Abstract
Objective:
To evaluate the safety and tolerability of aripiprazole adjunctive to standard antidepressant therapy (ADT) for patients with major depressive disorder (DSM-IV-TR criteria).
Method:
Data from 2 identical studies of aripiprazole augmentation (8 weeks of prospective ADT treatment followed by 6 weeks of randomized double-blind adjunctive treatment) were pooled. The incidence of treatment-emergent adverse events (TEAEs) and weight, electrocardiogram (ECG), and laboratory measurements were assessed during the 6-week phase, including time course, severity, resolution, and predictors. The studies were conducted from June 2004 to April 2006 and September 2004 to December 2006.
Results:
The safety analysis included 737 outpatients (aripiprazole, n = 371; placebo, n = 366). The majority of patients completed the trials (aripiprazole, 86%; placebo, 88%). Common TEAEs (≥ 5% and twice the placebo rate) with aripiprazole were akathisia (25%), restlessness (12%), insomnia (8%), fatigue (8%), blurred vision (6%), and constipation (5%). Most TEAEs were of mild to moderate severity (aripiprazole, 89%; placebo, 95%). TEAE rates in the aripiprazole and placebo groups were not affected by ADT, age, or gender. Discontinuation due to TEAEs was low (aripiprazole, 3%; placebo, 1%). Mean weight change was higher with aripiprazole versus placebo (1.73 kg vs 0.38 kg, P < .001). At endpoint, clinical laboratory parameters, vital signs, and ECG indices (including QTc interval) were similar between groups. Akathisia with aripiprazole generally occurred in the first 3 weeks (76%), was of mild to moderate severity (92%), and led to discontinuation in 3 patients (0.8%). Within the aripiprazole group, age (18–40 years) was the only positive predictor for akathisia.
Conclusions:
In this short-term post hoc analysis, aripiprazole as augmentation to ADT demonstrated a safety and tolerability profile similar to that in monotherapy studies in other psychiatric populations. Controlled long-term safety and efficacy data of aripiprazole as adjunctive to ADT are warranted.
Trial Registration:
clinicaltrials.gov Identifiers: NCT00095823 (CN138-139) and NCT00095758 (CN138-163)
Augmentation of antidepressant therapy (ADT) with an atypical antipsychotic is one treatment option for patients who do not obtain sufficient benefit from an adequate course of ADT.1,2 Aripiprazole is approved for use as an adjunctive treatment to ADT in adults with major depressive disorder (MDD) on the basis of results from 2 identical, large, multicenter, randomized, double-blind, placebo-controlled trials.3,4 These trials demonstrated the efficacy of adjunctive aripiprazole in patients with an inadequate response to a prospective 8-week trial of the same ADT and at least 1 historical ADT trial.3,4
Aripiprazole is a novel atypical agent with a unique pharmacologic profile that may make it particularly effective as an augmentation agent for the treatment of depression. Aripiprazole has potent partial-agonist activity at D2 and D3 receptors5,6 and demonstrates high affinity and partial-agonist activity at serotonin 5-HT1A receptors and antagonist activity at 5-HT2 receptors7,8—effects that may contribute to specific antidepressant action.
Augmentation of standard ADTs has the potential to induce or exacerbate adverse events (AEs). Treatment-emergent AEs (TEAEs) common with adjunctive atypical antipsychotic use include weight gain, sedation, extrapyramidal symptoms, metabolic disturbances (eg, diabetes and hyperlipidemia), and hyperprolactinemia, although risk varies between agents.9–11 Consideration of the relative risks and benefits of these agents may influence treatment selection. Understanding safety and tolerability issues may be particularly important early in treatment in order to improve clinical management and to promote good adherence. Finally, identification of groups of patients at increased risk for particular AEs may also assist drug selection, enhanced monitoring, and patient education. This pooled analysis used data from 2 identical studies of aripiprazole augmentation3,4 to provide a more comprehensive assessment of the safety and tolerability of aripiprazole adjunctive to standard ADT for patients with MDD.
Clinical Points
♦ Atypical antipsychotics as augmentation agents to antidepressant therapy can be used in patients with MDD who have an inadequate response to antidepressant monotherapy.
♦ The safety and tolerability profile of adjunctive aripiprazole in MDD is similar to that in monotherapy studies in other psychiatric populations.
♦ Akathisia was the most common side effect reported, usually of mild to moderate severity, and led to discontinuation in less than 1% of patients treated.
METHOD
Study Design
Details of the study methods have been described previously.3,4 Briefly, 2 identical multicenter, randomized, double-blind, placebo-controlled 14-week studies were conducted in the United States to investigate the efficacy and safety of adjunctive aripiprazole or placebo with standard ADT in patients with MDD who showed an inadequate response to at least 1 historical and 1 prospective ADT trial. The studies were conducted from June 2004 to April 2006 and September 2004 to December 2006. Both studies were conducted in accordance with the Declaration of Helsinki, and the ethics committee at each site approved the protocol. All patients provided written informed consent to participate.
Both studies consisted of 3 phases: a 7- to 28-day screening phase; an 8-week prospective treatment phase to establish inadequate antidepressant response with standard ADT given according to the label dosing guidelines (escitalopram, fluoxetine, paroxetine controlled release, sertraline, or venlafaxine extended release plus single-blinded placebo); and a 6-week randomization phase for patients who had an inadequate response at the end of the prospective ADT treatment phase.
