Abstract
Young children readily transmit and acquire nosocomial infections. Children are also vulnerable to endogenous infections as a result of the breakdown of their normal defences by disease, invasive procedures or therapy. The increasing acuity of illness in hospitalized children and therapeutic advances have resulted in a patient population that is increasingly at higher risk for nosocomial infections. Antibiotic resistance has emerged as a problem in some paediatric hospitals, usually in intensive care and oncology units. Infection rates are the highest in neonatal and paediatric intensive care units (where bloodstream infections are the most frequent), and are usually associated with intravascular devices. On general paediatric wards, respiratory and gastrointestinal infections predominate, reflecting the occurrence in the community. The surveillance of nosocomial infections identifies priorities for infection control activities and permits evaluation of interventions.
The prevention of transmission between patients and to personnel requires that certain measures be taken with all patients, and that additional precautions be taken with some infections, based on the route of transmission. The prevention of transmission from personnel involves ensuring that personnel are appropriately immunized and counselled about working with infections. The prevention of nosocomial infection also involves control of visitors, appropriate management of invasive procedures and devices, sterilization and disinfection of equipment, provision of a clean environment and adequate staffing. Severely immunocompromised children require extra protection, including ventilation systems that reduce the risk of exposure to filamentous fungi. Infection control in paediatrics is an evolving field that must adapt to changes in the paediatric patient population and in health care technology.
Keywords: Infection control, Nosocomial infections, Paediatric hospitals, Paediatric infections
RÉSUMÉ :
Les jeunes enfants transmettent et acquièrent facilement des infections nosocomiales. Les enfants sont également vulnérables aux infections endogènes par suite de l’effondrement de leurs défenses normales causé par la maladie, des interventions envahissantes ou un traitement. L’acuité croissante de la maladie chez les enfants hospitalisés et les progrès thérapeutiques résultent en une population de patients qui présente un risque plus élevé d’infections nosocomiales. L’antibiorésistance devient un problème dans certains hôpitaux pédiatriques, en général aux soins intensifs et dans les unités d’oncologie. Les taux d’infection sont les plus élevés dans les unités de soins intensifs pédiatriques et néonatales (où les infections sanguines sont les plus courantes) et s’associent généralement aux appareils intravasculaires. Dans les services de pédiatrie générale, les infections respiratoires et gastro-intestinales prédominent, ce qui reflète l’occurrence dans la collectivité. La surveillance des infections nosocomiales permet de repérer des priorités relativement aux activités de contrôle des infections ainsi que de procéder à une évaluation des interventions.
La prévention de la transmission entre les patients ou au personnel exige de prendre certaines mesures auprès de tous les patients et de prendre des précautions supplémentaires à l’égard de certaines infections, d’après la voie de transmission. La prévention de la transmission par le personnel exige une immunisation convenable du personnel et des conseils pertinents quant au travail avec des personnes infectées. La prévention des infections nosocomiales exige également un contrôle des visiteurs, une gestion convenable des interventions et des appareils envahissants, la stérilisation et la désinfection du matériel, la prestation d’un milieu propre et un personnel suffisant. Les enfants très immunocompromis ont besoin d’une protection supplémentaire, y compris des systèmes de ventilation qui réduisent le risque d’exposition aux champignons filamenteux. Le contrôle des infections en pédiatrie constitue un domaine en évolution qui doit s’adapter aux changements dans la population de patients pédiatriques et dans la technologie des soins de santé.
Children suffer significant morbidity and mortality from nosocomial infections. The consequences of these infections include prolongation of hospitalization, transfer to intensive care units, antibiotic therapy, placement or replacement of invasive devices and surgical procedures. The hospitalized paediatric population is changing. Organ and hematopoietic cell transplantation, more potent chemotherapy, human immunodeficiency virus infection, complex surgery for congenital malformations and the survival of extremely premature infants have resulted in an increasingly higher risk paediatric patient population. The transfer of many former inpatient care activities to ambulatory and day treatment centres has resulted in a higher acuity of illness in children who are hospitalized (1,2). The goal of infection control is to reduce the risk of acquiring infection in hospital to the lowest possible level. The present paper reviews the epidemiology of paediatric nosocomial infections and infection control strategies in acute care hospitals. It does not address the acquisition of infection in other health care settings for which there are limited data (3–6). The reader is referred to published recommendations for infection control in long term care, ambulatory care, home care and office practice (3,4).
YOUNG CHILDREN READILY TRANSMIT AND ACQUIRE INFECTIONS
Paediatric wards and hospitals are particularly suited to the transmission of infection. Infants and toddlers constitute a large proportion of the patients admitted. They frequently harbour infectious organisms and may shed pathogens, especially respiratory and gastrointestinal viruses, even if they are asymptomatic (1,7). Young children are also susceptible to many infections because they have not yet developed immunity. The close proximity of large numbers of infectious and susceptible hosts favours transmission. Behavioural characteristics of young children, such as incontinence, inadequate hygiene, frequent mouthing of hands and objects, drooling and direct contact between children during play, facilitate the spread of infection. Basic care requires frequent hands-on contact from health care personnel and parents. Multibed rooms, shared toys and playrooms, and visiting siblings contribute to the risk of transmission (1,2,8). Transmission rates increase with understaffing and overcrowding (9,10).
