Abstract
Objective:
To examine the diagnostic status of patients enrolled in the Factors Influencing Depression Endpoints Research (FINDER) study and symptomatic outcomes and baseline characteristics associated with remission 6 months after commencing antidepressant therapy.
Method:
Status of clinically diagnosed depressed patients was based on self-rated Hospital Anxiety and Depression Scale (HADS) scores. Five diagnostic categories were defined: noncaseness, mixed anxiety-depression (subthreshold depressive and anxious symptomatology), caseness for depression, caseness for anxiety, and caseness for comorbid anxiety-depression. Assessments included the Somatic Symptom Inventory and health-related quality of life (HRQoL) using the Medical Outcomes Study 36-item Short-Form Health Survey. Remission rates (based on HADS noncaseness for both depression and anxiety) and their associations with baseline characteristics were investigated. Patients were enrolled between May 2004 and September 2005.
Results:
Of the 3,353 patients enrolled, 66.4% met the HADS criteria for probable depressive disorder and 74.1% met the HADS criteria for probable anxiety disorder. Somatic symptom severity (painful and nonpainful) was highest and HRQoL was lowest in the comorbid anxiety-depression group. After 6 months, remission rates were 50.2% for caseness for depression, 40.4% for caseness for anxiety, and 40.6% for caseness for comorbid anxiety-depression. A lower number of previous depressive episodes, shorter current episode duration, lower painful and nonpainful somatic symptom scores, being married, a higher educational level, and working for pay were most consistently associated with higher remission rates.
Conclusions:
Physicians do not always differentiate between anxiety and depressive symptoms when making a clinical diagnosis of depression. At baseline, most enrolled patients had significant emotional depressive and anxious symptoms, as well as significant nonpainful and painful somatic symptomatology, and these factors were associated with outcome.
A strong relationship is expected between diagnosis and prescription pattern in psychiatry, but this is challenged by the available literature. The European Study on the Epidemiology of Mental Disorders (ESEMeD) investigated the relationship between the diagnosis of depressive or anxiety disorders and psychotropic medication use.1 The study found that less than one-third of subjects with a 12-month prevalence of major depressive disorder (MDD) were taking antidepressants, and the most important predictors of the use of antidepressants were seeking help for emotional problems and age rather than the presence of a formal DSM-IV diagnosis. This finding suggests that prescription of psychotropic medication may not always be in accordance with the licensed indications.
Clinicians do not always comply with diagnostic and treatment guidelines.2 One reason for this may be the limited ecological validity of the findings in antidepressant randomized controlled trials (RCTs), which aim to have a homogeneous patient sample by having multiple inclusion and exclusion criteria. However, these stringent criteria increase the likelihood that patients taking part in RCTs of antidepressants are not representative of the heterogeneous patient samples found in daily clinical practice.3 Two studies found that only about 14% of routine practice patients with MDD could be enrolled in RCTs, and that the main reason for exclusion was because of comorbid conditions.4–6
Another reason for noncompliance of clinicians with guidelines is the number of patients presenting with subthreshold conditions (eg, minor depressive disorder, recurrent brief depressive disorder, subsyndromal symptomatic depression, adjustment disorder with depressed mood).7,8 These conditions are highly prevalent and have a substantial impact on functioning.9 Given the limited data from RCTs for these subthreshold depressive disorders, evidence-based treatment recommendations cannot be given at this point.7 “Close monitoring and problem-solving therapy may be useful, and a treatment trial with one of the well-tolerated antidepressants is worth trying in more chronic and unremitting cases.”7(p82) The latter reason can logically result in off-label prescription of antidepressants.7
Clinical Points
♦ Physicians do not sufficiently differentiate between depressive and anxiety disorders.
♦ “Remission” 6 months after initiation of treatment is found in 50% of patients with a depressive disorder and in 40% of patients with an anxiety disorder.
♦ Sociodemographic variables (marital status, occupational status) are as powerful predictors as more clinical variables.
There are few studies of depressive disorder treatments in a naturalistic setting. The Factors Influencing Depression Endpoints Research (FINDER) study is a large (N = 3,468) European, prospective, observational study of health-related quality of life (HRQoL) outcomes in adult patients with a clinically diagnosed depressive episode at baseline (untreated) and at 3 and 6 months after commencing antidepressant medication.10–12 The only included psychometric tool allowing assessment of a formal diagnosis of a depressive or anxiety disorder in the FINDER study was the patient-rated Hospital Anxiety and Depression Scale (HADS), a well-known screening instrument.13 This questionnaire was originally used in the FINDER study as a patient-rated symptom severity scale.
