of this manuscript and Phebe Wilson, PhD of Sepracor for her assistance with the statistical analysis.
REFERENCES
- 1.Centers for Disease Control and Prevention. Health-related quality of life among adults with arthritis: Behavioral Risk Factor Surveillance System, 11 states, 1996–1998. MMWR Morb Mortal Wkly Rep. 2000;49(17):366–369. [PubMed] [Google Scholar]
- 2.Hagen KB, Kvien TK, Bjorndal A. Musculoskeletal pain and quality of life in patients with noninflammatory joint pain compared to rheumatoid arthritis: a population survey. J Rheumatol. 1997;24(9):1703–1709. [PubMed] [Google Scholar]
- 3.Hill CL, Parsons J, Taylor A, et al. Health related quality of life in a population sample with arthritis. J Rheumatol. 1999;26(9):2029–2035. [PubMed] [Google Scholar]
- 4.Devins GM, Edworthy SM, Paul LC, et al. Restless sleep, illness intrusiveness, and depressive symptoms in three chronic illness conditions: rheumatoid arthritis, end-stage renal disease, and multiple sclerosis. J Psychosom Res. 1993;37(2):163–170. doi: 10.1016/0022-3999(93)90083-r. [DOI] [PubMed] [Google Scholar]
- 5.Drewes AM, Nielsen KD, Hansen B, et al. A longitudinal study of clinical symptoms and sleep parameters in rheumatoid arthritis. Rheumatology (Oxford) 2000;39(11):1287–1289. doi: 10.1093/rheumatology/39.11.1287. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch M, Carlander B, Verge M, et al. Objective and subjective sleep disturbances in patients with rheumatoid arthritis: a reappraisal. Arthritis Rheum. 1994;37(1):41–49. doi: 10.1002/art.1780370107. [DOI] [PubMed] [Google Scholar]
- 7.Kirwan J, Heiberg T, Hewlett S, et al. Outcomes from the Patient Perspective Workshop at OMERACT 6. J Rheumatol. 2003;30(4):868–872. [PubMed] [Google Scholar]
- 8.Mahowald MW, Mahowald ML, Bundlie SR, et al. Sleep fragmentation in rheumatoid arthritis. Arthritis Rheum. 1989;32(8):974–983. doi: 10.1002/anr.1780320806. [DOI] [PubMed] [Google Scholar]
- 9.Nicassio PM, Wallston KA. Longitudinal relationships among pain, sleep problems, and depression in rheumatoid arthritis. J Abnorm Psychol. 1992;101(3):514–520. doi: 10.1037//0021-843x.101.3.514. [DOI] [PubMed] [Google Scholar]
- 10.Lavie P, Epstein R, Tzischinsky O, et al. Actigraphic measurements of sleep in rheumatoid arthritis: comparison of patients with low back pain and healthy controls. J Rheumatol. 1992;19(3):362–365. [PubMed] [Google Scholar]
- 11.Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53(6):911–919. doi: 10.1002/art.21584. [DOI] [PubMed] [Google Scholar]
- 12.Smith MT, Perlis ML, Smith MS, et al. Sleep quality and presleep arousal in chronic pain. J Behav Med. 2000;23(1):1–13. doi: 10.1023/a:1005444719169. [DOI] [PubMed] [Google Scholar]
- 13.Wittig RM, Zorick FJ, Blumer D, et al. Disturbed sleep in patients complaining of chronic pain. J Nerv Ment Dis. 1982;170(7):429–431. doi: 10.1097/00005053-198207000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Moldofsky H, Lue FA, Smythe HA. Alpha EEG sleep and morning symptoms in rheumatoid arthritis. J Rheumatol. 1983;10(3):373–379. [PubMed] [Google Scholar]
- 15.Roehrs T, Hyde M, Blaisdell B, et al. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29(2):145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 16.Smith MT, Edwards RR, McCann UD, et al. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 17.Drewes AM, Bjerregard K, Taagholt SJ, et al. Zopiclone as night medication in rheumatoid arthritis. Scand J Rheumatol. 1998;27(3):180–187. doi: 10.1080/030097498440787. [DOI] [PubMed] [Google Scholar]
- 18.Currie SR, Wilson KG, Pontefract AJ, et al. Cognitive-behavioral treatment of insomnia secondary to chronic pain. J Consult Clin Psychol. 2000;68(3):407–416. doi: 10.1037//0022-006x.68.3.407. [DOI] [PubMed] [Google Scholar]