CHILDREN ARE VULNERABLE TO ENDOGENOUS INFECTIONS
Infection may also result from an altered relationship between the host and endogenous microbial flora due to the breakdown of normal barriers to infection by invasive procedures, disease or therapy (1,2,11). Invasive procedures include the insertion of intravascular, urinary and peritoneal dialysis catheters, endotracheal tubes, nasogastric and gastrostomy tubes, as well as endoscopic and surgical procedures. Young children have higher rates of catheter-associated bloodstream infections (BSIs), urinary tract infections (UTIs), and certain surgical site infections than older children and adults (2,12,13).
NORMAL AND ABNORMAL FLORA: ANTIMICROBIAL RESISTANCE
Normal flora live on the skin and mucous membranes, and in the gastrointestinal tract. Generally, they do not cause disease, unless they are allowed access to sterile body sites. This flora consists mainly of Bifidobacteriium species, sother Gram-positive anaerobes, coagulasenegative staphylococci (CONS) and alpha-hemolytic streptococci with smaller numbers of coliforms and Bacteroides species. Normal flora prevents pathogens from establishing themselves by blocking receptors on host cells and by competing for nutrients. Abnormal flora is acquired rapidly in hospital, especially in intensive care units (ICUs). Antibiotics play a major role by destroying normal flora; disturbing the balance of anaerobes, coliforms and cocci in the gastrointestinal tract; and allowing coliforms, resistant organisms and yeasts to flourish (2,14). Antimicrobial resistance usually arises in intensive care and oncology units, where the high risk of serious infection and difficulty in making definitive microbiological diagnoses result in the widespread empirical use of broadspectrum antibiotics (1,2). This situation is especially important in neonatal intensive care units (NICUs), where 75% of infants may receive antibiotics (15).
Methicillin-resistant Staphylococcus aureus (MRSA) is an important cause of nosocomial infection in some paediatric institutions (1,2). MRSA colonizes the nares and may be transmitted among patients via hand contact. Community-acquired MRSA is found with increasing frequency in children (16). Strains of S aureus with intermediate susceptibility to vancomycin have recently been described and pose a therapeutic challenge (17). Vancomycin-resistant enterococcus colonizes the gastrointestinal tract, is excreted in the stool, and survives on contaminated objects and surfaces in the patient’s environment. Colonization is much more common than infection, but serious infections do occur, especially in immunocompromised patients (18,19). Strains of Escherichia coli and Klebsiella species have recently developed extended spectrum beta-lactamases, which confer resistance to most beta-lactam antibiotics. Outbreaks have been reported in paediatric hospitals (20,21). Other Gramnegative rods (Pseudomonas, Enterobacter, Serratia and Citrobacter species) with inducible beta-lactamases may develop resistance during treatment, especially with third-generation cephalosporins. Strains of Stenotrophomonas maltophilia, Burkholderia cepacia and Acinetobacter species that are resistant to multiple antibiotics are encountered with increasing frequency (2).
Controlling the emergence of antimicrobial resistant organisms (AROs) involves the judicious use of antibiotics, limiting the use of antibiotic regimens that favour resistance, optimizing the choice and duration of empirical antibiotic therapy, and monitoring for resistance.
MAGNITUDE OF THE PROBLEM: RATES AND INFECTIONS
Infection rates are highest in NICUs and paediatric intensive care units (PICUs), higher in paediatric hospitals than on paediatric wards in general hospitals, and lowest (usually less than 1%) in normal newborn nurseries (1).
The immune system of the normal neonate is immature. Prematurity results in more severe immune defects and a dependence on life-saving procedures such as mechanical ventilation, intravascular access devices and parenteral nutrition. Infants with serious congenital malformations or neonatal disease may require complex surgery or mechanical support. Protective normal flora has not yet developed, and neonates admitted to NICUs rapidly acquire abnormal flora. Reported infection rates in the past decade vary from 19 to 28/100 admissions or 10 to 22/1000 patient days (1,11). Data collected by the United States National Nosocomial Infection Surveillance (NNIS) system indicated that BSIs accounted for 40% of infections, followed in frequency by pneumonia and gastrointestinal infections. CONS, S aureus, enterococci, Enterobacter species and E coli were the organisms that were reported most frequently (22). Candida species are an important cause of BSIs and disseminated infections, especially in very low birthweight infants and those receiving parenteral lipid.