Patients were randomly assigned (1:1) to continue the same ADT plus either double-blind adjunctive placebo or adjunctive aripiprazole (2–20 mg/d). Aripiprazole was started at 5 mg/d and increased in weekly 5-mg/d increments to a maximum of 15 mg/d (patients receiving paroxetine or fluoxetine) or 20 mg/d (all other patients) on the basis of assessment of efficacy and clinical response. Aripiprazole doses could be decreased at any visit on the basis of tolerability except in the last week of double-blind treatment. Treatment of extrapyramidal symptoms (EPS) (benztropine ≤ 6 mg/d, propranolol ≤ 120 mg/d) was also permitted during the study except within 12 hours prior to administration of movement rating scales. Patients taking stable doses of hypnotics, including benzodiazepines and other sleep aids, for insomnia were required to discontinue medication at least 1 week prior to the prospective treatment phase.
Study Population
Subjects were outpatients aged 18–65 years who met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)12 criteria for a major depressive episode that had lasted ≥ 8 weeks. Patients were also required to have reported inadequate response to a previous adequate trial of ADT (defined as < 50% reduction in severity of depressive symptoms as determined by the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire [ATRQ]13 following at least 1 and no more than 3 ADT trials > 6 weeks’ duration [> 3 weeks for combination treatments] at the minimum dose specified in the ATRQ). Further details of inclusion and exclusion criteria have been reported previously.3,4
Assessments
The results of the primary efficacy endpoint (mean change from the end of the prospective treatment phase to the end of the randomized, double-blind phase in the Montgomery-Asberg Depression Rating Scale [MADRS]14 total score) are reported elsewhere.3,4 The safety and tolerability profile of aripiprazole was evaluated using reporting of TEAEs and serious AEs and body weight, vital sign, electrocardiogram (ECG), and laboratory measurements, as well as assessment of EPS using the Barnes Akathisia Rating Scale (BARS).15 TEAEs occurring during the adjunctive treatment phase were defined as AEs with an onset date on, or after, the start of adjunctive treatment. Patients were assessed weekly throughout the 6-week double-blind treatment phase (week 9 to week 14) for TEAEs, including time course, severity, discontinuations, and resolution. Common TEAEs were defined as those occurring in the adjunctive aripiprazole treatment group at an incidence ≥ 5% and twice the rate of placebo.
Analyses
For these analyses, data were pooled from patients who participated in the 2 aripiprazole studies of patients with MDD (studies CN138-139 and CN138-163). The safety sample includes all patients who received at least 1 dose of study medication (adjunctive aripiprazole or adjunctive placebo) during the 6-week, double-blind treatment phase. In these analyses, baseline was defined at the end of the prospective treatment phase just prior to receiving double-blind study medication. Continuous variables are described with mean, median, minimum and maximum, and standard error and categorical variables with frequency distributions. The Fisher exact test was used to compare the frequency of AEs between adjunctive aripiprazole and placebo in the total population. To test for differences in the AE (≥ 5%) profile of pooled adjunctive aripiprazole relative to placebo across gender, age, and ADT, an odds ratio (OR) estimate was calculated and compared across all categories using the Breslow-Day test for AEs.
Predictors of akathisia (gender, age group [18–40, 41–50, or > 50 years], ADT, response [≥ 50% decrease in MADRS total score during double-blind treatment], remission [MADRS total score ≤ 10 and at least 50% reduction in MADRS total score during double-blind treatment], and duration of current episode) were assessed for the adjunctive aripiprazole group only. Odds ratios and 95% CIs for each predictor comparing the akathisia and nonakathisia groups were estimated using logistic regression analysis. Odds ratios and 95% CIs for ORs in akathisia events between fluoxetine/paroxetine and other ADTs were calculated controlling for study and treatment. Mean change in body weight was assessed by analysis of covariance (ANCOVA) with double-blind treatment as main effect, study as a stratification effect, and baseline assessment as covariate. Mean changes from baseline in fractional exponent correction of the QT interval (QTcE = QT/RR0.36) were evaluated by ANCOVA controlling for baseline value.
RESULTS
Subject Disposition and Characteristics of Patients in the Randomization Phase
In total, 373 patients were randomly assigned to adjunctive aripiprazole treatment and 368 to adjunctive placebo treatment in the 2 studies. The percentage of randomized patients completing the studies was similar between adjunctive aripiprazole-treated (n = 322, 86%) and placebo-treated (n = 322, 88%) patients. Reasons for discontinuation (adjunctive aripiprazole versus adjunctive placebo) of patients in the randomized sample were due to lack of efficacy (1.6% vs 1.4%, P > .99), AEs (3.5% vs 1.6%, P = .16), consent withdrawn (2.2% vs 3.8%, P = .20), lost to follow-up (2.2% vs 2.5%, P = .81), or other reasons (3.8% vs 2.7%, P = .53). The safety sample consisted of 371 patients in the adjunctive aripiprazole group and 366 patients in the adjunctive placebo group; 4 patients did not receive study medication and were not included in the safety analysis (adjunctive aripiprazole, n = 2; adjunctive placebo, n = 2).
The baseline characteristics of patients assessed at screening and included in the safety analysis are shown in Table 1. There were no clinically relevant differences between the treatment groups. The majority of patients had 1 historical treatment failure prior to entering the 14-week trial (adjunctive aripiprazole, 68%; adjunctive placebo, 67%), and the mean duration of the current depressive episode was ∼45 months (∼4 years).
Table 1.
Baseline Demographics of Patients With Major Depressive Disorder Receiving Adjunctive Aripiprazole or Placebo (safety sample)
| Demographic | Placebo (n = 366) | Aripiprazole (n = 371) |
| Age, mean ± SE, y | 44.3 ± 0.6 | 45.5 ± 0.6 |
| Male/female, n (%) | 125/241 (34/66) | 134/237 (36/64) |
| Race, n (%) | ||
| White | 332 (91) | 327 (88) |
| Black | 24 (7) | 29 (8) |
| Asian | 4 (1) | 8 (2) |
| Other | 6 (2) | 7 (2) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 31 (8) | 17 (5) |
| Not Hispanic or Latino | 335 (92) | 354 (95) |
| No. of adequate antidepressant trials, n (%)a | ||
| 1 | 245 (67) | 254 (68) |
| 2 | 96 (26) | 94 (25) |
| 3 | 24 (7) | 21 (6) |
| 4 | 0 | 1 (< 1) |
Data missing for 1 patient in each treatment group.
