Abstract
To identify genes involved in macrophage development, we used the differential display technique and compared the gene expression profiles for human myeloid HL-60 leukemia cell lines susceptible and resistant to macrophage maturation. We identified a gene coding for a protein kinase, protein kinase X (PRKX), which was expressed in the maturation-susceptible, but not in the resistant, cell line. The expression of the PRKX gene was found to be induced during monocyte, macrophage, and granulocyte maturation of HL-60 cells. We also studied the expression of the PRKX gene in 12 different human tissues and transformed cell lines and found that, among these tissues and cell types, the PRKX gene is expressed only in blood. Among the blood cell lineages, the PRKX gene is specifically expressed in macrophages and granulocytes. Antisense inhibition of PRKX expression blocked terminal development in both the leukemic HL-60 cells and normal peripheral blood monocytes, implying that PRKX is a key mediator of macrophage and granulocyte maturation. Using the HL-60 cell variant deficient in protein kinase C-β (PKC-β) and several stable PKC-β transfectants, we found that PRKX gene expression is under control of PKC-β; hence PRKX is likely to act downstream of this PKC isozyme in the same signal transduction pathway leading to macrophage maturation.
Macrophages and granulocytes are blood cells that orchestrate the processes of inflammation and tissue repair and together with lymphocytes evoke immune responses. Their differentiation and maturation is initiated either extrinsically, by interaction of specific hematopoietic cytokines with appropriate receptors on progenitor cells or intrinsically, by stochastic processes (1–4). The progenitor cells then develop, via kinase-mediated signaling pathways, into one or another myeloid lineage, such as macrophages or granulocytes. Currently characterized signal transduction pathways are, to a large degree, shared by different lineages during maturation (5, 6).
The human myeloid leukemia HL-60 cell line is commonly used as a model system for studying myeloid cell development (7–15) because these cells can be induced to develop into macrophages (7, 8), monocytes (9), and granulocytes (10, 11) and exhibit useful functional and morphological markers upon maturation. Macrophage maturation in HL-60 cells is initiated upon activation of protein kinase C (PKC), which acts as both a specific receptor and a functional signal transduction protein (12, 13). We have developed a variant of HL-60 cells that is deficient in PKC-β, an abundant isoform of PKC in the HL-60 cell line (13, 14). This cell variant, HL-525, is resistant to macrophage maturation induced by a known PKC activator, phorbol 12-myristate 13-acetate (PMA), but sensitive to inducers of monocyte and granulocyte differentiation (12, 14).
To identify genes involved in macrophage development, we used HL-60 and HL-525 cells as a model system and used the mRNA differential display technique (16). By using this approach, we identified a myeloid-specific protein kinase, protein kinase X (PRKX), which we demonstrate to be a key mediator of macrophage and granulocyte development.
MATERIALS AND METHODS
Cells and Cell Culture.
Human myeloid leukemia cell line HL-60 was originally obtained from R. C. Gallo (National Cancer Institute, Bethesda, MD). The differentiation-resistant cell variant HL-525 was obtained by subculturing HL-60 cells 102 times in the presence of increasing concentrations (up to 3 μM) of PMA at 5- to 8-day intervals (12). Human monocytes, granulocytes, and lymphocytes were isolated from peripheral whole venous blood by Ficoll-Hypaque density gradient (1.077 g/ml) centrifugation as previously described (17). Contamination of the lymphocyte preparations with monocytes was minimized by allowing monocytes to adhere to tissue culture dishes during two successive 30-min incubations.
Cells were inoculated into 60-, 100-, or 150-mm dishes (Falcon) or 16-well Lab-Tek chamber slides (Nunc) at 1–1.5 × 105 cells/ml of RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Intergen, Purchase, NY), 100 units/ml penicillin, and 100 μg/ml streptomycin (GIBCO/BRL). The cells were incubated at 37°C in humidified air containing 8% CO2.
Induction of Cell Terminal Development and Maturation Markers.
For induction of terminal development, the cells were inoculated at 2 × 104 cells/ml in RPMI medium 1640. The cells were treated with 3–30 nM PMA (LC Laboratories, La Jolla, CA), 1 μM 1α,25-dihydroxy-vitamin D3 (vitD3; Calbiochem), 1.25% dimethyl sulfoxide (DMSO) (Sigma), or 250 units/ml macrophage colony-stimulating factor (M-CSF, BioSource International, Camarillo, CA). To measure maturation markers, the number of cells was determined by hemocytometer chamber counting, the fraction of attached and spread cells was determined by light microscopy, and the percentage of cells exhibiting the MAC-1 cell surface maturation antigen was determined by immunostaining as we have described previously (18). Phagocytosis was determined by the ability of cells to ingest fluorescent beads (18). The morphological changes associated with maturation were assessed according to ref. 18. The presence of hemoglobin in differentiating K-562 was determined by the benzidine-staining assay (19). As an additional myeloid maturation marker, cells were monitored by Northern blot analysis for the expression of X-linked chronic granulomatous disease (X-CGD) gene, a gene previously shown to be a marker of myeloid maturation (20).
Analysis of Differential Gene Expression.