This article examines the FINDER population from the perspective of their baseline HADS status (based on probable caseness, doubtful caseness, and noncaseness for depression and anxiety) and investigates the sociodemographic, clinical, and quality of life correlates of these patient subgroups. This article also investigates the symptomatic outcomes and the association of baseline characteristics with HADS diagnostic outcomes after 6 months of commencing antidepressant therapy.
METHOD
The FINDER study was a 6-month, prospective, observational study conducted in 12 European countries and designed to investigate the HRQoL of outpatients with depression initiating antidepressant treatment. Adult patients (N = 3,468) were eligible for enrollment when presenting within the normal course of care, were diagnosed by their physician (psychiatrist or general practitioner [GP], n = 1,818 and n = 1,650, respectively) as suffering from depression, and were about to commence antidepressant treatment for a first episode or new episode of recurrent depression. In order to keep the study as naturalistic as possible, no structured diagnostic criteria were requested for the diagnosis of depression. Assessments were performed at baseline and at 3 (± 1 month) and 6 months (± 1 month) during the follow-up period. Patients were enrolled between May 2004 and September 2005.
The detailed study design and methods have been reported elsewhere10 and are only briefly described here. The study was approved in all countries according to local requirements for ethics and/or regulatory approvals for observational studies, and all patients gave written informed consent.
Psychometric Instruments
Symptom scales assessed at baseline and at 3 and 6 months' follow-up.
The symptom scales used in the present study assessed both emotional and somatic symptoms. Emotional symptoms were assessed with the HADS,13 a well-documented screening instrument for anxiety and depression, in order to have a more detailed evaluation during treatment. For both the anxiety (HADS-A) and the depression (HADS-D) subscales, a score of 0–7 can be regarded as within a normal range (noncase), while a score ≥ 11 indicates a probable case; scores between 8 and 10 are suggested to be doubtful cases.
In an attempt to align all potential combinations of subscale ratings (for anxiety: noncase [A–], doubtful case [A?], and probable case [A+]; for depression: noncase [D–], doubtful case [D?], and probable case [D+]) with the more broadly used DSM-IV diagnostic categories, the following 5 subgroups were defined:
(1) noncaseness: D–A–, D–A?, or D?A–
(2) mixed anxiety-depression: D?A? (subthreshold depressive and anxious symptomatology)
(3) caseness for depression: D+A– or D+A?
(4) caseness for anxiety: D–A+ or D?A+
(5) caseness for comorbid anxiety-depression: D+A+
All patients with complete HADS ratings (at baseline as well as at 3- and 6-months' follow-up) were classified within these diagnostic categories.
The change in HADS symptoms of these patients were investigated as follows. Remission, traditionally defined as reaching a status of no significant symptomatology, was defined as D–A– at 3 and 6 months, independent of caseness status at baseline. Nonremission or still caseness was defined in different ways depending on the baseline status.
(1) For those with caseness for depression (D+A–, D+A?) at baseline, still caseness was defined as D+A+ (worsening to comorbid anxiety-depression) or D+A– or D+A?.
(2) For those with caseness for anxiety (D–A+, D?A+) at baseline, still caseness was defined as D+A+ (worsening to comorbid anxiety-depression) or D–A+ or D?A+.
(3) For those with caseness for comorbid anxiety-depression (D+A+) at baseline, 3 subgroups of still caseness were defined: caseness for depression (D+A–, D+A?), caseness for anxiety (D–A+, D?A+), and comorbid anxiety-depression (D+A+).
Somatic symptoms were assessed with the 28-item Somatic Symptom Inventory (SSI-28).14,15 The SSI is a patient self-report scale that assesses the degree to which various physical complaints have been bothersome to the patient. Each complaint is rated on a defined scale from 1 (not at all) to 5 (great deal). The pain subscale (SSI-pain) is derived by calculating the mean score over 7 pain-related items, and the somatic subscale (SSI-somatic) uses the remaining 21 items. Overall pain severity was also rated using a visual analog scale (VAS) from the extremes of experiencing “no pain” (0 mm) to experiencing pain “as severe as I can imagine” (100 mm). A threshold score of 30 mm has been identified to distinguish no or mild pain from moderate/severe pain.16,17
HRQoL scale assessed at baseline and at 3 and 6 months' follow-up.
HRQoL was assessed using the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36).18 The SF-36 consists of 36 questions that generate scores across 8 health domains (4 mental and 4 physical subscales) and 2 summary scores, the Physical Component Score (PCS) and the Mental Component Score (MCS). Each subscale is scored by summing the individual terms and transforming the scores into a 0 to 100 scale, with higher scores indicating better quality of life. Any score below 50 indicates worse than average quality of life.19 As several of the 8 health domains of the SF-36 are known to show considerable content overlap with symptom scales, this article reports on the 2 component summary scores and on the 3 domains that specifically assess functioning (role-physical, role-emotional, and social functioning).