A breakdown of the distribution and mean doses of each ADT received during the randomized treatment phase showed no differences between the adjunctive aripiprazole and adjunctive placebo groups (Table 2). The distribution of adjunctive aripiprazole dosing at endpoint was as follows: 2 mg/d, 4.9%; 5 mg/d, 37.8%; 10 mg/d, 25.1%; 15 mg/d, 18.4%; and 20 mg/d, 13.8%.
Table 2.
Antidepressant Therapy Assignment of Patients With Major Depressive Disorder Receiving Adjunctive Aripiprazole or Adjunctive Placebo (safety sample)
| Antidepressant Therapy | Placebo (n = 366) | Aripiprazole (n = 371) |
| Escitalopram | ||
| n (%) | 102 (27.9) | 118 (31.8) |
| Dose, mean ± SE (mg) | 19.3 ± 0.3 | 19.7 ± 0.1 |
| Fluoxetine | ||
| n (%) | 54 (14.8) | 53 (14.3) |
| Dose, mean ± SE (mg) | 37.8 ± 0.9 | 39.6 ± 0.4 |
| Paroxetine controlled release | ||
| n (%) | 28 (7.7) | 31 (8.4) |
| Dose, mean ± SE (mg) | 46.9 ± 1.0 | 48.4 ± 0.8 |
| Sertraline | ||
| n (%) | 77 (21.0) | 69 (18.6) |
| Dose, mean ± SE (mg) | 143.5 ± 1.9 | 140.6 ± 2.6 |
| Venlafaxine extended release | ||
| n (%) | 105 (28.7) | 100 (27.0) |
| Dose, mean ± SE (mg) | 214.3 ± 2.6 | 213.8 ± 2.7 |
Treatment-Emergent Adverse Events
The most commonly reported TEAEs (≥ 5% and twice the placebo rate) are shown in Table 3. Akathisia, restlessness, insomnia, fatigue, blurred vision, and constipation were the most commonly reported AEs with adjunctive aripiprazole. The majority of TEAEs were of mild to moderate severity in both the adjunctive placebo (94.5%) and adjunctive aripiprazole (88.7%) groups. The incidence of TEAEs when the adjunctive aripiprazole group was compared to the adjunctive placebo group was not significantly different (Breslow-Day test) between males or females, between age groups (Table 3), or among individual ADT groups (Table 4). The rate of discontinuation because of AEs was 3.0% (11/371) for the adjunctive aripiprazole group and 1.4% (5/366) for the adjunctive placebo group (safety sample). The AEs that led to discontinuation were (all n = 1 unless stated) restless legs syndrome, decreased libido, suicidal ideation, oral and pharyngeal pain, and depression in the adjunctive placebo group, and in the aripiprazole group, these were akathisia (n = 3), fatigue (n = 2), blurred vision (n = 2), abnormal coordination, sedation, somnolence, anxiety, insomnia, chest pain, pain, hyperhidrosis, rash, hematochezia, muscle twitching, restlessness, and urinary hesitation. Patients may have had more than 1 AE leading to discontinuation, but were counted in the overall total only once.
Table 3.
Treatment-Emergent Adverse Events That Occurred in the Adjunctive Aripiprazole Group at an Incidence ≥ 5% and Twice the Placebo Rate in Either the Total Safety Sample or in Groups Stratified by Gender or Age (safety sample)ab
| Total |
Male Patients |
Female Patients |
Age 18–50 Years |
Age > 50 Years |
||||||
| Adverse Event | Placebo (n = 366) | Aripiprazole (n = 371) | Placebo (n = 125) | Aripiprazole (n = 134) | Placebo (n = 241) | Aripiprazole (n = 237) | Placebo (n = 252) | Aripiprazole (n = 232) | Placebo (n = 114) | Aripiprazole (n = 139) |
| Akathisia | 4.4 | 24.8* | 3.2 | 20.1 | 5.0 | 27.4 | 5.6 | 28.4 | 1.8 | 18.7 |
| Restlessness | 1.9 | 12.1* | 1.6 | 17.2 | 2.1 | 9.3 | 1.6 | 10.3 | 2.6 | 15.1 |
| Insomnia | 2.5 | 8.1* | 0.8 | 9.7 | 3.3 | 7.2 | 3.2 | 6.9 | 0.9 | 10.1 |
| Fatigue | 4.1 | 8.4 | 4.8 | 8.2 | 3.7 | 8.4 | 4.8 | 9.5 | 2.6 | 6.5 |
| Blurred vision | 1.4 | 5.7* | 1.6 | 4.5 | 1.2 | 6.3 | 2.0 | 5.6 | 0 | 5.8 |
| Somnolence | 3.8 | 6.2 | 4.8 | 3.7 | 3.3 | 7.6 | 4.0 | 4.3 | 3.5 | 9.4 |
| Constipation | 1.9 | 4.6 | 2.4 | 3.0 | 1.7 | 5.5 | 2.0 | 5.2 | 1.8 | 3.6 |
| Sedation | 1.6 | 4.0 | 1.6 | 2.2 | 1.7 | 5.1 | 1.6 | 6.0 | 1.8 | 0.7 |
| Arthralgia | 2.7 | 4.0 | 4.0 | 2.2 | 2.1 | 5.1 | 1.2 | 3.9 | 6.1 | 4.3 |
Values are presented as %.