Total cellular RNA was isolated by centrifugation through a 5.7-M cesium chloride cushion as described by Chirgwin et al. (21) and treated with RNase-free DNase I (GenHunter, Nashville, TN) for 30 min at 37°C in a buffer containing 10 mM Tris⋅HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, and 0.001% gelatin. Differential display experiments were carried out by using RNAimage kits (GenHunter) according to the manufacturer’s instructions, using HL-60 and HL-525 cell total RNA as substrates. Differentially expressed cDNAs were excised from the gel, eluted with buffer containing 0.5 M sodium acetate, 10 mM magnesium acetate, 1 mM EDTA (pH 8), and 0.1% SDS, purified by using a QIAEX II kit (Qiagen, Chatsworth, CA), and reamplified by using the same set of primers and PCR. Differentially expressed cDNAs were cloned into a PcrTRAP vector (GenHunter) and partially sequenced by using the fmol DNA sequencing kit (Promega). The cDNA sequences were aligned with GenBank entries by using the blast sequence analysis program (22).
Poly(A)+ RNA for Northern blots was isolated by affinity chromatography by using an Oligo(dT) Cellulose column (GIBCO/BRL). Northern blot analysis was performed as previously described (14). To confirm the amounts of RNA loaded onto each lane, all blots were hybridized with a glyceraldehyde phosphate dehydrogenase probe. Tissue specificity of PRKX gene expression was studied by using a normal human tissue total RNA blot (Invitrogen) as well as RNA isolated from human blood cells and various cell lines. Either the coding region of PRKX cDNA (23) or the fragment isolated by differential display was used to obtain a probe for Northern blotting. Reverse transcription–PCR (RT-PCR) was performed as described earlier (24).
Anti-Sense Oligonucleotide Inhibition of PRKX Gene Expression.
Based on the analysis of PRKX mRNA secondary structure, we selected two unprotected pentadecamers, ANTISENSE1 (GGCGGCATCAACAGA) and ANTISENSE2 (AGGTCCGGGCACCGG), complementary to the regions of PRKX mRNA 14 and 102 nucleotides, respectively, upstream of the translation start codon (23). In control assays, the corresponding sense oligomers were used. To retard oligonucleotide degradation, the cells were pelleted, resuspended in serum-free RPMI medium 1640 containing the oligomers at 10 μM, and incubated for 8 hr. Bovine fetal serum and PMA (for HL-60) or M-CSF (for monocytes) were then added. After 2-hr incubation with PMA, a fraction of the HL-60 cells was lysed, total RNA was isolated, and the level of the X-CGD and PRKX transcripts was determined by Northern blot analysis. After 48-hr incubation with the inducers, the second fraction of cells was examined for appearance of myeloid maturation markers.
Plasmids and Transfection Analyses.
The 5′-untranslated region (UTR) of the PRKX cDNA was isolated as a SalI–NcoI fragment from +1 to +369 of the cDNA (23). This fragment was rendered blunt-ended with Pfu polymerase and ligated into the tetracycline responsive cytomegalovirus plasmid pTRE (CLONTECH) in the blunted EcoRI site. Plasmids expressing the PRKX 5′-UTR in the sense and antisense orientations were isolated, creating plasmids pTRE-PRKX-UTR and pTRE-PRKX-UTRα, respectively. For the creation of a plasmid expressing the cDNA as an antisense RNA, the BamHI–SalI fragment containing the PRKX-coding region and 5′-UTR was isolated, blunted, and cloned into the blunted EcoRI site of pTRE, creating plasmid pTRE-PRKX-cDNAα. To create an expression vector for PRKX, the aforementioned BamHI–SalI fragment was cloned into pTRE in the sense orientation. Plasmid pMV7-RP58 expressing a full length rat PKC-βI cDNA was a kind gift of I. B. Weinstein (Columbia University, New York).
For transient transfection analyses, cells were concentrated by centrifugation, washed twice in PBS, and resuspended in PBS at 2.5 × 107 cells/ml. Plasmids were sterilized by ethanol precipitation and resuspended in PBS. For each transfection, 5 μg of each plasmid was combined with PBS to a volume of 200 μl, mixed with 200 μl of the cell suspension in an 0.2-cm electroporation cuvette, and allowed to equilibrate for 10 min on ice. DNA concentrations were maintained equivalent between transfections by the addition of inert carrier plasmid. Electroporation was then performed by using a T820 ElecroSquarePorator square-wave electroporation device (BTX, San Diego) delivering two pulses of 500 V for 99 μsec/pulse. Cells were then diluted with 4 ml of ice-cold RPMI medium 1640 and incubated on ice for 10 min before plating. After 18 hr of recovery, PMA (10–30 nM) and/or tetracycline (10 μg/ml) was added to the cells, and the cells were incubated for 24 or 48 hr and then assayed for the aforementioned myeloid maturation markers.
RESULTS
Analysis of Differential Gene Expression.
To identify genes involved in macrophage development, we used the differential display technique (16) with RNA from HL-60 and HL-525 cells. The latter cell line has been developed earlier in our laboratory as a variant of HL-60 resistant to PMA-induced macrophage maturation (12, 14). We hypothesized that any genes proven to be suppressed or absent in the PMA-resistant cell line may be associated with macrophage development. To reduce a potential overload of genes whose expression is associated with the function of the mature cells rather than with the process of maturation induction, we used uninduced cells as a source of RNA.