Sociodemographic and psychiatric history variables at baseline.
The following sociodemographic variables were collected: age, gender, education (further or none/mandatory), marital status (married/domestic partner or other), occupational status (working for pay, unemployed, or other), number of dependents, body mass index (BMI), and current smoker.
The following clinical variables were collected: duration of current depressive episode, number of previous episodes of depression, age at first depressive episode, patients with at least 1 additional psychiatric illness in previous 2 years, and presence of any chronic medical condition.
Statistical Methods
All eligible patients with nonmissing HADS data at the first observation were included in the present analyses. Counts and percentages of patients falling into the defined diagnostic categories at baseline were calculated, and suitable summary statistics are presented for the characteristics of these patients. In the text, differences between the diagnostic categories at baseline are noted descriptively only since no direct statistical comparisons were made due to the multiple confounding factors that affect the characteristics observed. For patients with follow-up data on the HADS, percentages of still case and remitted patients, as defined above, were calculated.
For each of the 3 baseline categories of caseness for depression, caseness for anxiety, and caseness for comorbid anxiety-depression (ie, subgroups 3, 4, and 5), logistic regression analyses were performed to model each of the log odds of still caseness and of remission, as defined above, using backward elimination methods. Country was retained in all models even if not statistically significant. Results are reported using Wald χ2 statistics, and P values and odds ratios and their 95% CIs are presented for all statistically significant (P ≤ .05) independent variables in the model.
In the figures, independent variables are placed in order of the strength of the association with the dependent variable, with the most strongly associated variable at the top of the figure. The independent variables included in the models were as follows: age, gender, education (none/mandatory or further), occupational status (working for pay, unemployed, or other), marital status (married/domestic partner or other), BMI, number of dependents, smoking, country, number of previous episodes of depression, age at first MDD episode, any psychiatric illnesses in the 24 months before baseline, duration (at baseline) of the current MDD episode, baseline HADS anxiety and depression scores, VAS overall pain and SSI-somatic scores, and any chronic medical conditions.
RESULTS
Results at Baseline
Of the 3,468 eligible patients at baseline, 3,353 had nonmissing HADS ratings and were included in the current analyses.
Baseline HADS subgroups and diagnostic categories.
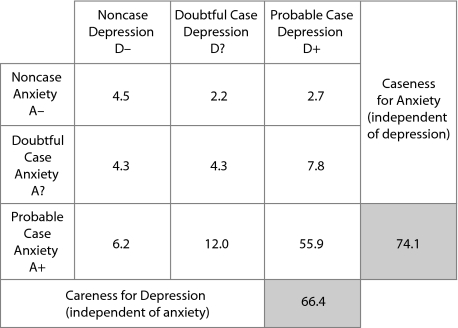
Tables 1A and 1B show the distribution of the 3,353 patients for each HADS caseness subgroup and HADS diagnostic category, respectively (status for depression: no, doubtful, or probable case and status for anxiety: no, doubtful, or probable case). Caseness for depression was identified in 66.4% of patients (irrespective of anxiety) and for anxiety in 74.1% of patients (irrespective of depression). When the HADS caseness subgroups were determined by investigator specialty (GP/psychiatrist), we observed some differences in the proportions of patients in the subgroups: probable case for depression was found in 59.8% and 72.5% of patients enrolled by GPs and psychiatrists, respectively; probable case for anxiety was found in 75.6% and 72.7% of patients enrolled by GPs and psychiatrists, respectively; and comorbid anxiety-depression was found in 52.6% and 58.9% of patients enrolled by GPs and psychiatrists, respectively. The sociodemographic and psychiatric history data for each diagnostic category are shown in Table 2.
Table 1A.
Baseline Distribution of All Patients by HADS Caseness Subgroups (N = 3,353)a
 |
aData are presented as %.
Abbreviation: HADS = Hospital Anxiety and Depression Scale.
Table 1B.
Baseline Distribution of All Patients by HADS Diagnostic Categories (N = 3,353)a
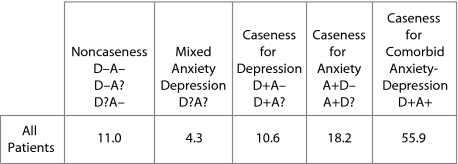 |
aData are presented as %.
Abbreviation: HADS = Hospital Anxiety and Depression Scale.
Table 2.