Adverse events for the composite data set. Extra adverse events with an incidence ≥ 5% and twice the placebo rate for adjunctive aripiprazole: 1 akathisia event, 1 restlessness event, and 3 fatigue events. Extra adverse events for adjunctive placebo: 1 blurred vision event and 2 fatigue events.
P < .05 vs adjunctive placebo (Fisher exact test comparing adjunctive aripiprazole and placebo in the total population only).
Table 4.
Treatment-Emergent Adverse Events by Antidepressant Therapy That Occurred in the Adjunctive Aripiprazole Group at an Incidence ≥ 5% and Twice the Placebo Rate With Any Antidepressant Therapy (safety sample)a
| Escitalopram |
Fluoxetine |
Paroxetine CR |
Sertraline |
Venflaxine XR |
||||||
| Adverse Event | Placebo (n = 102) | Aripiprazole (n = 118) | Placebo (n = 54) | Aripiprazole (n = 53) | Placebo (n = 28) | Aripiprazole (n = 31) | Placebo (n = 77) | Aripiprazole (n = 69) | Placebo (n = 105) | Aripiprazole (n = 100) |
| Akathisia | 4 (3.9) | 25 (21.2) | 2 (3.7) | 18 (34.0) | 2 (7.1) | 9 (29.0) | 4 (5.2) | 14 (20.3) | 4 (3.8) | 26 (26.0) |
| Restlessness | 2 (2.0) | 14 (11.9) | 2 (3.7) | 6 (11.3) | 0 | 3 (9.7) | 1 (1.3) | 8 (11.6) | 2 (1.9) | 14 (14.0) |
| Insomnia | 2 (2.0) | 10 (8.5) | 1 (1.9) | 5 (9.4) | 0 | 2 (6.5) | 2 (2.6) | 7 (10.1) | 4 (3.8) | 6 (6.0) |
| Fatigue | 8 (7.8) | 12 (10.2) | 2 (3.7) | 3 (5.7) | 1 (3.6) | 5 (16.1) | 1 (1.3) | 6 (8.7) | 3 (2.9) | 5 (5.0) |
| Blurred vision | 2 (2.0) | 5 (4.2) | 0 | 4 (7.5) | 1 (3.6) | 4 (12.9) | 1 (1.3) | 1 (1.4) | 1 (1.0) | 7 (7.0) |
| Constipation | 1 (1.0) | 6 (5.1) | 0 | 0 | 1 (3.6) | 2 (6.5) | 1 (1.3) | 1 (1.4) | 4 (3.8) | 8 (8.0) |
| Diarrhea | 8 (7.8) | 3 (2.5) | 2 (3.7) | 1 (1.9) | 1 (3.6) | 3 (9.7) | 1 (1.3) | 4 (5.8) | 4 (3.8) | 1 (1.0) |
| Somnolence | 4 (3.9) | 7 (5.9) | 3 (5.6) | 2 (3.8) | 3 (10.7) | 1 (3.2) | 2 (2.6) | 3 (4.3) | 2 (1.9) | 10 (10.0) |
| Urinary tract infection | 5 (4.9) | 6 (5.1) | 4 (7.4) | 4 (7.5) | 4 (14.3) | 5 (16.1) | 1 (1.3) | 5 (7.2) | 2 (1.9) | 2 (2.0) |
| Weight gain | 3 (2.9) | 4 (3.4) | 0 | 1 (1.9) | 0 | 2 (6.5) | 2 (2.6) | 2 (2.9) | 4 (3.8) | 3 (3.0) |
| Sedation | 4 (3.9) | 4 (3.4) | 0 | 2 (3.8) | 0 | 2 (6.5) | 1 (1.3) | 3 (4.3) | 1 (1.0) | 4 (4.0) |
| Disturbance in attention | 1 (1.0) | 4 (3.4) | 0 | 1 (1.9) | 0 | 0 | 2 (2.6) | 1 (1.4) | 1 (1.0) | 6 (6.0) |
| Dizziness | 2 (2.0) | 5 (4.2) | 1 (1.9) | 1 (1.9) | 0 | 1 (3.2) | 1 (1.3) | 4 (5.8) | 3 (2.9) | 3 (3.0) |
| Flatulence | 1 (1.0) | 2 (1.7) | 0 | 3 (5.7) | 1 (3.6) | 1 (3.2) | 1 (1.3) | 0 | 3 (2.9) | 2 (2.0) |
| Feeling jittery | 0 | 4 (3.4) | 0 | 0 | 1 (3.6) | 0 | 0 | 2 (2.9) | 1 (1.0) | 5 (5.0) |
Values are presented as n (%).
The incidence of serious AEs was low (< 1%) and similar between the adjunctive aripiprazole (n = 3, 0.8%) and adjunctive placebo (n = 5, 1.4%) groups (P = .73, Fisher exact test). The serious AEs with adjunctive aripiprazole included 1 case of cellulitis, 1 case of staphylococcal cellulitis, and 1 case of pneumonia, all deemed not related to study medication. Serious adverse events with placebo included 1 patient with exostosis, 1 patient with cellulitis and staphylococcal abscess, 1 patient with contusion and physical assault, 1 patient with gastroesophageal reflux disease, and 1 patient with accident at work. No seizure-related events or cases of neuroleptic malignant syndrome were reported, and no deaths occurred during either of the 2 studies. There were no suicide-related AEs reported in the adjunctive aripiprazole group, although 2 suicide-related events (suicidal ideation) were reported in the adjunctive placebo arm (incidence of 0.54%).
Extrapyramidal Symptom–Related Adverse Events
Of the spontaneous reports of EPS-related AEs, akathisia was the only event to occur in the adjunctive aripiprazole group at ≥ 5% and twice the rate of placebo (aripiprazole, n = 91, 24.5%; placebo, n = 16, 4.4%; P <.0001). Nonakathisia EPS–related events occurred at a low rate, and the rates did not differ significantly between treatment arms: aripiprazole, n = 31 (8.4%) and placebo, n = 20 (5.5%); there were no cases of tardive dyskinesia.