A total of 19 cDNAs showed differential expression between HL-60 and HL-525 cells. Each cDNA fragment was excised from the gel, purified, and reamplified by using the corresponding set of anchoring and arbitrary primers. The reamplified cDNAs were then used to screen HL-60 and HL-525 Poly(A)+ RNA blots for differential expression. Three of the 19 isolated cDNA fragments were confirmed to be differentially expressed by Northern blot analysis. For two of them (8.1 and 8.4), a transcript was observed in HL-60, but not in HL-525 cells, and for one of them (19.1) there was a quantitative difference; specifically, the signal on the HL-60 lane was approximately 10-fold stronger than that on the HL-525 lane. All three cDNAs were cloned; the results of sequence analysis indicate that cDNA 8.1 showed a 98% identity with the chronic granulomatous disease gene (X-CGD, GenBank accession no. X04011), cDNA 8.4 exhibited a 99% identity with the human protein kinase X1 (PRKX) gene (X85545), and cDNA 19.1 was identical to the human HLA class 1 antigen gene (X07061).
In further studies, we concentrated on the PRKX gene. This gene has been identified earlier based on its chromosomal location; its product, PRKX, was classified as a serine/threonine protein kinase; its biological function was not determined (23). It is noteworthy, however, that the differential expression of the other two genes, namely X-CGD and HLA class I, is consistent with the literature data. It has been previously shown (20) that the X-CGD gene is markedly induced in HL-60 cells during granulocytic and monocytic/macrophage maturation. The expression of the HLA genes has been shown to be potentiated by PKC (25), a major form of which, PKC-β, is abundant in HL-60, but not in HL-525 cells (12, 14).
Induction of PRKX Gene Expression During Maturation of HL-60 Cells.
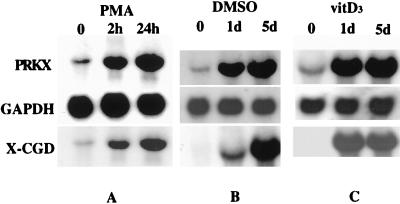
To study the expression of the PRKX gene during myeloid maturation, we induced HL-60 cells to mature along the macrophage, monocytic, and granulocytic pathways by treatment with the known inducers, PMA (7, 8), 1α,25-dihydroxy-vitamin D3 (vitD3) (9), and DMSO (10, 11), respectively. The mature phenotype was confirmed by growth inhibition, morphological changes, cell attachment and spreading, phagocytosis, and manifestation of a specific cell surface maturation antigen, MAC-1 (18). As an additional indicator of maturation, we used the expression of the X-CGD gene. This gene codes for a protein that is a part of the respiratory-burst oxidase complex specific to mature myeloid cells; its expression indicates maturation in HL-60 and related cell types (20). Northern blot analysis revealed that expression of the PRKX gene was markedly induced during HL-60 cell maturation along all three pathways (Fig. 1). The weak signal in uninduced HL-60 can be ascribed to the presence of a small fraction of spontaneously differentiated cells (7). It is noteworthy that PRKX gene expression also was induced in PMA-resistant HL-525 cells upon DMSO- and vitD3-induced maturation (data not shown).
Figure 1.
Northern blot of PRKX mRNA levels. Induction of the PRKX gene expression during macrophage (A), granulocytic (B), and monocytic (C) maturation of HL-60 cells. Glyceraldehyde phosphate dehydrogenase probe was used to quantify the RNA loaded onto each lane. The X-CGD gene expression was used as a marker of myeloid maturation.
Specificity of PRKX Gene Expression.
To determine whether PRKX is a blood cell-specific enzyme, we studied PRKX gene expression in several human tissues, including blood, heart, brain, kidney, liver, lung, pancreas, spleen, and muscle, as well as in a number of cell lines induced to differentiate with PMA, including AU-565 and MB-231 (26) (breast carcinoma), HT-1080 (27) (fibrosarcoma), and SK-MEL-131 (28) (melanoma). Among these tissues and cell lines, the PRKX gene was expressed only in blood cells (data not shown). These results are inconsistent with the data of Klink et al. (23), who observed a weak signal on poly(A)+ RNA Northern blots for PRKX mRNA in kidney, lung, and liver. This observation can be attributed to the presence of trace amounts of blood in the tissues used for RNA isolation. We did not observe such signals, most likely because we used total RNA rather than poly(A)+ RNA.
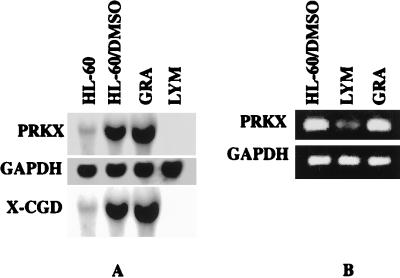
To gain further insight into the specificity of PRKX gene expression, we studied the expression of its gene in different blood cell lineages. First, lymphocytes and granulocytes were isolated from peripheral blood of healthy donors and PRKX gene expression was examined in these cells; granulocytic HL-60 cells were used as a positive control. It can be seen in Fig. 2A that the PRKX gene is expressed in granulocytes, whereas no PRKX transcript is detected in lymphocytes. We expected that there may be some contamination of our lymphocyte preparation by monocytes and granulocytes due to our method of fractionation. Such contamination was not evident by using Northern blot analysis. We therefore analyzed PRKX gene expression in lymphocytes and granulocytes by the more sensitive RT-PCR technique. Two sets of PRKX cDNA-directed primers were used, resulting in amplification of fragments of 466 and 231 bp. A series of dilutions was made to ensure that amplification of the fragments corresponded to the input of the RNA samples and number of cycles. The results of the RT-PCR analysis (Fig. 2B) are consistent with the Northern blots; we attribute the presence of a faint band on the lymphocyte lane to the expected monocyte/granulocyte contamination.