Sociodemographic and Psychiatric History Data for HADS Diagnostic Categories at Baseline
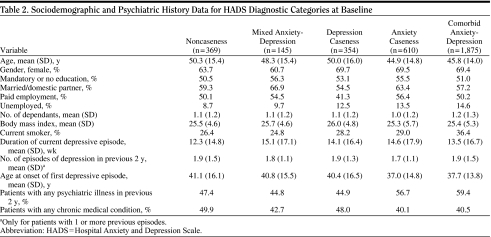 |
Baseline painful and nonpainful somatic symptom severity according to diagnostic category (Table 3).
Table 3.
Somatic Symptom Severity by HADS Diagnostic Categories at Baselinea,b
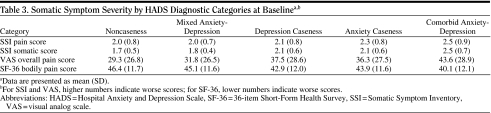 |
The comorbid anxiety-depression diagnostic category had the highest mean scores on the SSI-somatic, SSI-pain, and VAS overall pain severity subscales and the greatest impairment on the SF-36 bodily pain domain.
On the basis of the VAS overall pain severity subscale, moderate/severe pain was found in 40%, 43%, 51%, 54%, and 62% for noncaseness, mixed anxiety-depression, caseness for depression, caseness for anxiety, and caseness for comorbid anxiety-depression, respectively.
Baseline HRQoL (SF-36) according to diagnostic category (Table 4).
Table 4.
Health-Related Quality of Life (SF-36) by HADS Diagnostic Categories at Baselinea,b
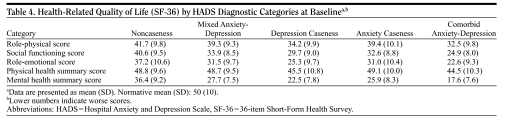 |
The mean PCS was only mildly impaired compared to population norms (from about 0.1 SD in the noncaseness subjects to 0.5 SD in the comorbid anxiety-depression subjects). On the contrary, the mean MCS was markedly impaired (from about 1.5 SDs in the noncaseness subjects to more than 3.0 SDs in the comorbid anxiety-depression subjects).
Physical functioning in the SF-36 (role-physical) was impaired from about 1.0 SD in the noncaseness subjects to more than 1.5 SDs in the comorbid anxiety-depression subjects. The functional impairment due to emotional problems (role-emotional) ranged from about 1.5 SDs in the noncaseness subjects to more than 2.5 SDs in the comorbid anxiety-depression subjects. The impairment in social functioning due to emotional or physical symptoms (social functioning) ranged from about 1.0 SD in the noncaseness subjects to about 2.5 SDs in the comorbid anxiety-depression subjects.
Results at Follow-Up
Still caseness and remission rates at 3 and 6 months' follow-up (Tables 5A, 5B, and 5C).
Tables 5A-C.
Still Caseness and Remission Proportions at 3 and 6 Months' Follow-Up for the Caseness Depression Category at Baselinea,b
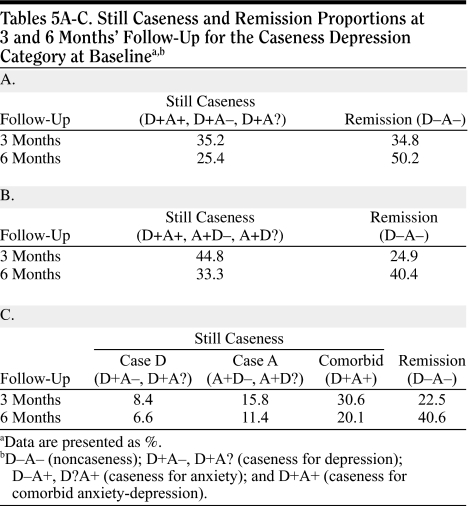 |
Remission rates were consistently higher at 6 months' follow-up than at 3 months' follow-up. Rates were also higher in the caseness for depression group (50%) than in the caseness for anxiety group (40%) or caseness for comorbid anxiety-depression group (41%). The caseness for anxiety and caseness for comorbid anxiety-depression groups had comparable remission rates. The percentage of patients with a moderate improvement (no longer fulfilling criteria for caseness but not fulfilling criteria for remission) was similar in the caseness for depression group, caseness for anxiety group, and caseness for comorbid anxiety-depression group (24%, 26%, and 21%, respectively). In noncaseness for depression subjects, the HADS-D and HADS-A scores changed (from baseline to 6 months) from 5.2 to 4.6 and from 9.6 to 6.7, respectively, and the SF-36 mental health score changed from 33.5 to 43.6. In noncaseness for anxiety subjects, the HADS-D and the HADS-A scores changed (from baseline to 6 months) from 8.0 to 5.4 and from 5.4 to 5.0, respectively, and the SF-36 mental health score changed from 32.7 to 43.5.