In PICUs that participated in the NNIS, mean infection rates were 6.1/100 admissions and 14.1/1000 patient days. Bloodstream, pulmonary and urinary infections were the most frequent, and were usually associated with invasive devices. The organisms most frequently associated with BSIs, pneumonia, UTIs and surgical site infections were CONS, Pseudomonas aeruginosa, E coli and S aureus, respectively (1,23).
In contrast to intensive care units, nosocomial infections on general paediatric wards reflect the occurrence in the community, and respiratory and gastrointestinal infections predominate. Rates are the highest in children younger than two years of age (1,24,25). Respiratory syncytial virus (RSV) is the major cause of nosocomial lower respiratory tract infection (26). Parainfluenza, influenza, adenovirus, rotavirus and other gastrointestinal viruses are other common nosocomial pathogens. Varicella, pertussis and measles are less common, but are readily transmitted in paediatric settings (1,2,7).
Children with cancer and transplant recipients are at risk for life-threatening nosocomial infections with respiratory viruses and with fungi, especially Candida and Aspergillus species (1,26–28). BSIs are frequent in children who require long term venous access catheters for chemotherapy, parenteral nutrition or dialysis (12). Children with bronchopulmonary dysplasia or cyanotic heart disease may have serious consequences from respiratory viral infections (1,7,26). Cystic fibrosis patients may acquire resistant strains of B cepacia in medical settings (2).
SURVEILLANCE IS ESSENTIAL
Surveillance of nosocomial infection is an essential component of infection control. Surveillance permits the identification of problem infections or patient populations, early detection of clusters and trends, and evaluation of control measures. To be effective, results must be analyzed and distributed promptly, and should direct action.
The use of standard definitions, methods of casefinding and denominators for rate calculations allow an institution to compare its performance internally over time and externally with published rates (29,30). The intensity of surveillance may vary from total hospital surveillance to surveillance of high risk populations (eg, patients in the ICU, immunocompromised children) or specific infections (eg, catheter-associated BSIs or UTIs, ventilator-associated pneumonia, infections related to surgery). On general paediatric wards, surveillance for viral respiratory and gastrointestinal infections may be performed during seasons of high prevalence in the community. Each institution should set priorities based on local epidemiological data and resources available.
Denominators used for rate calculations must be relevant. Infection rates calculated by admissions are not useful for intra- or interhospital comparisons because the infection risk is related to the duration of hospitalization. Infection rates/1000 patient days are preferable and are useful where patient populations are stable, but do not take into account variations in patient risk factors. Device-associated infection rates reported/1000 device days provide the best risk adjustment at present. For surgical site infections, rates have traditionally been stratified by surgeon and wound infection risk. More recently, procedure-specific infection rates stratified by a risk index that incorporates a severity of illness score, the wound infection risk and the duration of surgery have been used for adults (29). This risk index has not been validated in the paediatric population.
The NNIS regularly publishes mean, median and percentile rates of device-associated infections in PICUs and NICUs. NICU rates are stratified by birthweight group. Surgical site infection rates by procedure and risk index are also reported (31). These rates may be used as benchmarks for individual institutions to assess their performances.
Organism-specific surveillance is indicated for certain rare, but serious, nosocomial infections (eg, filamentous fungi, tuberculosis, Legionella) and for AROs. It is important to distinguish between infection and colonization with AROs because colonization rates will be influenced by the intensity of screening.
PREVENTION OF TRANSMISSION FROM PATIENTS
Recent guidelines for the prevention of transmission of infection published in the United States (32) and Canada (3) are based on the principles that certain precautions are required for the care of all patients, regardless of diagnoses, and are determined by the task performed; and that further measures are required for patients with certain infections, and are determined by the route of transmission of the infection.
Routes of transmission
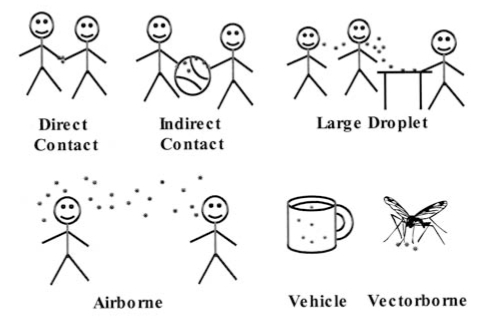
Understanding the routes of transmission of infection permits more efficient use of infection control precautions (3,32,33) (Figure 1).
Figure 1).
Routes of transmission of microorganisms
Contact transmission is the most frequent route of transmission in hospital, and includes direct contact (direct physical contact between infected and susceptible patients) and indirect contact (via contaminated intermediate surfaces such as the hands of personnel, bedrails, equipment and toys). Appropriate routine patient care practices should prevent most of the transmission by this route. Additional precautions (gloves, gowns and dedicated equipment) may be warranted for organisms of very low infective dose (eg, rotavirus) and for situations in which extensive contamination of the patient’s environment is expected (eg, watery diarrhea which cannot be contained within a diaper or a young child with respiratory infection and copious respiratory tract secretions). Respiratory and gastrointestinal viruses may remain viable on surfaces for several hours (1,7).