Analysis of Akathisia
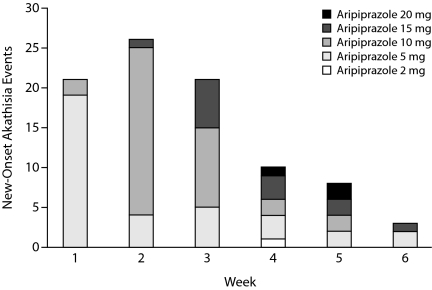
Of the 91 adjunctive aripiprazole treatment patients who experienced an onset of akathisia during the adjunctive treatment phase, the majority of cases occurred within the first 3 weeks of treatment (Figure 1) and were recorded as mild (49.5%) or moderate (42.9%) in severity on the basis of the investigator's attribution of severity grade. Only 7.7% of akathisia cases were recorded as severe. In the patients with akathisia, 42 aripiprazole-treated patients (46.2%) experienced mild distress at the time of greatest discomfort on the BARS subjective distress item. Moderate distress was reported by 38 patients (41.8%), 6 patients experienced severe distress (6.6%), and 5 patients reported no distress (5.5%). In patients experiencing akathisia, objective restlessness was rated as absent (objective item score of 0) in 27 patients (29.7%). At the time of maximum severity on the BARS scale, objective restlessness was rated occasional in 40 patients (44.0%), frequent in 22 patients (24.2%), and constant in 2 patients (2.2%).
Figure 1.
Number of New-Onset Akathisia Events for Adjunctive Aripiprazole Patients Experiencing Akathisia (n = 91) by Week of Treatmenta
aTwo adjunctive aripiprazole-treated patients with missing dosing information were not included.
Akathisia was transient in the majority of patients, with 52% of the akathisia events (47/91) resolving by study endpoint. This finding is supported by the distribution of BARS Global Clinical Assessment of Akathisia scores at the end of the adjunctive treatment phase for aripiprazole-treated patients who experienced akathisia (n = 91) (absent, 64.8%; questionable, 11.0%; mild akathisia, 17.6%; moderate or marked akathisia, 6.6%; severe akathisia, 0%).
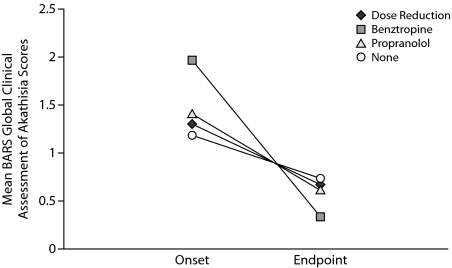
Interventions permitted and chosen for management of akathisia by study investigators included no intervention (38.5%), dose reduction only (31.9%), use of concomitant medications (benztropine only, 16.5%; propranolol only, 5.5%; benztropine and propranolol, 2.2%), or a combination of dose reduction and concomitant medications (6%). Resolution of akathisia events was achieved in 45% of the patients who received no intervention (17/38), 80% of patients who received dose reduction (24/30), 35% of patients who received benztropine (7/20), and 40% of patients who received propranolol (4/10). The median duration of akathisia events that resolved was between 8.5 and 13 days (dose reduction, 8.5 days; benztropine, 12 days; propranolol, 11 days; no intervention, 13 days).
Mean BARS Global Clinical Assessment of Akathisia scores for those experiencing akathisia (n = 91) at treatment onset and endpoint (week 14) are shown in Figure 2. Benztropine intervention tended to be associated with higher mean BARS Global Clinical Assessment of Akathisia scores at akathisia onset compared to other interventions, although by the end of the adjunctive treatment phase, mean BARS Global Clinical Assessment of Akathisia scores were below 1 (questionable akathisia) for all interventions.
Figure 2.
Change in Mean BARS Global Clinical Assessment of Akathisia Scores for Adjunctive Aripiprazole Patients Experiencing Akathisia (n = 91) by Interventiona
aBARS Global Clinical Assessment of Akathisia scores: 0 = absent, 1 = questionable, 2 = mild akathisia, 3 = moderate akathisia, 4 = marked akathisia, and 5 = severe akathisia.
Abbreviation: BARS = Barnes Akathisia Rating Scale.
Relationship of Akathisia to Response
Having akathisia or not did not influence rates of response (OR = 1.03; 95% CI, 0.61–1.72) or remission (OR = 0.85; 95% CI, 0.49–1.49) with adjunctive aripiprazole.
Potential Predictors of Akathisia
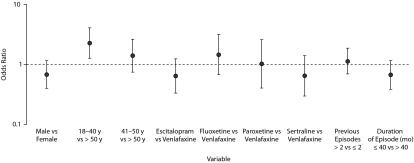
Younger age was a positive predictor for akathisia (risk was greatest in those < 40 years of age), while gender, number of previous episodes, and duration of current episode were not (Figure 3). Although no individual antidepressant was predictive of akathisia, we considered the possibility that the rate of akathisia might be higher when aripiprazole was added to potent 2D6 inhibitors (fluoxetine/paroxetine). However, post hoc comparison showed that rates of akathisia did not differ significantly with augmentation of fluoxetine/paroxetine versus other ADTs (32.2% versus 22.7%, OR = 1.27; 95% CI, 0.37–4.28).
Figure 3.
Predictors of Akathisia Events in Patients Treated With Adjunctive Aripiprazole (n = 371)a
aPredictors of akathisia (gender, age group [18–40, 41–50, or > 50 y], antidepressant therapy, number of previous episodes, and duration of current episode) were assessed for the adjunctive aripiprazole group (n = 371) only. Odds ratios and 95% CIs for each predictor comparing the akathisia and nonakathisia groups were estimated using logistic regression analysis.