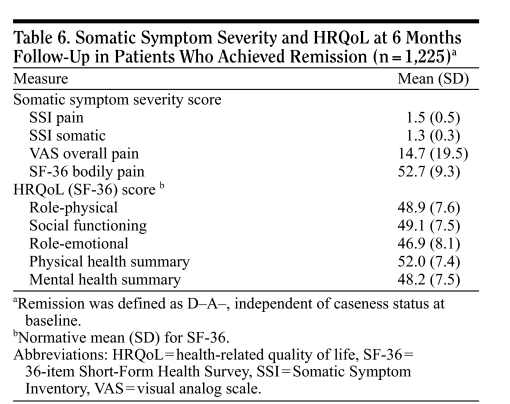
Somatic symptom severity and HRQoL at 6 months for patients in remission.
All patients in remission (from the 5 HADS diagnostic categories at baseline) were associated with normalization of symptomatic and HRQoL variables at 6 months' follow-up (Table 6), which provides some validation of the definition of remission used.
Table 6.
Somatic Symptom Severity and HRQoL at 6 Months Follow-Up in Patients Who Achieved Remission (n = 1,225)a
 |
Baseline factors associated with remission at 6 months' follow-up.
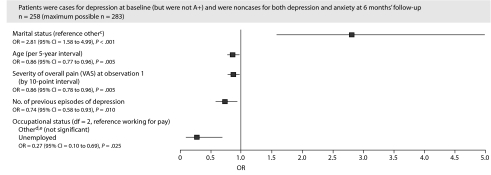
For the baseline caseness for depression category, a lower VAS overall pain severity score, a lower age, being married, working for pay, and a lower number of previous episodes of depression were associated with greater odds of achieving remission (Figure 1).
Figure 1.
Baseline Characteristics Associated With Categorical Status at 6 Months' Follow-Upa,b
aAn OR < 1 indicates decreased odds of remission.
bCountry not statistically significant (P = .252) but included in the model. Data from Norway were pooled with those from Sweden and Ireland with the United Kingdom to avoid problems of quasi separation during the modeling.
cOther includes divorced, legally separated, widowed, partner (living separately), and no relationship.
dOther includes retired, student, voluntary work, and keeping house (full-time).
eOther also significantly different from unemployed (OR = 2.58; 95% CI = 1.00 to 6.62).
Abbreviations: OR = odds ratio, VAS = visual analog scale.
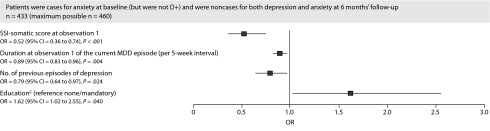
For the baseline caseness for anxiety category, a lower SSI nonpainful somatic subscore, a higher educational level, a shorter duration of the current episode of depression, and a lower number of previous episodes of depression were associated with greater odds of achieving remission (Figure 2).
Figure 2.
Baseline Characteristics Associated With Categorical Status at 6 Months' Follow-Upa,b
aAn OR < 1 indicates decreased odds of remission.
bCountry not statistically significant (P = .211) but included in the model.
cEducation includes further, university, and postgraduate education.
Abbreviations: MDD = major depressive disorder, OR = odds ratio, SSI = Somatic Symptom Inventory.
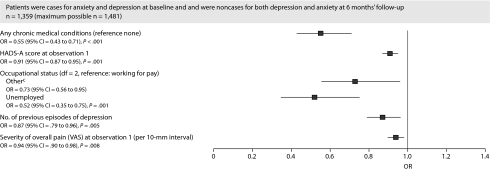
For the baseline caseness for comorbid anxiety-depression category, a lower baseline score on the HADS-A, a lower VAS overall pain score, working for pay, a lower number of previous episodes of depression, and the absence of a comorbid chronic medical condition were associated with greater odds of achieving remission (Figure 3).
Figure 3.
Baseline Characteristics Associated With Categorical Status at 6 Months' Follow-Upa,b
aAn OR < 1 indicates decreased odds of remission.
bCountry was statistically significant (P < .001).
cOther includes retired, student, voluntary work, and keeping house (full-time).
Abbreviations: HADS-A = Hospital Anxiety and Depression Scale-Anxiety, OR = odds ratio, VAS = visual analog scale.