Droplet transmission is important in paediatrics. Large droplets are expelled from the respiratory tract and deposited onto the respiratory mucous membranes of persons close to the infected child. Special ventilation is not required because large droplets do not stay suspended in the air but settle on surfaces close to the source patient. Surgical masks are recommended for those within 1 m of the patient. Some organisms transmitted by this route (eg, Haemophilus influenzae type b, Neisseria meningitidis and Bordetella pertussis) are very fragile and do not survive in the environment or on hands. Other organisms, such as RSV, influenza, parainfluenza and rhinovirus, survive long enough on surfaces to be picked up on the hands of patients or personnel. Thus, respiratory viruses may be transmitted by the inhalation of large droplets or by the inoculation of nasal mucosa or conjunctiva by contaminated hands (contact) (7,26).
Airborne transmission occurs when infectious particles survive in aerosols of small droplet nuclei or skin squames, which remain suspended in the air and are dispersed over large distances by air currents. Organisms may be carried around corners, through corridors, and in and out of windows (2). Control requires a negative pressure room with air exhausted outside of the building or passed through a high efficiency particulate air filter before recirculation. Special dust mist masks are recommended for susceptible persons who must enter a patient’s room. Airborne transmission is uncommon, but important, because varicella, measles and tuberculosis are spread by this route. Although children with tuberculosis rarely transmit the infection, their adult visitors may have contagious tuberculosis and should be assessed (34). Whether airborne transmission of influenza occurs is controversial, but contact and large droplet transmission appear to be the major routes of transmission (3,32).
Common vehicle transmission refers to the infection of several persons by a single contaminated source such as food, water or medication. Such transmission is rare but important because it often results in an explosive outbreak that requires urgent investigation and intervention.
Vectorborne transmission refers to the spread of infection by insects, and is prevented by proper hospital construction and maintenance.
Routine practices
Enhanced infection control measures in routine patient care practice should reduce transmission from all patients, including those with asymptomatic or unrecognized infections. The American Academy of Pediatrics states that “each neonate should be approached as though he or she harboured colonies of unique flora that should not be transmitted to any other neonate” (35). This may become a reasonable principle for all patients in acute care hospitals.
Recommendations for routine practices include hand cleansing before and after patient contact, and after touching contaminated objects; the use of gloves for direct hand contact with blood, body fluids, excretions, mucous membranes or nonintact skin, or items visibly soiled with these substances; the use of masks, eye protection and gowns during activities most likely to generate splashes of blood or other body fluids; procedures to prevent injury from used needles and other sharp objects; and cleaning equipment between patients. Hand washing is the most important measure in the prevention of transmission, but this procedure is frequently neglected (1,3). Compliance may be increased by measures that make hand cleansing easier, such as more convenient sink placements and the provision of waterless, alcohol-based hand rinses (36). Current formulations of waterless hand rinses generally have less adverse effects on hands than soap and water.
Published guidelines may be interpreted as indicating that gloves are needed for many aspects of routine infant care, such as diaper changing, feeding a drooling child or wiping a child’s nose. Precautions need to be adapted to specific situations. Gloves are not mandatory for routine diaper changes in children if the procedure can be done without direct hand contact with stool (3,33), and may not be warranted for feeding and nose-wiping if gross contamination of the hands is avoided and hands are washed afterward (4).
Adherence to enhanced routine practices is particularly relevant during the current era of increasing colonization with AROs. Whether it is more effective to take cultures of patients at risk for AROs on admission and isolate those patients with positive cultures or to upgrade practices for all patients is controversial. At present, targeted screening for specific AROs and the isolation of patients who are colonized is the usual practice. However, patients without evident risk factors may be colonized with AROs, and as new AROs emerge, it may become impossible to identify all risk factors or impractical to screen for multiple AROs.
Additional precautions
Preadmission screening for signs of infection or for recent exposure of nonimmune patients to varicella or measles is essential to the identification of patients who require additional precautions (Table 1). Decisions should initially be based on clinical presentation because waiting for the confirmation of a diagnosis provides an opportunity for transmission to occur. Additional measures may include special air handling, accommodation in a singlebed room, or the use of gloves, masks or gowns (Table 2).
TABLE 1:
| Airborne precautions |
| Clinical presentation |
|
| Specific etiology |
|
| Droplet precautions |
| Clinical presentation |
|
| Specific etiology |
|
| Contact precautions |
| Clinical presentation |
|
| Specific etiology |
|
This list is not exhaustive. For infections not listed, see reference 29;
Less stringent precautions are recommended for adults (see reference 29). Use paediatric precautions for children who are incontinent or too immature to be able to comply with handwashing, appropriate handling and disposal of respiratory secretions, purulent discharges and skin exudates, and maintenance of dressings in place. For older children who are continent and able to comply, recommendations for adults may be used;
Young children with tuberculosis are rarely infectious, but their adult visitors may be and should be assessed for cough.