Figure 2.
Expression of the PRKX gene in human granulocytes and lymphocytes analyzed by Northern blot analysis (A) and RT-PCR (B). Granulocytic HL-60 cells were used as a control. RT-PCR analysis did not reveal any PCR transcript in any of the tested human cell lines, including erythroid and megacaryocytic K-562 cells.
To investigate potential PRKX gene expression during megacaryocytic and erythroid differentiation, human K562 leukemia cells were induced to differentiate along these lineages by treatment with PMA (megacaryocytic) (29), or with guanosine, aphidicolin, or mycophenolic acid (erythroid) (30). No PRKX gene expression was detected in either untreated or treated K562 cells. It therefore appears that PRKX is specific for monocyte/macrophage and granulocyte lineages.
Inhibition of PRKX Gene Expression.
To gain further insight into the role of PRKX in macrophage and granulocyte development, we studied the effect of inhibiting PRKX gene expression on induction of maturation in HL-60 cells and normal human peripheral blood monocytes. We assumed that if PRKX is critical for terminal development, inhibition of its gene expression should block the maturation program and prevent the manifestation of the maturation markers. Two methods of targeted inhibition of gene expression were used.
First, we used antisense oligodeoxyribonucleotides to block translation of the PRKX mRNA, an approach that has been widely used to study the role of various genes in HL-60 cell proliferation and differentiation (for examples, see refs. 31–33). To choose the target site, the secondary structure of PRKX mRNA was predicted by using the foldrna program (34), and the antisense oligonucleotides were directed against predicted single stranded structures located near the translation start codon. We used pentadecamers, because this length is sufficient for theoretical uniqueness within the human genome, but at the same time short enough to ensure strong binding to single-stranded regions of mRNA and adequate binding to regions that have some secondary or tertiary structure limitations (32).
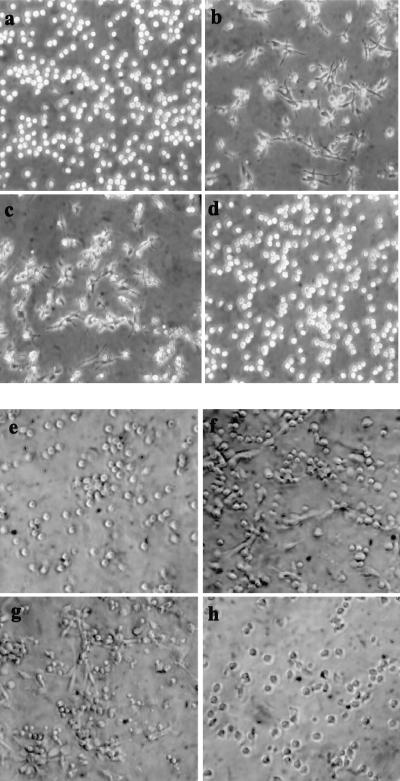
After treatment of HL-60 cells with PMA in the presence of antisense or sense oligonucleotides, we measured cell proliferation and assessed the maturation markers. The results indicate that after PMA was added, most of the cells incubated with the antisense oligomers continued to proliferate and failed to manifest markers of the macrophage phenotype, including cell adherence and spreading (Fig. 3), phagocytosis, and reactivity with the anti-MAC-1 antibody (Table 1). At the same time, the cells incubated with the sense oligonucleotide and treated with PMA behaved like the control PMA-treated cells, i.e., acquired the mature macrophage phenotype (Table 1; Fig. 3). The oligomers had a similar effect on DMSO-induced granulocytic maturation of HL-60 cells, as judged by changes in cell proliferation and reactivity with the specific antibody (Table 2) as well as by changes in cell morphology. In the absence of the maturation inducers, the oligonucleotides had little to no effect on cell proliferation.
Figure 3.
Light microscope images of HL-60 cells and human peripheral blood monocytes incubated with sense and antisense oligonucleotides to PRKX and induced to mature. (a) HL-60 cells; (b) HL-60 cells induced to mature with PMA; (c) HL-60 cells incubated with SENSE1 and induced with PMA; (d) HL-60 cells incubated with ANTISENSE1 and induced with PMA; (e) peripheral blood monocytes; (f) monocytes induced to mature with M-CSF; (g) monocytes incubated with SENSE1 and induced with M-CSF; (h) monocytes incubated with ANTISENSE1 and induced with M-CSF.
Table 1.