DISCUSSION
In this naturalistic sample of patients with a clinical diagnosis of depression whose physician had decided to commence antidepressant treatment, only 66.4% of enrolled patients were probable cases for depression on the basis of self-rating on the HADS, while 74.1% of patients were probable cases for anxiety. When looking at “pure” caseness using the diagnostic criteria, 10.6% were probable cases for depression only and 18.2% were probable cases for anxiety only. This finding suggests that, in daily clinical practice, physicians do not always make a differential diagnosis between depressive disorders and anxiety disorders when they start an antidepressant treatment and that anxiety was a stronger predictor of enrolling a patient in treatment than depression. The percentage of probable cases for depression was lower in the patients enrolled by GPs (59.8% vs 72.5%), while the percentage of probable cases for anxiety was similar between GPs and psychiatrists (75.6% vs 72.7%). Surprisingly, 11.0% of the enrolled patients were neither cases for depression nor anxiety (with only minor differences between patients enrolled by GPs and psychiatrists: 12.3% vs 9.9%).
The appropriateness of the prescription of antidepressants has been questioned previously.2 A Finnish primary care study20 showed that psychotropic medication was prescribed to 70% of patients with mental disorder symptoms (when a wider definition than a formal diagnosis was used) and to 13% of patients with no sign of mental disorder. It has been shown that physicians use methods other than formal DSM-IV or ICD-10 criteria to make prescription decisions.2 Our finding that the percentage of noncase subjects receiving an antidepressant is slightly higher in GP-treated patients than in psychiatrist-treated patients confirms previously published data.21
Caseness for comorbid anxiety-depression was found to be slightly lower (52.6% vs 58.9%) in patients enrolled by GPs compared to patients enrolled by psychiatrists. Published figures of depression and anxiety comorbidity vary between 39% in a Australian general population sample22 and 59% in a German general population sample,23 and between 51% and 62% (comorbidity also included alcohol abuse) in depressed outpatient samples.24,25
The lowest scores on SSI pain symptoms and VAS overall pain severity domains and the least impairment on the SF-36 bodily pain subscale at baseline were found in the noncaseness and the mixed anxiety-depression patients (with subthreshold symptoms), with intermediate scores in the depression or anxiety caseness patients and the highest scores (reflecting greatest impairment) in those with comorbid anxiety-depression. This finding confirms that patients suffering from depressive disorders as well as patients suffering from anxiety disorders have more painful physical symptoms.26–29
An interesting finding is that the nonpainful somatic symptoms (as assessed with the SSI) show a similar pattern, suggesting that their relationship to depression and with the findings of STAR*D.36 First, baseline depression severity as measured by the HADS-D was not significantly associated with remission at 6 months, although other more indirect measures of depression severity (like number of previous episodes or duration of current episode) were. Baseline anxiety severity as measured with the HADS-A was significantly associated with remission at 6 months but only for the caseness for comorbid anxiety-depression subjects.
Second, baseline somatic symptom severity was significantly associated with remission at 6 months. Overall, both painful and nonpainful somatic symptoms were highly correlated, suggesting that they could be regarded as 1 symptom cluster. The presence of chronic medical conditions was also associated with remission at 6 months but only for the caseness for comorbid anxiety-depression subjects.
Third, sociodemographic factors (lower age, a higher educational level, working for pay, and being married) were associated with better outcomes. These findings are clinically important, since neither pharmacotherapy nor psychotherapy can modify them.
The main limitation of the present study is that all results are based on patient-rated scales and that HADS-based diagnostic categories were constructed in order to reflect more standard diagnostic categories. However, the outcome and the regression models investigating the association between baseline variables and outcome closely reflect published data of studies in which more standard diagnostic categories were used.
In conclusion, these results indicate that physicians do not always differentiate between anxiety and depressive symptoms when making a clinical diagnosis of depression. Most enrolled patients had significant emotional-depressive and emotional-anxious symptoms, as well as significant nonpainful and painful somatic symptomatology at baseline. Clinical, symptomatic, and sociodemographic variables were associated with outcome in this study.
Potential conflicts of interest: Dr Demyttenaere has served as a consultant to and on the speakers or advisory boards of and has received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Cyberonics, Eli Lilly, GlaxoSmithKline, Lundbeck, Otsuka, Servier, Takeda, and Wyeth and has received grant/research support from GlaxoSmithKline and Eli Lilly. Dr Dantchev has served on the speakers or advisory boards of Eli Lilly and Boehringer Ingelheim. Dr Grassi has served on the speakers or advisory boards of Eli Lilly and Boehringer Ingelheim. Dr Montejo has served as a consultant to Eli Lilly, Boehringer Ingelheim, GlaxoSmithKline, Bristol-Myers Squibb, and AstraZeneca; has received grant/research support from Lundbeck, AstraZeneca, Eli Lilly, and Servier; has received honoraria from AstraZeneca, Bristol-Myers Squibb, Servier, and Eli Lilly; and has served on the speakers or advisory boards of Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Servier. Dr Perahia and Ms Reed are employees and stock shareholders in Eli Lilly. Ms Quail is an employee of Eli Lilly. Dr Tylee has received honoraria from and served on the speakers or advisory boards of Eli Lilly and Boehringer Ingelheim. Dr Bauer has served as a consultant to AstraZeneca, Eli Lilly, GlaxoSmithKline, Servier Deutschland, and Wyeth and on the advisory boards of Eli Lilly and Boehringer Ingelheim; has received grant/research support from Stanley Medical Research Institute, NARSAD, Eli Lilly, and AstraZeneca; and has received honoraria from AstraZeneca, Eli Lilly, GlaxoSmithKline, Pfizer, and Wyeth. Dr Verhaeghen reports no financial or other affiliations relevant to the subject of this article.