Body Weight and Laboratory Measurements
Mean (SD) weight change from baseline over the adjunctive treatment phase was +1.73 kg (2.39) for adjunctive aripiprazole versus +0.38 kg (2.00) for adjunctive placebo (P < .001). Clinically significant weight gain (≥ 7% increase in body weight from baseline) was different between the 2 treatment arms (adjunctive aripiprazole, 5.2% vs adjunctive placebo, 0.6%; P < .001).
The incidences of potentially clinically relevant serum chemistry or hematology laboratory abnormalities and serum electrolyte measurements were similar between the treatment groups. The incidence of treatment-emergent prolactin levels greater than the upper limit of normal was similar between aripiprazole (7.0%) and placebo (5.7%). Median percentage change in prolactin levels decreased from baseline to endpoint in the aripiprazole group (–18.3%) compared to no change with placebo, although these changes were not clinically relevant. Mean (SE) change from baseline in serum prolactin levels was –1.43 ng/mL (0.42) for adjunctive aripiprazole versus 0.03 ng/mL (0.44) for adjunctive placebo (P = .02). One aripiprazole-treated subject discontinued because of a weight increase; no other patients discontinued because of AEs related to abnormal laboratory measurement.
Vital Signs and Electrocardiograms
The incidences of potentially clinically relevant vital signs and ECG measurements were similar between the treatment groups, and no patients discontinued because of a vital sign or ECG abnormality.
The mean decreases in QTcE interval from baseline to endpoint observed with adjunctive aripiprazole treatment (–0.23 ms; mean baseline, 404.5 ms; n = 341) were similar to the changes seen with placebo (–0.18 ms; mean baseline, 405.9 ms; n = 325) (P = .964). The percentage of patients experiencing an increase in QTcE interval ≥ 30 ms was similar between adjunctive aripiprazole treatment (4.0%) and placebo (3.0%) treatment (P = .537). Only 1 patient (0.3%) who received adjunctive aripiprazole treatment experienced an increase in QTcE interval ≥ 60 ms. The percentage of patients experiencing a QTcE interval greater than 450 ms was also similar between adjunctive aripiprazole treatment (1.7%) and placebo treatment (1.2%) (P = .753), and no patients experienced a QTcE interval greater than 500 ms.
DISCUSSION
The results of this post hoc pooled analysis showed that aripiprazole, when used as augmentation to ADT, was safe and well tolerated for the treatment of patients with MDD who had shown an inadequate response to previous ADT. Completion rates were high and the rate of discontinuation due to AEs was low (3.5%, randomized sample). Few patients reported serious adverse events, and no aripiprazole-treated patients reported the emergence or worsening of suicidal ideation. The tolerability profile of adjunctive aripiprazole was mostly similar to that seen in monotherapy studies in other psychiatric populations: a low rate of somnolence and sedation and no vital sign abnormalities. Other than in patients with schizophrenia, akathisia is the most common AE reported across studies in bipolar mania, bipolar depression, and MDD. Furthermore, the safety and tolerability profile of adjunctive aripiprazole did not differ by type of ADT augmented.
Akathisia occurred at a higher rate with adjunctive aripiprazole than with adjunctive placebo; however, the majority of these events were mild to moderate in severity, generally resulted in minimal subjective distress, and rarely led to discontinuation. Akathisia did not interfere with depression response; patients with akathisia were just as likely to remit as those without. In half the affected cases, akathisia resolved by the end of the trial. Dose reduction appeared to be the most effective intervention, with 80% of cases resolving. In 45% of cases with no intervention, akathisia resolved with time. It should be noted, however, that choice of intervention was based on the investigator's assessment and was without random assignment, and the data suggested that concomitant medications may have been selected in more severe cases (Figure 2). Finally, it should be noted that the scheduled dosing increases may have contributed to a higher rate of akathisia than might be achieved with more flexible dosing.
The rate of akathisia associated with aripiprazole treatment reported here in patients with MDD (25%) is higher than that observed in trials of schizophrenia (10%)16 or bipolar mania (15%).17 It may be hypothesized that depressed patients may be more vulnerable to this adverse effect, and higher rates of extrapyramidal symptoms have been shown to be associated with bipolar depression than with schizophrenia.18 Possibly, patients already receiving agents that block serotonin uptake may be more vulnerable to akathisia, especially as selective serotonin reuptake inhibitors may induce akathisia or restlessness.19,20 The higher rates of akathisia reported here may reflect a synergistic interaction between aripiprazole and serotonin reuptake–blocking agents.
Analysis of predictors found few variables associated with increased risk of akathisia. Risk was increased in younger patients, particularly those less than 40 years of age. In younger patients, consideration of a lower starting dose of aripiprazole (2 mg/d) may help to minimize the risk of akathisia during initial treatment. Rates of akathisia with adjunctive aripiprazole did not vary significantly among the individual antidepressants. Although rates of akathisia were not significantly higher when aripiprazole was added to robust 2D6 inhibitors fluoxetine or paroxetine compared to the other antidepressants, 2D6 inhibitors would be expected to raise aripiprazole concentrations, and lower aripiprazole doses may be advisable when adding the drug to fluoxetine or paroxetine.