Effect of antisense oligonucleotides to PRKX on PMA-induced macrophage maturation of HL-60 cells
| HL-60 cells treated with | Cell number, × 104 cells/ml | Adherent and spread cells, % | Phagocytic cells, % | Cells reacting with anti-MAC1 antibody, % |
|---|---|---|---|---|
| — | 125 ± 11 | <1 | 6 ± 3 | <1 |
| PMA | 47 ± 5 | 72 ± 13 | 91 ± 5 | 83 ± 7 |
| PMA + SENSE1 | 40 ± 12 | 76 ± 15 | 84 ± 5 | 77 ± 4 |
| PMA + ANTISENSE1 | 117 ± 25 | 3 ± 1 | 5 ± 1 | 5 ± 2 |
| PMA + SENSE2 | 41 ± 6 | 82 ± 3 | 79 ± 3 | 88 ± 3 |
| PMA + ANTISENSE2 | 93 ± 16 | 5 ± 1 | 12 ± 3 | <1 |
In the absence of the differentiation inducers, the oligonucleotides neither affected cell proliferation nor induced appearance of any maturation markers. The data represent the mean results of three independent experiments ± SD.
Table 2.
Effect of antisense oligonucleotides to PRKX on DMSO induced granulocyte maturation of HL-60 cells
| HL-60 cells treated with | Cell number, ×104 cells/ml | Cells reacting with anti-MAC1 antibody, % |
|---|---|---|
| – | 57 ± 5 | <1 |
| DMSO | 7 ± 2 | 53 ± 4 |
| DMSO + SENSE1 | 8 ± 3 | 49 ± 5 |
| DMSO + ANTISENSE1 | 51 ± 6 | 11 ± 4 |
| DMSO + SENSE2 | 11 ± 3 | 61 ± 6 |
| DMSO + ANTISENSE2 | 61 ± 6 | 6 ± 1 |
In the absence of the differentiation inducers, the oligonucleotides neither affected cell proliferation nor induced appearance of any maturation markers. The data represent the mean results of three independent experiments ± SD.
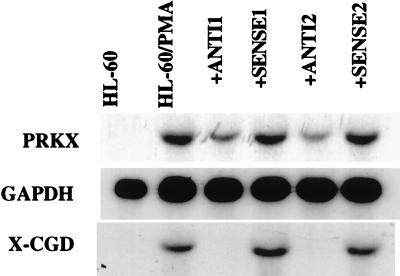
Northern blot analysis was used to confirm that the antisense oligonucleotides indeed inhibited PRKX gene expression in the HL-60 cell system. The level of PRKX mRNA in PMA-treated cells incubated with the antisense oligomers was substantially decreased compared with those incubated with the sense oligonucleotides and the control PMA-treated cells (Fig. 4), demonstrating that the antisense oligonucleotides indeed affected the expression of the PRKX gene. The mechanism of this inhibition may involve RNase H-catalyzed hydrolysis of the RNA–DNA duplex formed by PRKX mRNA and the antisense oligonucleotide (35). This assumption is consistent with the observed decrease in the PRKX transcript level. The expression of the X-CGD myeloid maturation marker gene also was not induced in the cells incubated with the antisense oligonucleotides (Fig. 4), validating our conclusion on their ability to block terminal development in HL-60 cells.
Figure 4.
PRKX gene expression in untreated and in PMA-treated HL-60 cells incubated with either sense or antisense oligonucleotides. Expression of the X-CGD gene was used as a myeloid maturation marker.
To confirm our observations, we used an additional method of targeted inhibition of gene expression by antisense RNA (36). We constructed tetracycline-responsive expression vectors expressing either the 5′-UTR of the PRKX cDNA or the entire coding region as an antisense RNA and transfected them into HL-60 cells. PMA treatment of these transiently transfected cells resulted in typical morphological and functional maturation when synthesis of the antisense RNA was suppressed; inducing expression of either of the antisense constructs led to a 50% reduction in the appearance of the maturation markers. Inducing the sense RNA construct had no effect on these markers. Because transfection efficiencies in HL-60 cells also are ≈50%, this finding further reinforced our conclusion that PRKX gene expression is essential for macrophage differentiation in HL-60 cells.
In human peripheral blood monocytes, macrophage maturation may be promoted by M-CSF (37). To extend our observation to normal blood cells, we studied the effect of the same antisense and sense oligonucleotides used for HL-60 cells, on M-CSF-induced maturation of peripheral blood monocytes. Monocytes incubated with the antisense oligomers did not respond to M-CSF induction, whereas the cells incubated with the sense oligonucleotides behaved similarly to the control M-CSF-treated monocytes, i.e., developed into macrophages (Table 3; Fig. 3). Thus, our results indicate that PRKX is critical for macrophage maturation of both leukemic (HL-60) and normal progenitor (blood monocytic) cells.
Table 3.
Effect of antisense oligonucleotides to PRKX on M-CSF-induced macrophage maturation of human peripheral blood monocytes
| Monocytes treated with | Adherent and spread cells, % | Phagocytic cells, % |
|---|---|---|
| — | 12 ± 7 | 48 ± 5 |
| M-CSF | 53 ± 11 | 89 ± 5 |
| M-CSF + SENSE1 | 57 ± 8 | 92 ± 5 |
| M-CSF + ANTISENSE1 | 17 ± 6 | 43 ± 5 |
| M-CSF + SENSE2 | 52 ± 9 | 89 ± 7 |
| M-CSF + ANTISENSE2 | 15 ± 6 | 46 ± 3 |
In the absence of the differentiation inducers, the oligonucleotides neither affected cell proliferation nor induced appearance of any maturation markers. The data represent the mean results of three independent experiments ± SD.