Funding/support: FINDER was funded by Eli Lilly and Company Limited, Windlesham, United Kingdom, and Boehringer Ingelheim GmbH, Ingelheim, Germany.
Disclaimer: Dr Perahia and Mss Quail and Reed are Eli Lilly employees, and Drs Demyttenaere, Dantchev, Grassi, Montejo, Tylee, and Bauer have received economic compensation for participation in the FINDER Advisory Board.
Previous presentation: Results for this analysis have been previously presented at the Annual Meeting of the European College of Neuropsychopharmacology; March 11–14, 2007; Nice, France, and the 16th European Congress of Psychiatry; April 5–9, 2008; Nice, France.
Acknowledgments
The authors take full responsibility for the content of the article but thank Deirdre Elmhirst, PhD (Eli Lilly and Company Limited, Windlesham, United Kingdom), for her assistance in preparing the first draft of the manuscript and collating the comments of the authors and other named contributors.
REFERENCES
- 1.Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Clinical factors influencing the prescription of antidepressants and benzodiazepines: results from the European study of epidemiology of mental disorders (ESEMeD) J Affect Disord. 2008;110(1–2):84–93. doi: 10.1016/j.jad.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Linden M, Lecrubier Y, Bellantuono C, et al. The prescription of psychotropic drugs by primary care physicians: an international collaborative study. J Clin Psychopharmacol. 1999;19(2):132–140. doi: 10.1097/00004714-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Yastrubetskaya O, Chiu E, O'Connel S. Is good clinical research practice for clinical trials good clinical practice? Int J Geriatr Psychiatry. 1997;12(2):227–231. doi: 10.1002/(sici)1099-1166(199702)12:2<227::aid-gps549>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman M, Mattia JL, Posternak MA. Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice? Am J Psychiatry. 2002;159(3):469–473. doi: 10.1176/appi.ajp.159.3.469. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman M, Chelminski I, Posternak MA. Generalizability of antidepressant trials: differences between depressed psychiatric outpatients who would or would not qualify for an efficacy trial. Am J Psychiatry. 2005;162(7):1370–1372. doi: 10.1176/appi.ajp.162.7.1370. [DOI] [PubMed] [Google Scholar]
- 6.Keitner GI, Posternak MA, Ryan CE. How many subjects with major depressive disorder meet eligibility requirements of an antidepressant efficacy trial? J Clin Psychiatry. 2003;64(9):1091–1093. doi: 10.4088/jcp.v64n0915. [DOI] [PubMed] [Google Scholar]
- 7.Bauer M, Whybrow PC, Angst J, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 2: maintenance treatment of major depressive disorder and treatment of chronic depressive disorders and subthreshold depressions. World J Biol Psychiatry. 2002;3(2):69–86. doi: 10.3109/15622970209150605. [DOI] [PubMed] [Google Scholar]
- 8.Cuijpers P, Smit F. Subthreshold depression as a risk indicator for major depressive disorder: a systematic review of prospective studies. Acta Psychiatr Scand. 2004;109(5):325–331. doi: 10.1111/j.1600-0447.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- 9.Judd LL, Schettler PJ, Akiskal HS. The prevalence, clinical relevance, and public health significance of subthreshold depressions. Psychiatr Clin North Am. 2002;25(4):685–698. doi: 10.1016/s0193-953x(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Cebrian A, Bauer M, Montejo AL, et al. Factors Influencing Depression Endpoints Research (FINDER): study design and population characteristics. Eur Psychiatry. 2008;23(1):57–65. doi: 10.1016/j.eurpsy.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Bauer M, Monz BU, Montejo AL, et al. Prescribing patterns of antidepressants in Europe: results from the Factors Influencing Depression Endpoints Research (FINDER) study. Eur Psychiatry. 2008;23(1):66–73. doi: 10.1016/j.eurpsy.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Reed C, Monz BU, Perahia DG, et al. Quality of life outcomes among patients with depression after 6 months of starting treatment: results from FINDER. J Affect Disord. 2009;113(3):296–302. doi: 10.1016/j.jad.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 14.Barsky AJ, Wushak G, Klerman GL. Hypochondriasis: an evolution of the DSM-III criteria in medical outpatients. Arch Gen Psychiatry. 1986;43(5):493–500. doi: 10.1001/archpsyc.1986.01800050099013. [DOI] [PubMed] [Google Scholar]