These recommendations are for possible Haemophilus influenzae type b (HIB) infection, and are not necessary if the child has received HIB vaccine;
It is controversial whether influenza can be transmitted by the airborne route. It may be prudent to use negative pressure rooms for patients with known or suspected influenza who must be accommodated in high risk areas such as oncology or bone marrow transplant units; RSV Respiratory syncytial virus
TABLE 2:
Additional (transmission-based) precautions
| Transmission category | Single room | Special ventilation | Precautions | Gown | Dedicated equipment | |
|---|---|---|---|---|---|---|
| Gloves | Masks | |||||
| Airborne | Yes | Yes | No | Yes | No | No |
| Droplet | Preferable* | No | No | Yes, if close to patient† | No | No |
| Contact | Preferable* | No | Yes | No | Yes‡ | Yes |
Not required if the child can be confined to an incubator, crib or bed. Children infected with the same pathogen may be cohorted;
If within 1 m of the source patient. Eye protection (face shields, goggles or glasses) may provide additional protection against infection with respiratory viruses;
For extensive contact with patient, or contaminated items or surfaces
A negative pressure room is essential for airborne precautions. For large droplet and contact precautions, single-bed rooms are preferable because they facilitate the physical separation of patients and control of the activities of their visitors, and deter the sharing of toys and equipment. However, many paediatric hospitals or wards do not have the numbers of single rooms required for optimal isolation. One Canadian study showed that isolation was required for 15% of all bed days (37). Another study showed that between 6% and 31% of children admitted per month required isolation (38). At the Montreal Children’s Hospital, Montreal, Quebec the author and her colleagues observed a need for the isolation of 62% of children admitted to paediatric medicine wards between October, 1996 and March, 1997 (unpublished data). Precautions may be taken in a shared room if a child can be confined to bed or crib, the space allocated to an individual child and family is clearly defined, and families and visitors are able to understand and comply with infection control measures.
Gloves are recommended for contact precautions. Whether gloves provide added benefit over careful handwashing is questionable, but glove use may compensate if handwashing is inadequate or not done. Wearing gloves may also deter personnel from inadvertently touching their mouths, noses or eyes during patient care. Masks protect personnel from the acquisition of infection by inhalation or splashes, and may also help to keep the hands away from the nose and mouth. Eye shields may give added protection against viruses that infect via the conjunctiva (7). Gowns protect the clothing and forearms in situations of extensive contact with a patient’s infective secretions, colonized skin or contaminated environmental surfaces (3,32).
Postexposure prophylaxis
Postexposure prophylaxis may be indicated for patients, families or personnel when precautions have not been taken and significant exposure has occurred. Immunoprophylaxis is indicated for some nonimmune individuals exposed to varicella, measles or hepatitis A. Antibiotic prophylaxis may be indicated after exposure to meningococcal or invasive H influenzae type b infection, pertussis or tuberculosis. Personnel should be aware of policies for prophylaxis after occupational exposure to bloodborne viruses.
PREVENTION OF TRANSMISSION FROM PERSONNEL
It is important that all personnel who care for children, including physicians, be immune to vaccine-preventable diseases such as measles, rubella, mumps, varicella, hepatitis B, polio and diphtheria, and receive yearly influenza vaccination (33). Patients may not be immune to these diseases because of their young age or illness, and personnel are at risk for occupational exposure. The above infections may be more severe in adults than in children, and immunization protects both personnel and their patients. The transmission of pertussis in hospitals is often associated with atypical pertussis in personnel, and vaccination should be considered when the acellular vaccine becomes available to adults. Personnel should undergo pre-employment assessment for tuberculosis exposure. Occupational health services play an essential role in the implementation of the above measures.
Personnel may acquire and transmit infections, such as respiratory viral infections, which may be minor in a healthy adult but have severe consequences in a young or immunocompromised patient (26). Counselling about work restrictions and measures to prevent transmission while working with an infection is required.
PREVENTION OF TRANSMISSION FROM VISITORS
Parents and families need to be informed about infection control issues and advised of the hazards of bringing visitors with infections into the hospital. Parents should be questioned about recent exposures and visiting siblings should be assessed for signs of transmissible infections. This assessment is particularly important for siblings visiting immunocompromised patients or patients in ICUs. Young children should be supervised by responsible adults, should not visit children other than their own siblings and should not use patients’ playrooms or toys (33). Adults with infections should be advised of precautions to take if they must visit. Appropriate management of visitors may be hampered by liberal visiting policies, coupled with insufficient personnel to provide monitoring, assessment and education.