- 19.Lavie P, Nahir M, Lorber M, et al. Nonsteroidal antiinflammatory drug therapy in rheumatoid arthritis patients: lack of association between clinical improvement and effects on sleep. Arthritis Rheum. 1991;34(6):655–659. doi: 10.1002/art.1780340605. [DOI] [PubMed] [Google Scholar]
- 20.Simon GE, VonKorff M, Barlow W, et al. Predictors of chronic benzodiazepine use in a health maintenance organization sample. J Clin Epidemiol. 1996;49(9):1067–1073. doi: 10.1016/0895-4356(96)00139-4. [DOI] [PubMed] [Google Scholar]
- 21.Walsh JK, Muehlbach MJ, Lauter SA, et al. Effects of triazolam on sleep, daytime sleepiness, and morning stiffness in patients with rheumatoid arthritis. J Rheumatol. 1996;23(2):245–252. [PubMed] [Google Scholar]
- 22.Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30(8):959–968. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59(11):1052–1060. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Pollack M, Kinrys G, Krystal A, et al. Eszopiclone coadministered with escitalopram in patients with insomnia and comorbid generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):551–562. doi: 10.1001/archpsyc.65.5.551. [DOI] [PubMed] [Google Scholar]
- 25.Soares CN, Joffe H, Rubens R, et al. Eszopiclone in patients with insomnia during perimenopause and early postmenopause: a randomized controlled trial. Obstet Gynecol. 2006;108(6):1402–1410. doi: 10.1097/01.AOG.0000245449.97365.97. [DOI] [PubMed] [Google Scholar]
- 26.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 27.Lorig K, Chastain RL, Ung E, et al. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32(1):37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 28.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38(6):727–735. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 29.Hochberg MC, Chang RW, Dwosh I, et al. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35(5):498–502. doi: 10.1002/art.1780350502. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Snow KK, Kosinski M, et al. Boston, MA: The Health Institute, New England Medical Center; 1993. SF-36 Health Survey Manual and Interpretation Guide. [Google Scholar]
- 31.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the Health Assessment Questionnaire, disability and pain scales. J Rheumatol. 1982;9(5):789–793. [PubMed] [Google Scholar]
- 32.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 33.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26(7):793–799. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 34.Kosinski M, Keller SD, Ware JE, Jr, et al. The SF-36 Health Survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: relative validity of scales in relation to clinical measures of arthritis severity. Med Care. 1999;37(suppl 5):MS23–MS39. doi: 10.1097/00005650-199905001-00003. [DOI] [PubMed] [Google Scholar]
- 35.Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations 48. Sleep. 2007;30(8):959–968. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobkirk D, Rhodes M, Haslock I. Night medication in rheumatoid arthritis, II: combined therapy with indomethacin and diazepam. Rheumatol Rehabil. 1977;16(2):125–127. doi: 10.1093/rheumatology/16.2.125. [DOI] [PubMed] [Google Scholar]
- 37.Sharma BK, Haslock I. Night medication in rheumatoid arthritis, III: the use of sulindac. Curr Med Res Opin. 1978;5(6):472–475. doi: 10.1185/03007997809111917. [DOI] [PubMed] [Google Scholar]
- 38.Moldofsky H, Lue FA, Mously C, et al. The effect of zolpidem in patients with fibromyalgia: a dose-ranging, double-blind, placebo-controlled, modified crossover study. J Rheumatol. 1996;23(3):529–533. [PubMed] [Google Scholar]
- 38.Zammit GK, McNabb LJ, Caron J, et al. Efficacy and safety of eszopiclone across 6 weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20(12):1979–1991. doi: 10.1185/174234304x15174. [DOI] [PubMed] [Google Scholar]