Macrophage Maturation in HL-525 Cells Transiently Transfected with an Expression Vector Encoding PRKX.
To further extend our observations on the role of PRKX in macrophage development, we used the HL-60 cell variant, HL-525, which is deficient in PKC-β and PRKX and resistant to PMA-induced macrophage maturation (14). We transiently transfected the cells with vectors expressing either the PRKX or PKC-β cDNA, treated them with PMA, and measured the maturation markers (Table 4). In the absence of PMA the fraction of cell exhibiting the macrophage-specific cell surface antigen and showing phagocytosis in various transfections was ≤3%. In the presence of PMA, the control vector yielded a 2- to 3-fold increase over this percentage, whereas the vectors encoding either PRKX or PKC-β caused 22–32% of the cells to express the maturation markers. Cotransfection of the PRKX- and PKC-β-encoding plasmids resulted in a similar level of PMA-induced maturation markers. When analyzing these transfection data, one should bear in mind that the efficiency of transient transfections is <50% by using these conditions in HL-525 cells. Thus, restoring of PRKX expression renders macrophage maturation resistant cells HL-525 sensitive to PMA-induced maturation. However, the PMA requirement suggests that PRKX expression in itself is not sufficient for macrophage maturation and that PMA-dependent events are necessary to induce the maturation program.
Table 4.
PMA-induced macrophage maturation in HL-525 cells transfected with expression vectors encoding PRKX and PKC-β
| Cells reacting with anti-MAC1 antibody, % | Phagocytic cells, % | |
|---|---|---|
| pTRE | 10 ± 1 | 5 ± 3 |
| PRKX | 26 ± 4 | 22 ± 1 |
| PKC-β | 32 ± 7 | 31 ± 3 |
| PRKX + PKC-β | 36 ± 5 | 35 ± 5 |
In the absence of PMA, 1.9–2.6% of cells reacted with the antibody, and 1.8–2.5% of cells displayed phagocytosis. The data represent the mean results of three independent experiments ± SD.
Expression of the PRKX Gene in Stable HL-525/PKC-β Transfectants.
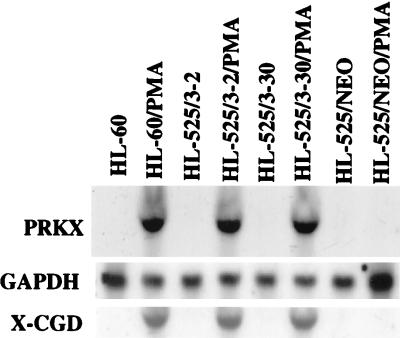
Expression of the PKC-β gene and susceptibility to PMA-induced macrophage maturation can be restored in HL-525 cells by stable transfection with expression vectors that contain PKC-β cDNA (13). To determine whether PRKX expression is under the control of PKC-β, we used two such PKC-β transfectants, HL525/3–2 and HL-525/3–30, as well as a control HL-525/NEO transfectant, which carries the expression plasmid containing only the neomycin resistance gene. HL-60 cells were used as a control. Treatment of the HL-60, HL-525/3–2, and HL-525/3–30 cells with PMA for 24 hr brought about PRKX gene expression (Fig. 5), as well as macrophage maturation, as judged by inhibition of cell proliferation, appearance of cell adherence and spreading (data not shown), and the induction of the X-CGD myeloid maturation marker gene (Fig. 5), whereas the NEO transfectants did not respond to PMA. These data indicate that reestablishing PKC-β expression in HL-525 cells results in restoration of PMA-induced PRKX gene expression and maturation, and thus implicating this PKC isozyme in the control of PRKX gene expression during macrophage maturation.
Figure 5.
Induction of the PRKX gene expression by PMA in HL-60 cells and in HL-525 stably transfected with an expression plasmid containing a full-length PKC-β cDNA (HL-525/3–2; HL-525/3–30) or a plasmid containing only the neomycin resistance gene (HL-525/NEO).
DISCUSSION
Lineage-specific blood cell differentiation and maturation are multistage processes initiated either in a cell-intrinsic or cell-extrinsic fashion and involving a signal transduction cascade that entails different protein kinases (1–4). The precise mechanisms whereby blood lineages, such as macrophages and granulocytes, develop are largely unknown. In this study we identified a kinase, which plays a critical role in the signal transduction pathway leading to macrophage and granulocyte maturation. The cDNA, which we isolated codes for the previously identified PRKX (23), which did not previously have an assigned biological function. Klink et al. (23) have classified PRKX as a serine/threonine kinase related to the catalytic subunit of cAMP-dependent protein kinases.
Our results indicate that the PRKX gene expression is markedly induced as HL-60 cells mature along the monocyte, macrophage, and granulocyte lineages. We also studied its expression in blood, heart, brain, kidney, liver, lung, pancreas, spleen, and muscle, as well as in several transformed cells lines and found that among these tissues and cell lines, the gene only is expressed in blood. Furthermore, we showed that expression of the PRKX gene is characteristic for certain myeloid blood lineages, namely monocytes, macrophages, and granulocytes, by excluding the lymphoid, erythroid, and megacaryocytic lineages.