PREVENTION OF INFECTIONS FROM INVASIVE PROCEDURES AND DEVICES
BSIs are the major device-associated infections in paediatrics (1,2,22,23,31). The infection of nontunnelled vascular catheters is usually due to the migration of microorganisms from the skin at the insertion site along the catheter tract and into the blood stream. With tunnelled catheters, contamination of the hub is important. Contamination of the infused fluid and hematogenous seeding of the catheter from a distant site are less common routes of infection. The risk of infection is reduced by the use of maximal sterile barriers, including full sterile surgical drapes during insertion, aseptic techniques for dressing changes, and appropriate handling of infusates and infusions sets. Infection rates are lower with Broviac-type catheters, which are tunnelled through subcutaneous tissue, and rates are the lowest with completely implanted ports (12). Coating catheters with antiseptics or antibiotics reduces the risk of early BSIs and may be beneficial for short term use in high risk patients. There is concern about the potential for the selection of resistant strains with antibiotic coated catheters, although this result has not been documented. New catheter materials that impede microbial adherence are being investigated.
UTIs are acquired by the spread of organisms along the catheter from the urethral meatus. Control involves using catheters only when essential and removing them as early as possible. Aseptic techniques for catheter insertion and handling slow the colonization process, but infection is inevitable with prolonged catheterization. In one PICU, infection rates were reduced by a policy of automatic catheter removal at 48 to 72 h, unless there was a specific order to continue catheterization (13). Antiseptic coated catheters are being evaluated.
Ventilator-associated pneumonia is less common in children than adults (1,31), perhaps in part due to underdiagnosis. Prevention involves sterile technique for suctioning, positioning the patient to prevent aspiration, early weaning and the decontamination of equipment (1,2). The use of selective decontamination of the gastrointestinal tract or prophylactic antibiotics is controversial (2).
Most surgical wound infections result from the contamination of the surgical site with skin flora of the patient or operating room personnel during surgery. The risk of contamination may be reduced by preoperative antiseptic baths, appropriately administered preoperative prophylactic antibiotics, sterile surgical procedures and techniques that minimize tissue damage (1,2). New surgical techniques via laparoscopy result in less tissue damage, a shorter postoperative stay and reduced infection rates, but may introduce new risks. The intricate equipment required may be difficult to sterilize, and outbreaks of infection have been associated with faulty processing (2).
As new devices or procedures are introduced, the potential for an effect on nosocomial infections should be assessed and monitoring systems should be set up.
IMMUNOCOMPROMISED CHILDREN REQUIRE PROTECTION
Immunocompromised children need to be protected from exposure to other patients with transmissible infections, especially respiratory viruses. They should be accommodated in single rooms or assigned carefully selected roommates. Handwashing before patient contact is essential, and the use of an antiseptic agent is sometimes recommended. The routine use of gloves, gowns or masks is controversial and may not be beneficial. Equipment should be reserved for the patient or disinfected before use (2,27).
Children with prolonged severe neutropenia, hematopoietic cell transplantation, recent organ transplantation and some other severe immune deficiencies require protection from exposure to fungal spores in dust and air. Rooms with positive pressure air flow and high efficiency particulate air filtration reduce exposure to airborne fungi, and should be considered for patients at highest risk. The patient’s room should be maintained free of dust by frequent cleaning, and all articles brought into the room should be clean and dust-free (1,2,27).
THE INANIMATE ENVIRONMENT AND INFECTION
Hospital design should permit the safe management of patients with infectious illnesses and patients who require protection by providing sufficient numbers and adequate placement of negative and positive pressure rooms; appropriate air filtration and ventilation; sufficient space to prevent crowding; and adequate numbers and placement of sinks and toilets (3,27,39). The provision of only single-bed rooms facilitates the implementation of infection control measures and the protection of high risk patients, obviates the need for transfers when infections occur, and provides privacy for patients and families (38).
Prevention of the acquisition of infection from the inanimate environment involves appropriate cleaning, disinfection and sterilization of equipment, cleaning of patient care areas, maintenance of ventilation and plumbing, and measures to protect patients from dust and contaminated air during construction or renovation (39,40).
CONCLUSIONS
Control of nosocomial infections in children is an ongoing challenge. Hospitalized children today have an increasing severity of illness and an increasing degree of immunocompromise. As certain infection risks have been reduced by immunizations, improved treatment and technological advances, new risks have emerged. Transmission of infection between patients is theoretically achievable with current procedures, but implementation may be hampered by inadequate facilities, insufficient personnel, a lack of an appreciation of the impact of nosocomial infections and issues of feasibility. Balancing the benefits and costs of prevention, preventing infections associated with invasive procedures and devices, and protecting immunocompromised patients are major challenges.
Effective infection control programs require specifically trained infection control practitioners, the involvement of physicians, nurses and administrators, and strategies to educate hospital personnel. Programs must be visible and proactive, and must evolve with the changing epidemiology of paediatric nosocomial infections.