Aripiprazole augmentation was associated with greater weight gain than ADT alone in this 6-week adjunctive phase of the study in patients with MDD. This weight gain is in contrast to observations in studies with aripiprazole monotherapy in patients with schizophrenia, schizoaffective disorder, or bipolar disorder in which aripiprazole has been shown to have a low potential for weight gain.16,21–29 Furthermore, aripiprazole also demonstrated a low potential for weight gain when used as adjunctive treatment in patients with bipolar mania29 or bipolar depression.30 It is possible that concomitant prescription of aripiprazole with antidepressants may increase risk of weight gain. It is important to note that, even in this population already at risk for metabolic disturbance (more than 50% of patients had a baseline body mass index > 30 kg/m2), change in weight did not translate into a worsening of metabolic parameters, such as lipid or glucose levels.
The findings reported here are strengthened by the relatively large numbers of patients with MDD included in this pooled analysis, providing greater statistical power to detect treatment differences. The findings should, however, be considered in light of several limitations, such as the post hoc nature of this analysis. Furthermore, although dose reduction of aripiprazole on the basis of tolerability was permitted up to the last week of double-blind treatment, investigators were encouraged to increase the aripiprazole dose in weekly increments of 5 mg/d up to the maximum dose for the given ADT. As a result, the findings are not informative about the best dosing strategy to avoid adverse events. Finally, this short-term study does not allow for conclusions about the long-term safety of adjunctive aripiprazole in this population. Such studies are currently underway.
In conclusion, this post hoc analysis extends previous findings demonstrating that aripiprazole is safe and generally well tolerated as an augmentation strategy to standard ADT in patients with MDD with a history of an inadequate response to antidepressant medication. Akathisia and weight gain associated with adjunctive aripiprazole were clinically manageable and seldom led to treatment discontinuation.
Drug names: aripiprazole (Abilify), benztropine (Cogentin and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), paroxetine (Paxil, Pexeva, and others), propranolol (Inderal and others), sertraline (Zoloft and others), venlafaxine (Effexor and others).
Potential conflicts of interest: Dr Nelson in the last 12 months has served as a consultant to or on the advisory boards of Bristol-Myers Squibb, Corcept, Eli Lilly, Forest, Medtronic, Merck, Orexigen, Otsuka, Sanofi-Aventis, and Sierra Neuropharmaceuticals; has received lecture honoraria from Eli Lilly Global, Otsuka Asia, and Schering Plough Japan; and has received grant/research support from the National Institute of Mental Health (NIMH) and Health Resources and Services Administration. Dr Thase has served as a consultant to AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest, GlaxoSmithKline, MedAvante, Neuronetics, Novartis, Schering-Plough, Shire, Supernus, Takeda, Transcept, and Wyeth-Ayerst; has received grant/research support from Eli Lilly, GlaxoSmithKline, National Institute of Mental Health, and Sepracor; has served on the speakers’ boards for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, and Wyeth-Ayerst; has equity holdings in MedAvante; and receives royalty income from American Psychiatric Publishing, Guilford Publications, Herald House, Oxford University Press, and W.W. Norton & Company. His wife is employed as the senior medical director for Advogent. Dr Trivedi in the last 12 months has served as a consultant to AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Evotec, Fabre-Kramer, Forest, GlaxoSmithKline, Janssen, Johnson & Johnson, Medtronic, Neuronetics, Otsuka, Pfizer, Shire, and Wyeth-Ayerst and has received grant/research support from the Agency for Healthcare Research and Quality, NIMH, National Institute on Drug Abuse (NIDA), and Targacept. Dr Fava has received grant/research support from Abbott, Alkermes, Aspect Medical Systems, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest, Ganeden, GlaxoSmithKline, Johnson & Johnson, Lichtwer Pharma GmbH, Lorex, the National Alliance for Research on Schizophrenia and Depression, National Center for Complimentary and Alternative Medicine, NIDA, NIMH, Novartis, Organon, PamLab, Pfizer, Pharmavite, Roche, Sanofi-Aventis, Shire, Solvay, Synthelabo, and Wyeth-Ayerst Laboratories; has served as a consultant to or on the advisory boards of Abbott, Amarin, Aspect Medical Systems, AstraZeneca, Auspex, Bayer AG, Best Practice Project Management, Biovail, BrainCells, Bristol-Myers Squibb, Cephalon, Clinical Trials Solutions, CNS Response, Compellis, Cypress, Dov, Eli Lilly, EPIX, Fabre-Kramer, Forest, GlaxoSmithKline, Grunenthal GmBH, Janssen, Jazz, Johnson & Johnson, Knoll, Labopharm, Lorex, Lundbeck, MedAvante, Merck, Methylation Sciences, Neuronetics, Novartis, Nutrition 21, Organon, PamLab, Pfizer, PharmaStar, Pharmavite, Precision Human Biolaboratory, Roche, Sanofi-Aventis, Sepracor, Solvay, Somaxon, Somerset, Synthelabo, Takeda, Tetragenex, Transcept, Vanda, and Wyeth-Ayerst; has served on the speakers bureaus of Advanced Meeting Partners, American Psychiatric Association, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest, GlaxoSmithKline, Imedex, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed-Elsevier, Novartis, Organon, Pfizer, PharmaStar, Primedia, Reed-Elsevier, UBC, and Wyeth-Ayerst; has equity holdings in Compellis and MedAvante; receives copyright royalties for the MGH CPFQ, SFI, ATRQ, DESS, and SAFER; and has patent applications for SPCD and for a combination of azapirones and bupropion in major depressive disorder. Drs Han, Carlson, Marcus, and Berman are employees of Bristol-Myers Squibb. Dr Tran is an employee of Otsuka America Pharmaceutical, Inc. Dr Pikalov is a former employee of Otsuka America Pharmaceutical, Inc. Dr Qi is a former employee of Bristol-Myers Squibb.
Funding/support: This study was supported by Bristol-Myers Squibb (Princeton, New Jersey) and Otsuka Pharmaceutical Co, Ltd (Tokyo, Japan). Editorial support for the preparation of this manuscript was provided by Ogilvy Healthworld Medical Education (London, United Kingdom); funding for editorial support was provided by Bristol-Myers Squibb.