To establish a functional rather than a correlative role for PRKX in macrophage and granulocyte differentiation, we investigated the effects of inhibiting PRKX gene expression by antisense oligodeoxyribonucleotides and RNA on induction of terminal development in HL-60 cells and normal human peripheral blood monocytes. Our results indicate that inhibition of PRKX gene expression blocks macrophage and granulocyte maturation, establishing this protein kinase as a crucial mediator of terminal development along these lineages. On the other hand, restoring PRKX expression rendered the PKC-β-deficient and maturation-resistant cell line HL-525 sensitive to PMA-induced macrophage maturation; no macrophage maturation was observed in the absence of PMA. The requirement for PMA suggests that PRKX expression in itself is not sufficient for macrophage maturation; PKC activation is needed. The HL-525 cell variant is deficient in PKC-β, an abundant PKC isozyme in the HL-60 cell line, but it expresses other PKC isozymes [e.g., PKC-α (14)], which may act as this activator.
Our earlier data (13, 14) and the results obtained in this work demonstrate the existence of two protein kinases essential for macrophage maturation, PKC-β and PRKX. These kinases could be elements of the same signal transduction cascade or they could belong to alternative pathways. In an attempt to establish the relationship between these two enzymes in the signal transduction cascade, we studied PRKX gene expression in an HL-60 cell variant, HL-525, which is deficient in PKC-β and resistant to PMA-induced macrophage maturation and in HL-525 cells stably transfected with a PKC-β-expressing plasmid (13). These transfectants regain susceptibility to PMA-induced macrophage maturation (13). Our results indicate that PMA-induced PRKX gene expression, which is deficient in HL-525 cells, is restored in the transfectants, suggesting a requirement for PKC-β for induction of PRKX gene expression during macrophage maturation. These data also imply that in HL-60 and related cell types, PRKX acts downstream of PKC-β in the same signal transduction pathway. It is likely that PRKX functions downstream of other PKC isozymes during monocytic and granulocytic maturation of HL-60 cells. It is noteworthy that the inducers of these lineages in HL-60, vitD3, and DMSO also induce the expression of PKC α, γ, δ, and ɛ (38–40). In this context, various isozymes of PKC are expressed in mature myeloid cells (38–41).
Thus, we identified a myeloid-specific protein kinase and showed it to be a key mediator of macrophage and granulocyte maturation. PRKX is unique in its specificity to macrophages and granulocytes; many components of the known signal transduction pathways are common to different cell lineages (5, 6).
Identifying appropriate inducers and elucidating the process whereby blood cell lineages develop from multipotential progenitors is fundamental to understanding and treating blood diseases including leukemia, a disorder associated with blocked blood cell differentiation. In this context, it has been recently shown that PMA has therapeutic efficacy in controlling myeloid leukemia, particularly in patients that were refractory to other chemotherapies (42). Most likely, its mode of action involves induction of maturation via sequential induction of PKC-β and PRKX gene expression.
Many questions related to the mode of PRKX action remain to be addressed, including the nature of its substrates. However, our results suggest that PRKX is a critical component of the intracellular signal transduction process that leads to development of macrophages and granulocytes from both normal and leukemic progenitor cells.
Acknowledgments
We thank Dr. G. Rappold for providing the PRKX full-length cDNA clone and Drs. F. Collart, M. Bhattacharhya, and F. Stevens for critical reading of the manuscript. This work was supported by the U.S. Department of Energy, Office of Biological and Environmental Research under Contract W-31-109-ENG-38.
ABBREVIATIONS
- PRKX
protein kinase X
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- DMSO
dimethyl sulfoxide
- vitD3
1α,25-dihydroxy-vitamin D3
- M-CSF
macrophage colony-stimulating factor
- UTR
untranslated region
- X-CGD
X-linked chronic granulomatous disease
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Metcalf D. Blood. 1998;92:345–348. [PubMed] [Google Scholar]
- 2.Enver T, Heyworth C M, Dexter T M. Blood. 1998;92:348–351. [PubMed] [Google Scholar]
- 3.Sachs L. Int J Dev Biol Suppl. 1996;1:61S–62S. [PubMed] [Google Scholar]
- 4.Orkin S H. Curr Opin Genet Dev. 1996;6:597–602. doi: 10.1016/s0959-437x(96)80089-x. [DOI] [PubMed] [Google Scholar]
- 5.Metcalf D. Blood. 1993;82:3515–3523. [PubMed] [Google Scholar]