Footnotes
Internet addresses are current at the time of publication
REFERENCES
- 1.Jarvis WR, Robles B. Nosocomial infections in pediatric patients. Adv Pediatr Infect Dis. 1997;12:243–95. [PubMed] [Google Scholar]
- 2.Huskins WC, Goldmann DA. Nosocomial infections. In: Feigin RD, Cherry JD, editors. Textbook of Pediatric Infectious Diseases. 4th edn. Philadelphia: Saunders; 1998. pp. 2545–85. [Google Scholar]
- 3.Health Canada Steering Committee on Infection Control Guidelines Routine practices and additional precautions for preventing the transmission of infection in health care Can Commun Dis Rep 1999Suppl 25S41–142.<http://www.hc-sc.gc.ca/hpb/lcdc/dpg_e.html#infection> (Version current at October 12, 2001) [PubMed]
- 4.American Academy of Pediatrics Committee on Infectious Diseases and Committee on Practice and Ambulatory Medicine Infection control in physician’s offices Pediatrics 20001051361–9.10835084 [Google Scholar]
- 5.Vermaat JH, Rosebrugh E, Ford-Jones EL, Ciano J, Kobayashi J, Miller G. An epidemiologic study of nosocomial infections in a pediatric long-term care facility. Am J Infect Control. 1993;21:183–8. doi: 10.1016/0196-6553(93)90029-4. [DOI] [PubMed] [Google Scholar]
- 6.Lobovits AM, Freeman J, Goldmann DA, McIntosh K. Risk of illness after exposure to a pediatric office. N Engl J Med. 1985;313:425–8. doi: 10.1056/NEJM198508153130706. [DOI] [PubMed] [Google Scholar]
- 7.Graman PS, Hall CB. Epidemiology and control of nosocomial viral infections. Infect Dis Clin North Am. 1989;3:815–41. [PubMed] [Google Scholar]
- 8.Harris JS. Pediatric nosocomial infections: Children are not little adults. Infect Control Hosp Epidemiol. 1997;18:739–42. doi: 10.1086/647526. [DOI] [PubMed] [Google Scholar]
- 9.Haley RW, Cushion NB, Tenover FC, et al. Eradication of endemic methicillin-resistant Staphylococcus aureus infections from a neonatal intensive care unit. J Infect Dis. 1995;171:614–24. doi: 10.1093/infdis/171.3.614. [DOI] [PubMed] [Google Scholar]
- 10.Archibald LK, Manning ML, Bell LM, Banerjee S, Jarvis WR. Patient density, nurse-to-patient ratio and nosocomial infection risk in a pediatric cardiac intensive care unit. Pediatr Infect Dis J. 1997;16:1045–8. doi: 10.1097/00006454-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Moore DL. Nosocomial infections in newborn nurseries and neonatal intensive care units. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. 2nd edn. Philadelphia: Lippincott, Williams and Wilkins; 1999. pp. 665–93. [Google Scholar]
- 12.Salzman MB, Rubin LG. Intravenous catheter-related infections. Adv Pediatr Infect Dis. 1995;10:337–68. [PubMed] [Google Scholar]
- 13.Davies HD, Ford Jones EL, Sheng RY, Leslie B, Matlow AG, Gold R. Nosocomial urinary tract infections at a pediatric hospital. Pediatr Infect Dis J. 1992;11:349–54. doi: 10.1097/00006454-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Jarvis WR. The epidemiology of colonization. Infect Control Hosp Epidemiol. 1996;17:47–52. doi: 10.1086/647189. [DOI] [PubMed] [Google Scholar]
- 15.Lee SK, McMillan DD, Ohlsson A, et al. Variations in practice and outcomes in the Canadian NICU network: 1996–1997. Pediatrics. 2000;106:1070–9. doi: 10.1542/peds.106.5.1070. [DOI] [PubMed] [Google Scholar]
- 16.Hussain FM, Boyle-Vavra S, Bethel C, Daum RS. Current trends in community-acquired methicillin-resistant Staphylococcus aureus at a tertiary care pediatric facility. Pediatr Infect Dis J. 2000;19:1163–6. doi: 10.1097/00006454-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Hunstad DA, St Geme JW. Staphylococcus aureus with reduced susceptibility to glycopeptide antibiotics. Pediatr Infect Dis J. 2000;19:1093–5. doi: 10.1097/00006454-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Gray JW, George RH. Experience of vancomycin-resistant enterococci in a children’s hospital. J Hosp Infect. 2000;45:11–8. doi: 10.1053/jhin.1999.0724. [DOI] [PubMed] [Google Scholar]
- 19.Hayden MK. Insights into the epidemiology and control of infection with vancomycin-resistant enterococci. Clin Infect Dis. 2000;31:1058–65. doi: 10.1086/318126. [DOI] [PubMed] [Google Scholar]
- 20.Podschun R, Ullmann U. Klebsiella spp as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebuck JA, Olsen KM, Fey PD, Langnas AN, Rupp ME. Characterization of an outbreak due to extended-spectrum ß-lactamase-producing Klebsiella pneumoniae in a pediatric intensive care unit transplant population. Clin Infect Dis. 2000;31:1368–72. doi: 10.1086/317474. [DOI] [PubMed] [Google Scholar]