- 15.Barsky AJ, Wushak G, Klerman GL. Psychiatric comorbidity in DSM-III-R hypochondriasis. Arch Gen Psychiatry. 1992;49(2):101–108. doi: 10.1001/archpsyc.1992.01820020021003. [DOI] [PubMed] [Google Scholar]
- 16.Kelly AM. The minimum clinically significant difference in visual analogue scale pain does not differ with severity of pain. Emerg Med J. 2001;18(3):205–207. doi: 10.1136/emj.18.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1-2):95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, Health Institute; 1993. [Google Scholar]
- 19.Ware JE, Kosinski M, Dewey JE, et al. Lincoln, RI: Quality Metric Incorporated; 2001. How to Score Version 2 of the SF-36 Health Survey. [Google Scholar]
- 20.Joukamaa M, Sohlman B, Lehtinen V. The prescription of psychotropic drugs in primary health care. Acta Psychiatr Scand. 1995;92(5):359–364. doi: 10.1111/j.1600-0447.1995.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 21.Mojtabai R. Datapoints: prescription patterns for mood and anxiety disorder in a community sample. Psychiatr Serv. 1999;50(12):1557. doi: 10.1176/ps.50.12.1557. [DOI] [PubMed] [Google Scholar]
- 22.Hunt C, Slade T, Andrews G. Generalized anxiety disorder and major depressive disorder comorbidity in the national survey of mental health and well-being. Depress Anxiety. 2004;20(1):23–31. doi: 10.1002/da.20019. [DOI] [PubMed] [Google Scholar]
- 23.Carter RM, Wittchen HU, Pfister H, et al. One year prevalence of subthreshold and threshold DSM-IV generalized anxiety disorder in a national representative sample. Depress Anxiety. 2001;13(2):78–88. doi: 10.1002/da.1020. [DOI] [PubMed] [Google Scholar]
- 24.Fava M, Rankin M, Wright E, et al. Anxiety disorders in major depression. Compr Psychiatry. 2000;41(2):97–102. doi: 10.1016/s0010-440x(00)90140-8. [DOI] [PubMed] [Google Scholar]
- 25.Rush AJ, Zimmerman M, Wisniewski SR, et al. Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord. 2005;87(1):43–55. doi: 10.1016/j.jad.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Sartorius N, Ustun T, Costa e Silva J, et al. An international study of psychological problems in primary care: preliminary report from the World Health Organization collaborative project on psychological problems in general health care. Arch Gen Psychiatry. 1993;50(10):819–824. doi: 10.1001/archpsyc.1993.01820220075008. [DOI] [PubMed] [Google Scholar]
- 27.McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111(1–2):77–83. doi: 10.1016/j.pain.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: prevalence, work loss and help seeking. J Affect Disord. 2006;92(2–3):185–193. doi: 10.1016/j.jad.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and anxiety: prevalence, work-loss and help seeking. J Affect Disord. 2008;109(3):264–272. doi: 10.1016/j.jad.2007.12.231. [DOI] [PubMed] [Google Scholar]
- 30.Vaccarino AL, Sills TL, Evans KR, et al. Prevalence and association of somatic symptoms in patients with major depressive disorder. J Affect Disord. 2008;110(3):270–276. doi: 10.1016/j.jad.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Boulanger JP, Fournier M, Rosales D, et al. Mixed anxiety and depression: from theory to practice. J Clin Psychiatry. 1997;57(suppl 8):27–34. [PubMed] [Google Scholar]
- 32.Judd LL, Kessler RC, Paulus MP, et al. Comorbidity as a fundamental feature of generalized anxiety disorders: results from the National Comorbidity Study (NCS) Acta Psychiatr Scand. 1998;98(suppl 393):6–11. doi: 10.1111/j.1600-0447.1998.tb05960.x. [DOI] [PubMed] [Google Scholar]
- 33.Aberg-Wistedt A, Agren H, Ekselius L, et al. Sertraline versus paroxetine in major depression: clinical outcome after six months of continuous therapy. J Clin Psychopharmacol. 2000;20(6):645–652. doi: 10.1097/00004714-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Nelson JC. Anxious depression and response to treatment. Am J Psychiatry. 2008;165(3):297–299. doi: 10.1176/appi.ajp.2007.07121927. [DOI] [PubMed] [Google Scholar]
- 35.Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- 36.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]