- 6.Socolovsky M, Lodish H F, Daley G Q. Proc Natl Acad Sci USA. 1998;95:6573–6575. doi: 10.1073/pnas.95.12.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huberman E, Callaham M F. Proc Natl Acad Sci USA. 1979;76:1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rovera G, Santoli D, Damsky C. Proc Natl Acad Sci USA. 1979;76:2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murao S I, Gemmell M A, Callaham M F, Anderson N L, Huberman E. Cancer Res. 1983;43:4989–4996. [PubMed] [Google Scholar]
- 10.Collins S J, Ruscetti F W, Gallagher R E, Gallo R C. Proc Natl Acad Sci USA. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitman T R, Selonick S E, Collins S J. Proc Natl Acad Sci USA. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homma Y, Henning-Chubb C B, Huberman E. Proc Natl Acad Sci USA. 1986;83:7316–7319. doi: 10.1073/pnas.83.19.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonetti D A, Henning-Chubb C, Yamanishi D T, Huberman E. J Biol Chem. 1994;269:23230–23235. [PubMed] [Google Scholar]
- 14.Tonetti D A, Horio M, Collart F R, Huberman E. Cell Growth Differ. 1992;3:739–745. [PubMed] [Google Scholar]
- 15.Kharbanda S, Saleem A, Emoto Y, Stone R, Rapp U, Kufe D. J Biol Chem. 1994;269:872–878. [PubMed] [Google Scholar]
- 16.Liang P, Pardee A B. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 17.Laouar A, Bauvois B. Immunol Lett. 1992;34:257–266. doi: 10.1016/0165-2478(92)90222-a. [DOI] [PubMed] [Google Scholar]
- 18.Laouar A, Collart F, Huberman E. In: Cell Biology: A Laboratory Handbook. Celis J E, editor. San Diego: Academic; 1997. [Google Scholar]
- 19.Scher W, Friend C. Cancer Res. 1978;38:841–849. [PubMed] [Google Scholar]
- 20.Royer-Pokora B, Kunkel L M, Monaco A P, Goff S C, Newburger P E, Baehner R L, Cole F S, Curnutte J T, Orkin S H. Nature (London) 1986;322:32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- 21.Chirgwin J A, Przybyla R, MacDonald R, Rutter R. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 22.Altschul S F, Gish W, Miller W, Myers E, Lipman J L. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.Klink A, Schiebel K, Winkelmann M, Rao E, Horshemke B, Luedecke H-J, Claussen U, Scherer G, Rappold G. Hum Mol Genet. 1995;4:869–878. doi: 10.1093/hmg/4.5.869. [DOI] [PubMed] [Google Scholar]
- 24.Xie B, Laouar A, Huberman E. J Biol Chem. 1998;273:11583–11588. doi: 10.1074/jbc.273.19.11583. [DOI] [PubMed] [Google Scholar]
- 25.Lin H-Y, Thakore H R, Davis F B, Martino L J, Davis P J. Interferon Cytokine Res. 1996;16:17–24. doi: 10.1089/jir.1996.16.17. [DOI] [PubMed] [Google Scholar]
- 26.Bacus S S, Kiguchi K, Chin D, King C R, Huberman E. Mol Carcinogen. 1990;3:350–362. doi: 10.1002/mc.2940030607. [DOI] [PubMed] [Google Scholar]
- 27.Rasheed S, Nelson-Rees W A, Toth E M, Arnstein P, Gardner M B. Cancer (Philadelphia) 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 28.Kiguchi K, Collart F R, Henning-Chubb C, Huberman E. Cell Growth Differ. 1990;1:259–270. [PubMed] [Google Scholar]
- 29.Berger S R, Zutter M M, Sturgill-Kocsycki S, Santoro S A. Exp Cell Res. 1992;202:28–35. doi: 10.1016/0014-4827(92)90400-3. [DOI] [PubMed] [Google Scholar]
- 30.Rutherford T, Clegg J B, Higgs D R, Jones R W, Thompson J, Weatherall D J. Proc Natl Acad Sci USA. 1981;78:348–352. doi: 10.1073/pnas.78.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen H Q, Hoffman-Liebermann B, Liebermann D. Cell. 1993;72:197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 32.Wickstrom E L, Bacon T A, Gonzalez A, Freeman D L, Lyman G H, Wickstrom E. Proc Natl Acad Sci USA. 1988;85:1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng G, Ariga T, Gu X-B, Yu R K. Proc Natl Acad Sci USA. 1995;92:8670–8674. doi: 10.1073/pnas.92.19.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuker M. Methods Enzymol. 1989;180:262–288. doi: 10.1016/0076-6879(89)80106-5. [DOI] [PubMed] [Google Scholar]
- 35.Dash P, Lotan I, Knapp M, Kandel E R, Goelet P. Proc Natl Acad Sci USA. 1987;84:7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izant J G, Weintraub H. Science. 1985;229:345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- 37.Becker S, Warren M K, Haskill S. J Immunol. 1987;139:3703–3709. [PubMed] [Google Scholar]
- 38.Seibenhener M L, Wooten M W. Exp Cell Res. 1993;207:183–188. doi: 10.1006/excr.1993.1178. [DOI] [PubMed] [Google Scholar]
- 39.Obeid L M, Okazaki T, Karolak L A, Hannun Y A. J Biol Chem. 1990;265:2370–2374. [PubMed] [Google Scholar]
- 40.Makowske M, Ballester R, Cayre Y, Rosen O M. J Biol Chem. 1988;263:3402–3410. [PubMed] [Google Scholar]
- 41.Sergeant S, McPhail L C. J Immunol. 1997;159:2877–2885. [PubMed] [Google Scholar]
- 42.Han Z T, Zhu X X, Yang R Y, Sun J Z, Tian G F, Liu X J, Cao G S, Newmark H L, Conney A H, Chang R L. Proc Natl Acad Sci USA. 1998;95:5357–5361. doi: 10.1073/pnas.95.9.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]