- 22.Gaynes RP, Edwards JR, Jarvis WR, Culver DH, Tolson JS, Martone WJ, the National Nosocomial Infections Surveillance System Nosocomial infections among neonates in high-risk nurseries in the United States. Pediatrics. 1996;98:357–61. [PubMed] [Google Scholar]
- 23.Richards MJ, Edwards JR, Culver DH, Gaynes RP, the National Nosocomial Infections Surveillance System Nosocomial infections in pediatric intensive care units in the United States. Pediatrics. 1999;103:e39. doi: 10.1542/peds.103.4.e39. [DOI] [PubMed] [Google Scholar]
- 24.Welliver RC, McLaughlin S. Unique epidemiology of nosocomial infection in a children’s hospital. Am J Dis Child. 1984;138:131–5. doi: 10.1001/archpedi.1984.02140400017004. [DOI] [PubMed] [Google Scholar]
- 25.Ford-Jones EL, Mindorff CM, Langley JM, et al. Epidemiologic study of 4684 hospital-acquired infections in pediatric patients. Pediatr Infect Dis J. 1989;8:668–75. doi: 10.1097/00006454-198910000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Hall CB. Nosocomial respiratory syncytial virus infections: The “cold war” has not ended. Clin Infect Dis. 2000;31:590–6. doi: 10.1086/313960. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients: Recommendations of CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow Transplantation MMWR Mortal Morb Wkly Rep 200491–125.<http://www.cdc.gov/ncidod/hip/Guide/guide.htm> (Version current at October 12, 2001) [PubMed] [Google Scholar]
- 28.Green M, Michaels MG. Infectious complications of solid-organ transplantation in children. Adv Pediatr Infect Dis. 1992;7:181–204. [PubMed] [Google Scholar]
- 29.Emori TG, Culver DH, Horan TC, et al. National nosocomial infections surveillance system (NNIS): Description of surveillance methods. Am J Infect Control. 1991;19:19–35. doi: 10.1016/0196-6553(91)90157-8. [DOI] [PubMed] [Google Scholar]
- 30.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 31.Hospital Infections Program, National Center for Infectious Diseases, Centers for Disease Control and Prevention National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992-April 2000, issued June 2000. Am J Infect Control. 2000;28:429–48. doi: 10.1067/mic.2000.110544. [DOI] [PubMed] [Google Scholar]
- 32.Garner JS, the Hospital Infection Control Practices Advisory Committee Guideline for isolation precautions in hospitals Infect Control Hosp Epidemiol 19961753–80.<http://www.cdc.gov/ncidod/hip/Guide/guide.htm> (Version current at October 12, 2001) [DOI] [PubMed] [Google Scholar]
- 33.American Academy of Pediatrics . Infection control for hospitalized children. In: Pickering LK, editor. Red Book: Report of the Committee on Infectious Diseases. 25th edn. Elk Grove Village: American Academy of Pediatrics; 2000. 2000. pp. 127–38. [Google Scholar]
- 34.Aznar J, Hassan S, Romero J, Alejo A, Gracia A, Palomares JC. Nosocomial transmission of tuberculosis infection in pediatrics wards. Pediatr Infect Dis J. 1995;14:44–8. doi: 10.1097/00006454-199501000-00009. [DOI] [PubMed] [Google Scholar]
- 35.American Academy of Pediatrics, American College of Obstetricians and Gynecologists . Infection control. In: Hauth JC, Merenstein GB, editors. Guidelines for Perinatal Care. 4th edn. Elk Grove Village: American Academy of Pediatrics; 1997. pp. 251–77. [Google Scholar]
- 36.Pittet D. Improving compliance with hand hygiene in hospitals. Infect Control Hosp Epidemiol. 2000;21:381–6. doi: 10.1086/501777. [DOI] [PubMed] [Google Scholar]
- 37.Kim M-HM, Mindorff C, Patrick ML, Gold R, Ford-Jones EL. Isolation usage in a pediatric hospital. Infect Control. 1987;8:195–9. doi: 10.1017/s0195941700065930. [DOI] [PubMed] [Google Scholar]
- 38.Langley JM, Hanakowski M, Bortolussi R. Demand for isolation beds in a pediatric hospital. Am J Infect Control. 1994;22:207–11. doi: 10.1016/0196-6553(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 39.Bartley JM. APIC State-of-the-art report: The role of infection control during construction in health care facilities. Am J Infect Control. 2000;28:156–69. [PubMed] [Google Scholar]
- 40.Health Canada. Infection Control Guidelines Hand washing, cleaning, disinfection and sterilization in health care Can Commun Dis Rep 199824S81–55.<http://www.hc-sc.gc.ca/hpb/lcdc/dpg_e.html#infection> (Version current at October 12, 2001) [PubMed] [Google Scholar]