Previous presentations: Presented at the Annual Meeting of the American College of Neuropsychopharmacology; December 8–12, 2007; Boca Raton, Fla, and the 117th Annual Meeting of the American Psychiatric Association; May 3–8, 2008; Washington, DC.
REFERENCES
- 1.Fava M, Rush AJ. Current status of augmentation and combination treatments for major depressive disorder: a literature review and a proposal for a novel approach to improve practice. Psychother Psychosom. 2006;75(3):139–153. doi: 10.1159/000091771. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Practice Guideline for the Treatment of Patients With Major Depressive Disorder (revision) Am J Psychiatry. 2000;157(suppl 4):1–45. [PubMed] [Google Scholar]
- 3.Berman RM, Marcus RN, Swanink R, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):843–853. doi: 10.4088/jcp.v68n0604. [DOI] [PubMed] [Google Scholar]
- 4.Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28(2):156–165. doi: 10.1097/JCP.0b013e31816774f9. [DOI] [PubMed] [Google Scholar]
- 5.Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302(1):381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 7.Jordan S, Koprivica V, Chen R, et al. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT(1A) receptor. Eur J Pharmacol. 2002;441(3):137–140. doi: 10.1016/s0014-2999(02)01532-7. [DOI] [PubMed] [Google Scholar]
- 8.Jordan S, Koprivica V, Dunn R, et al. In vivo effects of aripiprazole on cortical and striatal dopaminergic and serotonergic function. Eur J Pharmacol. 2004;483(1):45–53. doi: 10.1016/j.ejphar.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Shelton RC, Williamson DJ, Corya SA, et al. Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry. 2005;66(10):1289–1297. doi: 10.4088/jcp.v66n1012. [DOI] [PubMed] [Google Scholar]
- 10.Rapaport MH, Gharabawi GM, Canuso CM, et al. Effects of risperidone augmentation in patients with treatment-resistant depression: results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology. 2006;31(11):2505–2513. doi: 10.1038/sj.npp.1301113. [DOI] [PubMed] [Google Scholar]
- 11.Thase ME, Corya SA, Osuntokun O, et al. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry. 2007;68(2):224–236. doi: 10.4088/jcp.v68n0207. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. Fourth Edition, Text Revision. [Google Scholar]
- 13.Chandler GM, Iosifescu DV, Pollack MH, et al. Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ) CNS Neurosci Ther. 2009 doi: 10.1111/j.1755-5949.2009.00102.x. Sept 21 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery SA, Asberg MC. A new depression rating scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 15.Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 16.Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res. 2003;61(2–3):123–136. doi: 10.1016/s0920-9964(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 17.Marcus RN, Carson WH, McQuade RD, et al. New York, NY: New research poster 796 presented at: 157th Annual Meeting of the American Psychiatric Association; 2004. Overview of safety and tolerability of aripiprazole in acute mania. May 1–6. [Google Scholar]
- 18.Gao K, Kemp DE, Ganocy SJ, et al. Antipsychotic-induced extrapyramidal side effects in bipolar disorder and schizophrenia: a systematic review. J Clin Psychopharmacol. 2008;28(2):203–209. doi: 10.1097/JCP.0b013e318166c4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane RM. SSRI-induced extrapyramidal side effects and akathisia: implications for treatment. J Psychopharmacol. 1998;12(2):192–214. doi: 10.1177/026988119801200212. [DOI] [PubMed] [Google Scholar]
- 20.Gerber PE, Lynd LD. Selective serotonin-reuptake inhibitor–induced movement disorders. Ann Pharmacother. 1998;32(6):692–698. doi: 10.1345/aph.17302. [DOI] [PubMed] [Google Scholar]
- 21.Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry. 2003;160(9):1651–1658. doi: 10.1176/appi.ajp.160.9.1651. [DOI] [PubMed] [Google Scholar]
- 22.Keck PE, Jr, McElroy SL. Aripiprazole: a partial dopamine D2 receptor agonist antipsychotic. Expert Opin Investig Drugs. 2003;12(4):655–662. doi: 10.1517/13543784.12.4.655. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 24.Newcomer JW. Second-Generation (atypical) Antipsychotics and Metabolic Effects: a Comprehensive Literature Review. CNS Drugs. 2005;19(suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 25.Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763–771. doi: 10.4088/jcp.v63n0903. [DOI] [PubMed] [Google Scholar]
- 26.Kasper S, Lerman MN, McQuade RD, et al. Efficacy and safety of aripiprazole vs haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol. 2003;6(4):325–337. doi: 10.1017/S1461145703003651. [DOI] [PubMed] [Google Scholar]
- 27.Pigott TA, Carson WH, Saha AR, et al. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry. 2003;64(9):1048–1056. doi: 10.4088/jcp.v64n0910. [DOI] [PubMed] [Google Scholar]
- 28.Sachs G, Sanchez R, Marcus R, et al. Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: a 3-week placebo-controlled study. J Psychopharmacol. 2006;20(4):536–546. doi: 10.1177/0269881106059693. [DOI] [PubMed] [Google Scholar]
- 29.Vieta E, T'joen C, McQuade RD, et al. Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially non-responsive to valproate/lithium monotherapy: a placebo-controlled study. Am J Psychiatry. 2008;165(10):1316–1325. doi: 10.1176/appi.ajp.2008.07101560. [DOI] [PubMed] [Google Scholar]
- 30.Ketter TA, Wang PW, Chandler RA, et al. Adjunctive aripiprazole in treatment-resistant bipolar depression. Ann Clin Psychiatry. 2006;18(3):169–172. doi: 10.1080/10401230600801176. [DOI] [PubMed] [Google Scholar]